Beruflich Dokumente
Kultur Dokumente
Quiz 1 Name: Student Number: Group:: QUIZ1/JUNE-OCT2012/CHM257
Hochgeladen von
cookiesandmilk0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
21 Ansichten2 SeitenWrite a Lewis structure of NO3and determine the formal charge on nitrogen atom. State the types of hybridization of bolded carbon atom in the following compounds. Predict the bond angle of the hybrid orbitals.
Originalbeschreibung:
Originaltitel
Quiz 1
Copyright
© Attribution Non-Commercial (BY-NC)
Verfügbare Formate
DOC, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenWrite a Lewis structure of NO3and determine the formal charge on nitrogen atom. State the types of hybridization of bolded carbon atom in the following compounds. Predict the bond angle of the hybrid orbitals.
Copyright:
Attribution Non-Commercial (BY-NC)
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
21 Ansichten2 SeitenQuiz 1 Name: Student Number: Group:: QUIZ1/JUNE-OCT2012/CHM257
Hochgeladen von
cookiesandmilkWrite a Lewis structure of NO3and determine the formal charge on nitrogen atom. State the types of hybridization of bolded carbon atom in the following compounds. Predict the bond angle of the hybrid orbitals.
Copyright:
Attribution Non-Commercial (BY-NC)
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 2
QUIZ1/JUNE-OCT2012/CHM257
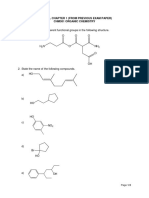
QUIZ 1 NAME: STUDENT NUMBER: GROUP: 1. Write a Lewis structure of NO3- and determine the formal charge on nitrogen atom.
(2 marks) 2. State the types of hybridization of bolded carbon atom in the following compounds. Predict the bond angle of the hybrid orbitals. i) CH3 O C OH
ii)
CH3 CH3CHCH3
(4 marks) 3. Consider the following organic compound. O CH3CCH2CH2C i) CCH3
Draw the skeleton structure for the above compound.
QUIZ1/JUNE-OCT2012/CHM257
ii)
Identify two functional groups present in this compound.
(3 marks) 4. Write the IUPAC names of the following compounds. i)
CH3 CH3CH2CH2CHCHCH3 CH2CH2CH2CH3
(2 marks) ii)
Br
(2 marks) 5. Arrange these following alkanes in order of increasing boiling point. Butane, decane and hexane
(2 marks)
Das könnte Ihnen auch gefallen
- Hsslive-Xi-Chem-Prvs-Qn-12. Organic Chemistry Some Basic PrinciplesDokument7 SeitenHsslive-Xi-Chem-Prvs-Qn-12. Organic Chemistry Some Basic PrinciplesLayanNoch keine Bewertungen
- Tutorial 1 Chapter 1: Introduction To Organic Chemistry: C C H CN H HDokument4 SeitenTutorial 1 Chapter 1: Introduction To Organic Chemistry: C C H CN H HRAIN9393Noch keine Bewertungen
- Hsslive Xi Chemistry QB CH 12. Organic Chemistry Some Basic PrinciplesDokument7 SeitenHsslive Xi Chemistry QB CH 12. Organic Chemistry Some Basic PrinciplesAshly Santhosh KNoch keine Bewertungen
- Tutorial 1Dokument3 SeitenTutorial 1sylvieNoch keine Bewertungen
- Worksheet For Organic SectionDokument17 SeitenWorksheet For Organic SectionPramudith Liyanage100% (2)
- Hsslive XI Chemistry QB CH 13. HydrocarbonsDokument5 SeitenHsslive XI Chemistry QB CH 13. Hydrocarbonsanumaria bijuNoch keine Bewertungen
- Plus 1 - Chemistry PYQ HydrocarbonsDokument6 SeitenPlus 1 - Chemistry PYQ Hydrocarbonssivaranjini S.VNoch keine Bewertungen
- Work Sheet-Some Basic Concepts in Organic ChemistryDokument2 SeitenWork Sheet-Some Basic Concepts in Organic ChemistryHarshitha MenonNoch keine Bewertungen
