Beruflich Dokumente
Kultur Dokumente
S
Hochgeladen von
Ehsanul Haque Nirjhar0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
14 Ansichten1 Seitemath
Copyright
© Attribution Non-Commercial (BY-NC)
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenmath
Copyright:
Attribution Non-Commercial (BY-NC)
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
14 Ansichten1 SeiteS
Hochgeladen von
Ehsanul Haque Nirjharmath
Copyright:
Attribution Non-Commercial (BY-NC)
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 1
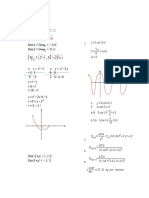
Kittel Problem 1.3 Show that the c/a ratio for an ideal hexagonal close-packed structure is 8/3 = 1.633.
If c/a is signicantly larger than this value, the crystal structure may be thought of as composed of planes of closely packed atoms, the planes being loosely packed.
h. . .height of the equilateral triangle a. . .lattice constant 1 a= 4 3 a 4
h=
a2 (a/2)2 =
Distance x from an atom to the middle of the triangle: x= 2 2 h= 3 3 1 x= a 3 c 2 = a x2 = 2 c = a 1 a2 a2 = 3 8 3 2 a 3 3 a 4
Das könnte Ihnen auch gefallen
- Ay 127: Midterm Solution: Samaporn Tinyanont February 20, 2017Dokument4 SeitenAy 127: Midterm Solution: Samaporn Tinyanont February 20, 2017TmusicKenanNoch keine Bewertungen
- Cambridge Physics PhD Tutor Optimization ProblemsDokument5 SeitenCambridge Physics PhD Tutor Optimization ProblemsHin Wa LeungNoch keine Bewertungen
- Find The Values of The Six Circular Functions ofDokument1 SeiteFind The Values of The Six Circular Functions ofGiorno GiovannaNoch keine Bewertungen
- Calculus 2 Tutorial 2Dokument7 SeitenCalculus 2 Tutorial 2Rumbidzai KambaNoch keine Bewertungen
- PCweek4 2015Dokument11 SeitenPCweek4 2015Roy VeseyNoch keine Bewertungen
- Fisika Zat Padat I Muhammad Arohman F1B118006Dokument7 SeitenFisika Zat Padat I Muhammad Arohman F1B118006Rohman muhNoch keine Bewertungen
- Dispersion relation of a two-dimensional hexagonal latticeDokument3 SeitenDispersion relation of a two-dimensional hexagonal latticeThanh NguyenNoch keine Bewertungen
- Ejercicios Propuestos Movimiento CurvilineoDokument2 SeitenEjercicios Propuestos Movimiento CurvilineoSaday CastrilloNoch keine Bewertungen
- Iit, Review Final Examination, Math 333Dokument4 SeitenIit, Review Final Examination, Math 333Quỳnh Trang TrầnNoch keine Bewertungen
- Kittel Charles Introduction To Solid StaDokument60 SeitenKittel Charles Introduction To Solid Stahareg bogaleNoch keine Bewertungen
- Binomial Theorem Solution PDFDokument10 SeitenBinomial Theorem Solution PDFNiranjanNoch keine Bewertungen
- Formule Puteri Radicali Si LogaritmiDokument3 SeitenFormule Puteri Radicali Si LogaritmiAdrian CocosNoch keine Bewertungen
- 165 175 Bpas e June2020Dokument11 Seiten165 175 Bpas e June2020Jade Bong NatuilNoch keine Bewertungen
- Maths SL PracticeDokument3 SeitenMaths SL PracticeGAVUENoch keine Bewertungen
- Introducción A La Física de Estado Sólido - Charles Kittel - 8va Edición PDFDokument60 SeitenIntroducción A La Física de Estado Sólido - Charles Kittel - 8va Edición PDFvlae do ventoNoch keine Bewertungen
- Chapter 2. Review of Matrix Algebra: X A X X A X A X A XDokument4 SeitenChapter 2. Review of Matrix Algebra: X A X X A X A X A Xsakeriraq81Noch keine Bewertungen
- Solucionário Introdução A Física Do Estado Sólido Charles Kittel 460 Hmwk1 KitellDokument4 SeitenSolucionário Introdução A Física Do Estado Sólido Charles Kittel 460 Hmwk1 KitellJosias Santos100% (2)
- Introduction To Solid State Physics 8Th Edition - Solution Manual-Kittel Charles PDFDokument64 SeitenIntroduction To Solid State Physics 8Th Edition - Solution Manual-Kittel Charles PDFresuscitatNoch keine Bewertungen
- Jawaban MTK SalsaDokument2 SeitenJawaban MTK SalsaSalsabila ArianiNoch keine Bewertungen
