Beruflich Dokumente
Kultur Dokumente
LTO and CPR Processing
Hochgeladen von
verkieCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
LTO and CPR Processing
Hochgeladen von
verkieCopyright:
Verfügbare Formate
2/5/2014
LTO and CPR Processing
Downloadables
LTO and CPR Processing
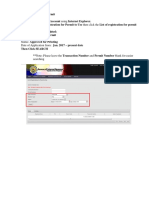
Download. There are two application forms: License to Operate (LTO), and Certificate of Product Registration (CPR). Make sure you have the right form. Remember that a valid LTO is required for a CPR. Only one establishment per LTO application form. Only one product per CPR application form. Fill up Form. To facilitate the process, please provide as much information as possible. Avoid abbreviations. Use 'Not Applicable' when appropriate. Double check the spelling. Multiple applications in a single email are acceptable. Use the the full name of the applicant establishment as subject. Don't forget to indicate as well the region where the establishment is based. Email. In the XLS application form, the worksheet 'Email' composes the subject and body of the email that should be sent to pair@fda.gov.ph. Copy completely and paste the appropriate fields onto the email. Include CCs as needed. The XLS file does not need to be attached but it will be required during submission. Scheduling. The FDA will determine the schedule of applications according to the priority of the Centers.
http://www.fda.gov.ph/industry-corner/downloadables/237-fda-public-assistance-information-and-receiving-pair/111583-lto-and-cpr-processing?tmpl=component 1/2
2/5/2014
LTO and CPR Processing
A quota will be set for the total number of applications that can be scheduled in a day. Multiple applications sent in a single email may be scheduled over separate days. Requests for specific schedules will not be accommodated. Receiving will be scheduled within 10 working days of receipt of application email. Pay. A copy of the DTL provided by FDA and a copy of the application form are required to process payment. Indicate in the application form the RSN provided. The RSN is indicated in the DTL. The amount that is due is indicated at the bottom of the application form. Check that the RSN indicated in the Document Tracking Log is indicated in the proof of payment. Check. The requirements for every application differs. Be sure that you have a checklist of requirements and that you have all the necessary documents. Don't forget to have the declaration form notarized. A softcopy of all documents pertinent to the application is required to be stored in a USB device to facilitate transfer. Include an XLS copy of the accomplished application form. Please keep your USB devices free of malicious software. Submission. Only applications scheduled for the day will be accommodated. Hard copies will no longer be required at submission. Don't forget to get back the USB devices used to transfer documents. Remember the RSN number of each application. Use the RSN to follow-up through pair@fda.gov.ph. Should you fail to complete submission on the set date, queue for another schedule through pair@fda.gov.ph using the RSN. Attachments:
FDA Application Form 28 Jan 2014 - CPR FDA Application Form - LTO
http://www.fda.gov.ph/industry-corner/downloadables/237-fda-public-assistance-information-and-receiving-pair/111583-lto-and-cpr-processing?tmpl=component
2/2
Das könnte Ihnen auch gefallen
- Reflection: Marketing in The Age of Alexa: Power To The PlatformDokument2 SeitenReflection: Marketing in The Age of Alexa: Power To The PlatformmykeeccruzNoch keine Bewertungen
- Joint AffidavitDokument1 SeiteJoint AffidavitMarlette BaltazarNoch keine Bewertungen
- Joint Sworn AffidavitDokument1 SeiteJoint Sworn AffidavitjparrojadoNoch keine Bewertungen
- QC Government - Transfer of OwnershipDokument24 SeitenQC Government - Transfer of OwnershipLorish ArguellesNoch keine Bewertungen
- Lifetime UPAA Member - UP Alumni AssociationDokument2 SeitenLifetime UPAA Member - UP Alumni AssociationNexBengsonNoch keine Bewertungen