- CBSE Class 12 Chemistry Coordination CompoundsDokument2 SeitenCBSE Class 12 Chemistry Coordination CompoundsDeepa PaulNoch keine Bewertungen
- CBSE Class 12 Chemistry Coordination Compounds PDFDokument2 SeitenCBSE Class 12 Chemistry Coordination Compounds PDFDeepa PaulNoch keine Bewertungen
- Classroom - Sep 7, 2022 at 9:50 AMDokument3 SeitenClassroom - Sep 7, 2022 at 9:50 AMEeshika KalotyNoch keine Bewertungen
- Chem Q.bank Xi 2022Dokument16 SeitenChem Q.bank Xi 2022rishikaa.saxenaNoch keine Bewertungen
- Tutorial Chapter 1 chm301Dokument2 SeitenTutorial Chapter 1 chm301NURUL AINUN MUHAMMAD NORNoch keine Bewertungen
- 2000-2019 Nesa Chemistry Advanced Level-1Dokument269 Seiten2000-2019 Nesa Chemistry Advanced Level-1Jeff AlbaNoch keine Bewertungen
- Chemical Bonding Year 11 Jan 2020Dokument4 SeitenChemical Bonding Year 11 Jan 2020Subesh ShanmugamNoch keine Bewertungen
- Tutorial 4, 5 & 6 CHM361Dokument4 SeitenTutorial 4, 5 & 6 CHM3612021819542Noch keine Bewertungen
- Exercise Final Chem 1Dokument5 SeitenExercise Final Chem 1Travis PhelpsNoch keine Bewertungen
- Mcqs Chemistry Sample PracticeDokument3 SeitenMcqs Chemistry Sample PracticeWajid Ali0% (1)
- Apch11 EstersDokument2 SeitenApch11 Estersloly62006Noch keine Bewertungen
- Previous Hse Questions From The Chapter "Co-Ordination Compounds"Dokument3 SeitenPrevious Hse Questions From The Chapter "Co-Ordination Compounds"SOny binuNoch keine Bewertungen
- Action Center 1 Constitutional IsomersDokument1 SeiteAction Center 1 Constitutional IsomersooitzandyooNoch keine Bewertungen
- Organic Chemistry Structure and BondingDokument13 SeitenOrganic Chemistry Structure and BondingHossNoch keine Bewertungen
- ICH602S Tutorial 18 September 2017Dokument1 SeiteICH602S Tutorial 18 September 2017Maria GaingosNoch keine Bewertungen
- Tutorial 1A: Basic Chemistry ConceptsDokument4 SeitenTutorial 1A: Basic Chemistry ConceptsWeijuan YuenNoch keine Bewertungen
- Sch3u Review 20923 42 04Dokument4 SeitenSch3u Review 20923 42 04limichael000Noch keine Bewertungen
- Chemistry 231 Spring 2023 Exam 1.docx63ed278f8b08f4482Dokument7 SeitenChemistry 231 Spring 2023 Exam 1.docx63ed278f8b08f4482francisNoch keine Bewertungen
- Indian Institute of Technology, Kharagpur: Answer All QuestionsDokument3 SeitenIndian Institute of Technology, Kharagpur: Answer All QuestionsAnurag TiwariNoch keine Bewertungen
- Chemistry QP - FYDokument2 SeitenChemistry QP - FYmuneerkkmullaNoch keine Bewertungen
- CHAPTER 1 - Covalent Bonding and Shapes of MoleculesDokument10 SeitenCHAPTER 1 - Covalent Bonding and Shapes of MoleculeslorrainebarandonNoch keine Bewertungen
- Work Book (Phase - IV) : SubjectiveDokument21 SeitenWork Book (Phase - IV) : SubjectiveAshwani Kumar SinghNoch keine Bewertungen
- AL Chemistry 1996 Paper 1+2Dokument12 SeitenAL Chemistry 1996 Paper 1+2api-3734333Noch keine Bewertungen
- Sample Paper Gr11Dokument3 SeitenSample Paper Gr11Enoca AJNoch keine Bewertungen
- S.5 Bot Ii Chem 2 2019 Revision Past PapersDokument5 SeitenS.5 Bot Ii Chem 2 2019 Revision Past PapersMaama PhionaNoch keine Bewertungen
- Sch3u Exam Review Ws s2018 PDFDokument4 SeitenSch3u Exam Review Ws s2018 PDFwdsfNoch keine Bewertungen