- A Circle of Radius 3 Can Be Expressed in A Number of Ways: Planar CurvesDokument21 SeitenA Circle of Radius 3 Can Be Expressed in A Number of Ways: Planar CurvestrijrajNoch keine Bewertungen
- Semana 15 CalculoDokument10 SeitenSemana 15 CalculoJavier GarciaNoch keine Bewertungen
- Finding the Shrinking Hexagram Area FormulaDokument24 SeitenFinding the Shrinking Hexagram Area FormulagedNoch keine Bewertungen
- JEE Main B.Arch Sample Paper 2020 Set ADokument29 SeitenJEE Main B.Arch Sample Paper 2020 Set Ach.venkateshNoch keine Bewertungen
- Phpa 339 U9Dokument22 SeitenPhpa 339 U9iiojnjnNoch keine Bewertungen
- EJERCICIOS DE CAMBIO DE VARIABLEDokument19 SeitenEJERCICIOS DE CAMBIO DE VARIABLET. MiesNoch keine Bewertungen
- 2013 JC2 H2 Math Post MYE Revision Paper Solns For Students PDFDokument9 Seiten2013 JC2 H2 Math Post MYE Revision Paper Solns For Students PDFDouglas TanNoch keine Bewertungen
- Math Project WorkDokument19 SeitenMath Project Workrakesh9988Noch keine Bewertungen
- Idz 8.1 Variant 1 (Idz - KZ)Dokument3 SeitenIdz 8.1 Variant 1 (Idz - KZ)Miras BolatovNoch keine Bewertungen
- Tarea 2-AlejandrafajardoDokument7 SeitenTarea 2-AlejandrafajardoMoviLed DCNoch keine Bewertungen
- Chapter 4Dokument40 SeitenChapter 4Asadullah MemonNoch keine Bewertungen
- Matrix Matrix OpnDokument20 SeitenMatrix Matrix OpnVinod NambiarNoch keine Bewertungen
- Parametric Equations: Harvey Mudd College Math TutorialDokument3 SeitenParametric Equations: Harvey Mudd College Math TutorialArtist RecordingNoch keine Bewertungen
- Tarea 11Dokument6 SeitenTarea 11Yole HerediaNoch keine Bewertungen
- Solutions To Assignment 1: Lecturer: Zhou ZhangDokument5 SeitenSolutions To Assignment 1: Lecturer: Zhou Zhang杨胜Noch keine Bewertungen
- ACESORIADokument2 SeitenACESORIAjv680802Noch keine Bewertungen
- Formulae ChartDokument13 SeitenFormulae Chartsaic27927Noch keine Bewertungen
- HB C11 ISM 05 Final Odd Even PDFDokument124 SeitenHB C11 ISM 05 Final Odd Even PDFMarkNoch keine Bewertungen
- Xy X Xy X: Dom F Domg Dom F Domg y 3 Cos (8 X)Dokument3 SeitenXy X Xy X: Dom F Domg Dom F Domg y 3 Cos (8 X)Ana Moreyra SalasNoch keine Bewertungen
- Xy X Xy X: Dom F Domg Dom F Domg y 3 Cos (8 X)Dokument3 SeitenXy X Xy X: Dom F Domg Dom F Domg y 3 Cos (8 X)Ana Moreyra SalasNoch keine Bewertungen
- PC1 - Material 1 (2018 - 3) analysis of functionsDokument3 SeitenPC1 - Material 1 (2018 - 3) analysis of functionsAna Moreyra SalasNoch keine Bewertungen
- Xy X Xy X: Dom F Domg Dom F Domg y 3 Cos (8 X)Dokument3 SeitenXy X Xy X: Dom F Domg Dom F Domg y 3 Cos (8 X)Ana Moreyra SalasNoch keine Bewertungen
- Xy X Xy X: Dom F Domg Dom F Domg y 3 Cos (8 X)Dokument3 SeitenXy X Xy X: Dom F Domg Dom F Domg y 3 Cos (8 X)Ana Moreyra SalasNoch keine Bewertungen
- PC1 - Material 1 (2018 - 3) analysis of functionsDokument3 SeitenPC1 - Material 1 (2018 - 3) analysis of functionsAna Moreyra SalasNoch keine Bewertungen
- A A A A Axax Xa A A - . - . C C C C C C: Bab 1 Eksponen & Logaritma 4. Pangkat PecahanDokument13 SeitenA A A A Axax Xa A A - . - . C C C C C C: Bab 1 Eksponen & Logaritma 4. Pangkat Pecahansmkmaarifnu srddNoch keine Bewertungen
- Calculus For Business Economics and The Social and Life Scienc Brief Edition 11th Edition Hoffmann Solutions ManualDokument22 SeitenCalculus For Business Economics and The Social and Life Scienc Brief Edition 11th Edition Hoffmann Solutions Manualstockeadishureejw100% (12)
- MTH4100 Calculus I: Lecture Notes For Week 3 Thomas' Calculus, Sections 1.5, 1.6, 2.1, 2.2 and 2.4Dokument14 SeitenMTH4100 Calculus I: Lecture Notes For Week 3 Thomas' Calculus, Sections 1.5, 1.6, 2.1, 2.2 and 2.4Roy VeseyNoch keine Bewertungen
- GeometriaDokument3 SeitenGeometriakenisita valverdeNoch keine Bewertungen
- Lesson 4 - Matrices Part 2Dokument13 SeitenLesson 4 - Matrices Part 2ace orellanoNoch keine Bewertungen
- Chapter 3. Determinants and DiagonalizationDokument30 SeitenChapter 3. Determinants and DiagonalizationTrungkeeperNoch keine Bewertungen
- Qdoc - Tips Ece633f09hw2solutionsDokument13 SeitenQdoc - Tips Ece633f09hw2solutionsTrần Trọng TiếnNoch keine Bewertungen
- Math TutorialDokument5 SeitenMath TutorialJoseph WhelliusNoch keine Bewertungen
- Review of Matrices: 1.1 DefinitionsDokument17 SeitenReview of Matrices: 1.1 DefinitionsAlexander ChenNoch keine Bewertungen
- Em04 IlDokument10 SeitenEm04 IlAdri adriNoch keine Bewertungen
- Week 1 Functions and Their GraphsDokument12 SeitenWeek 1 Functions and Their GraphsCynthia PlazaNoch keine Bewertungen
- Print TakeDokument6 SeitenPrint TakeDr. Anuradha ChugNoch keine Bewertungen
- Algebra problems and factorizationsDokument2 SeitenAlgebra problems and factorizationsAngeln Calsin CariNoch keine Bewertungen
- CircleDokument2 SeitenCircleabdul shaggyNoch keine Bewertungen
- Pertemuan 3 Dimensi 3 IPA A Selasa Kamis Ka IrfanDokument47 SeitenPertemuan 3 Dimensi 3 IPA A Selasa Kamis Ka IrfanMuhammad Irfan Arsyad PrayitnoNoch keine Bewertungen
- A Course of Mathematics for Engineers and ScientistsVon EverandA Course of Mathematics for Engineers and ScientistsNoch keine Bewertungen
- 16.810 L8 OptimizationDokument58 Seiten16.810 L8 OptimizationsaravanaNoch keine Bewertungen
- Imp Gas PDFDokument42 SeitenImp Gas PDFharimadhavareddyNoch keine Bewertungen
- HPC PDFDokument1 SeiteHPC PDFMohamed Ashraf HindyNoch keine Bewertungen
- Peter Jensen PolymersDokument25 SeitenPeter Jensen PolymersUday KiranNoch keine Bewertungen
- Windlens PresentationDokument15 SeitenWindlens PresentationMohamed Ashraf HindyNoch keine Bewertungen
- Partial Answers to Chapter End ProblemsDokument71 SeitenPartial Answers to Chapter End ProblemsNiyat PatelNoch keine Bewertungen
- How To Write Your First Research Paper: Focus: Education - Career AdviceDokument10 SeitenHow To Write Your First Research Paper: Focus: Education - Career AdviceRicardo ChavarriaNoch keine Bewertungen
- Lattices and Crystal StructuresDokument4 SeitenLattices and Crystal StructuresMohamed Ashraf HindyNoch keine Bewertungen
- HCPDokument4 SeitenHCPIshan BaruahNoch keine Bewertungen
- 14 Vib ExpDokument5 Seiten14 Vib ExpMohamed Ashraf HindyNoch keine Bewertungen
- Bravais Lattice and Basis Define Crystal StructureDokument8 SeitenBravais Lattice and Basis Define Crystal StructureMohamed Ashraf HindyNoch keine Bewertungen
- Design of Formula SAE Brake SystemsDokument6 SeitenDesign of Formula SAE Brake SystemsFuinha120194Noch keine Bewertungen
- Crystal StructuresDokument12 SeitenCrystal StructuresMohamed Ashraf HindyNoch keine Bewertungen
- Pa 00307Dokument2 SeitenPa 00307Mohamed Ashraf HindyNoch keine Bewertungen
- Functions Staticitcs ExcelDokument15 SeitenFunctions Staticitcs Exceljcar727Noch keine Bewertungen
- Static Force AnalysisDokument16 SeitenStatic Force AnalysisMohamed Ashraf HindyNoch keine Bewertungen
- Automotive Piston RingDokument68 SeitenAutomotive Piston RingARVINDA KUMARNoch keine Bewertungen
- CombustionDokument27 SeitenCombustionMohamed Ashraf Hindy100% (1)
- Defects in The ProductDokument3 SeitenDefects in The ProductMohamed Ashraf HindyNoch keine Bewertungen
- L1 Renewable EnergyDokument25 SeitenL1 Renewable EnergyMohamed Ashraf HindyNoch keine Bewertungen
- Defects in The ProductDokument3 SeitenDefects in The ProductMohamed Ashraf HindyNoch keine Bewertungen
- The Suspension System: (Absorb Then Dissipate)Dokument17 SeitenThe Suspension System: (Absorb Then Dissipate)Mohamed Ashraf HindyNoch keine Bewertungen
- Sand casting processDokument2 SeitenSand casting processMohamed Ashraf HindyNoch keine Bewertungen
- Edpt 401-Cad Lab In-Class Project (Autocad)Dokument2 SeitenEdpt 401-Cad Lab In-Class Project (Autocad)Mohamed Ashraf HindyNoch keine Bewertungen