- CH 12 Organic WSDokument5 SeitenCH 12 Organic WSRaviNoch keine Bewertungen
- Hsslive-9. Co-Ordination CompoundsDokument8 SeitenHsslive-9. Co-Ordination Compoundssindhumv631Noch keine Bewertungen
- TS - JR - Ipe Chemistry Important Questions - 01-03-2023Dokument6 SeitenTS - JR - Ipe Chemistry Important Questions - 01-03-2023bittu060606Noch keine Bewertungen
- Chm092 Tutorial 4Dokument4 SeitenChm092 Tutorial 4Ain Syakirah AzleeNoch keine Bewertungen
- TS JR (Pre-Final-2) (Chemstry Q P) Ex DT 17-04-2021Dokument2 SeitenTS JR (Pre-Final-2) (Chemstry Q P) Ex DT 17-04-2021AbhiNoch keine Bewertungen
- T12 Introduction To Organic Chemistry 27-34Dokument8 SeitenT12 Introduction To Organic Chemistry 27-34饶宝珍Noch keine Bewertungen
- Carbon and Its CompoundsDokument27 SeitenCarbon and Its CompoundstechvipreshNoch keine Bewertungen
- CHEM 333 - Principles of Organic Chemistry I Exam 1 Spring 200X Student's Name: 75min ID #: - Total: 100ptsDokument3 SeitenCHEM 333 - Principles of Organic Chemistry I Exam 1 Spring 200X Student's Name: 75min ID #: - Total: 100ptsNo WoNoch keine Bewertungen
- Chemistry Cbse Mock TestDokument10 SeitenChemistry Cbse Mock TestHrityush ShivamNoch keine Bewertungen
- Sukellimo Chem Pp1 Qns Teacher Co KeDokument15 SeitenSukellimo Chem Pp1 Qns Teacher Co Keianmutunga5070Noch keine Bewertungen
- NYA Winter 08 Unit Test 2bDokument6 SeitenNYA Winter 08 Unit Test 2bDr. Michael Lautman100% (3)
- SCH 2108 Organic Chemistry KisiiDokument4 SeitenSCH 2108 Organic Chemistry KisiiCaleb MumohNoch keine Bewertungen
- Chemistry Unit ReviewDokument4 SeitenChemistry Unit ReviewnishilgeorgeNoch keine Bewertungen
- Final Exam Review Fall 2009 AnswersDokument14 SeitenFinal Exam Review Fall 2009 AnswersCharisma SubaNoch keine Bewertungen
- Chem Exam Style QN PDFDokument13 SeitenChem Exam Style QN PDFChirisuu PantsuNoch keine Bewertungen
- ChemistryDokument14 SeitenChemistryGutsy Studs7Noch keine Bewertungen
- Chemistry SQP XII PDFDokument14 SeitenChemistry SQP XII PDFIshikaGuptaNoch keine Bewertungen
- Q Names1 PDFDokument4 SeitenQ Names1 PDFClement Charles100% (1)
- Problem Set 1 PDFDokument2 SeitenProblem Set 1 PDFLouisNoch keine Bewertungen
- Coord CompDokument3 SeitenCoord CompShivaanee SKNoch keine Bewertungen
- Chem 0000Dokument5 SeitenChem 0000chikondikosamu24Noch keine Bewertungen
- NFTF Tutorial Chapter 5 Q LatestDokument3 SeitenNFTF Tutorial Chapter 5 Q LatestHoneySingerYugenNoch keine Bewertungen
- HYdrocarbons PDFDokument24 SeitenHYdrocarbons PDFKumar PANKAJNoch keine Bewertungen
- TUTORIAL 1 AlcoholDokument6 SeitenTUTORIAL 1 Alcohol2023837078Noch keine Bewertungen
- Second Terminal Examination, 2017: Chemistry Time - 3:00 Hrs. Class XI M.M. - 70Dokument5 SeitenSecond Terminal Examination, 2017: Chemistry Time - 3:00 Hrs. Class XI M.M. - 7049. Bhavy PatelNoch keine Bewertungen
- Hyrdogen Storage TechnologiesVon EverandHyrdogen Storage TechnologiesMehmet SankirNoch keine Bewertungen
- Class 11 Chapter 4 Chemical Bonding and Molecular StructureDokument139 SeitenClass 11 Chapter 4 Chemical Bonding and Molecular Structureprateek yadavNoch keine Bewertungen
- Molecular Geometry and Bonding TheoriesDokument24 SeitenMolecular Geometry and Bonding TheoriesHendri KurniawanNoch keine Bewertungen
- MilitaryArchitectureinEnglandDuringtheMiddleAges 10061529Dokument417 SeitenMilitaryArchitectureinEnglandDuringtheMiddleAges 10061529jurebieNoch keine Bewertungen
- Chemical Bonding PDFDokument14 SeitenChemical Bonding PDFTai PanNoch keine Bewertungen
- Molecular Orbitals and Hybridisation: Organic ChemistryDokument28 SeitenMolecular Orbitals and Hybridisation: Organic ChemistryWinni TanNoch keine Bewertungen
- General Organic Chemistry For Iit/aipmt/aieeeDokument7 SeitenGeneral Organic Chemistry For Iit/aipmt/aieeeIshan Khanna71% (14)
- Chemical Bonding Package SolutionsDokument17 SeitenChemical Bonding Package Solutionspriyanshu rajputNoch keine Bewertungen
- Chemical Bonding WS Packet Margie Core 2013Dokument4 SeitenChemical Bonding WS Packet Margie Core 2013Lama DebanaNoch keine Bewertungen
- NEET Question PaperDokument6 SeitenNEET Question Papergk7936Noch keine Bewertungen
- Sri Chaitanya IIT Academy, India: KEY Sheet PhysicsDokument6 SeitenSri Chaitanya IIT Academy, India: KEY Sheet PhysicsO SNoch keine Bewertungen
- Lecture 5the Periodic Table PDFDokument23 SeitenLecture 5the Periodic Table PDFMohammedNoch keine Bewertungen
- Lecture 1 NotesDokument46 SeitenLecture 1 NotesIncNoch keine Bewertungen
- Solid State Bonding in SolidsDokument6 SeitenSolid State Bonding in SolidsbookregtestNoch keine Bewertungen
- General Chemistry I - Q2 M7.1 Ionic & Covalent BondsDokument22 SeitenGeneral Chemistry I - Q2 M7.1 Ionic & Covalent BondseliNoch keine Bewertungen
- Periodic Trends Gizmo AssessmentDokument3 SeitenPeriodic Trends Gizmo AssessmentDhruv VmNoch keine Bewertungen
- Che 91164 RevisionDokument0 SeitenChe 91164 Revisionapi-218511741Noch keine Bewertungen
- Chemical Bond Assig (Ans) 04 11 20Dokument4 SeitenChemical Bond Assig (Ans) 04 11 20Rushikesh ThoratNoch keine Bewertungen
- Atomic Radii in Crystals - Slater 1969Dokument7 SeitenAtomic Radii in Crystals - Slater 1969Chelsea ClarkNoch keine Bewertungen
- TAPAYAN GeneralChemistry1 Q2 Module-2-1Dokument37 SeitenTAPAYAN GeneralChemistry1 Q2 Module-2-1Raquel Leigh TorresNoch keine Bewertungen
- پیوندهای شیمیائی - ساختار لویس - هندسه مولکولی - قطبیت مولکولهاDokument24 Seitenپیوندهای شیمیائی - ساختار لویس - هندسه مولکولی - قطبیت مولکولهاapi-3706290Noch keine Bewertungen
- Unit 3Dokument26 SeitenUnit 3Himangshu SarmahNoch keine Bewertungen
- Qualitative Treatment of Molecular Orbital TheoryDokument27 SeitenQualitative Treatment of Molecular Orbital TheoryIfiok UsoroNoch keine Bewertungen
- Data Discovery StudioDokument15 SeitenData Discovery Studioazzatul amiraNoch keine Bewertungen
- Structural EffectsDokument45 SeitenStructural EffectsOrlando Angelo Cerezo100% (1)
- Chapter 6 Shapes of Molecules and Intermolecular ForcesDokument9 SeitenChapter 6 Shapes of Molecules and Intermolecular Forcesnoreen doraniNoch keine Bewertungen
- Edudigm: Chemistry For IIT-JEE & Other Entrance ExamsDokument32 SeitenEdudigm: Chemistry For IIT-JEE & Other Entrance Examstapas kunduNoch keine Bewertungen
- Hosmane 2017Dokument16 SeitenHosmane 2017murat tosunNoch keine Bewertungen
- Organo MetallicDokument25 SeitenOrgano MetallicbutiayundaNoch keine Bewertungen
- Organic Chemistry 1Dokument324 SeitenOrganic Chemistry 1Bellony Sanders100% (7)
- CMC Chapter 07Dokument101 SeitenCMC Chapter 07MattNoch keine Bewertungen