- PhilHealth Circular No. 0035, s.2013 Annex 9 Referral FormDokument1 SeitePhilHealth Circular No. 0035, s.2013 Annex 9 Referral FormChrysanthus HerreraNoch keine Bewertungen
- Authority Letter To Collect Documents PDFDokument1 SeiteAuthority Letter To Collect Documents PDFvishnnu9696969Noch keine Bewertungen
- BCMT Module 5 - Monitoring and Evaluating Tech4ED CentersDokument30 SeitenBCMT Module 5 - Monitoring and Evaluating Tech4ED CentersCabaluay NHS0% (1)
- Demand To Explain AsaytonaDokument1 SeiteDemand To Explain AsaytonaMarceliano Monato IIINoch keine Bewertungen
- Memo ReconstitutionDokument5 SeitenMemo ReconstitutionAljeane TorresNoch keine Bewertungen
- Joint Affidavit of Two Disinterested PersonsDokument2 SeitenJoint Affidavit of Two Disinterested PersonsRodjard Pacete100% (1)
- Legal Policies For Retail StoresDokument19 SeitenLegal Policies For Retail StoresTermsFeedNoch keine Bewertungen
- 19ICCC Invitation Letter - Mr. Julcon AraizDokument2 Seiten19ICCC Invitation Letter - Mr. Julcon AraizJulcon Avanceña AraizNoch keine Bewertungen
- Edpms Service Request Form 2Dokument1 SeiteEdpms Service Request Form 2BRENDA BALILINoch keine Bewertungen
- Affidavit of CorrectionDokument1 SeiteAffidavit of Correctionsuigeneris28Noch keine Bewertungen
- RMC No 68-2015Dokument4 SeitenRMC No 68-2015Pedro Brugada100% (1)
- Steps On Printing The Final PermitDokument2 SeitenSteps On Printing The Final PermitRaijin ThunderkegNoch keine Bewertungen
- BIR ICC and BCCDokument1 SeiteBIR ICC and BCCDarlyn EtangNoch keine Bewertungen
- Eprs Ver.3.0Dokument48 SeitenEprs Ver.3.0Balanghai FuntoursNoch keine Bewertungen
- Most Outstanding Kapampangan AwardsDokument1 SeiteMost Outstanding Kapampangan AwardsMary Grace BlankerNoch keine Bewertungen
- NTP Irr - SignedDokument24 SeitenNTP Irr - SignedPalanas A. VinceNoch keine Bewertungen
- CNC SofitelDokument1 SeiteCNC SofitelVholts Villa VitugNoch keine Bewertungen
- GR Pharma Limited - Ghana - MEDOPHARM-DISTRIBUTION AGT-V3 - 22-04-2020Dokument17 SeitenGR Pharma Limited - Ghana - MEDOPHARM-DISTRIBUTION AGT-V3 - 22-04-2020shoban008Noch keine Bewertungen
- Chapter 16Dokument48 SeitenChapter 16AudreyMae100% (1)
- Deed of Donation (Thesis)Dokument2 SeitenDeed of Donation (Thesis)Lean LuluNoch keine Bewertungen
- People vs. David - Petition For Review - Court of Appeals - 09-04-2017 PDFDokument26 SeitenPeople vs. David - Petition For Review - Court of Appeals - 09-04-2017 PDFLlamas TugononNoch keine Bewertungen
- Moa Solar ProjectDokument2 SeitenMoa Solar ProjectcarlomaderazoNoch keine Bewertungen
- Application For New Authorization of PETC IT Service ProviderDokument1 SeiteApplication For New Authorization of PETC IT Service ProviderAnonymous dtceNuyIFINoch keine Bewertungen
- OM BasicsDokument18 SeitenOM BasicsprashantpardheNoch keine Bewertungen
- Jollibee Foods Corporation - SEC Form 20-IS - 19june2020 PDFDokument573 SeitenJollibee Foods Corporation - SEC Form 20-IS - 19june2020 PDFIrene SalvadorNoch keine Bewertungen
- Strategy AeropostaleDokument57 SeitenStrategy Aeropostalezeeshan25% (4)
- Referral Letter Format - From HRDokument4 SeitenReferral Letter Format - From HRAshley CeliaNoch keine Bewertungen
- How To Apply For SSS Death BenefitDokument4 SeitenHow To Apply For SSS Death BenefitShilalah Openiano100% (1)
- Golf Tournament LetterDokument1 SeiteGolf Tournament LettergslaboardNoch keine Bewertungen
- NPS Investigation Form No. 01, S. 2008Dokument2 SeitenNPS Investigation Form No. 01, S. 2008Tim ArroyoNoch keine Bewertungen
- Affidavit Financial SupportDokument2 SeitenAffidavit Financial SupportciryajamNoch keine Bewertungen
- Application Form For Import Validation PDFDokument1 SeiteApplication Form For Import Validation PDFSucculent City0% (1)
- NTC - Letter of IntentDokument1 SeiteNTC - Letter of IntentElvan Mae Rita ReyesNoch keine Bewertungen
- Reply To Ombudsman Dated 7 April 2007Dokument8 SeitenReply To Ombudsman Dated 7 April 2007jolanda041Noch keine Bewertungen
- Loan Agreement: Spouse ofDokument1 SeiteLoan Agreement: Spouse ofAntonio J. David IINoch keine Bewertungen
- Fuel Supply Agreement and IssuesDokument5 SeitenFuel Supply Agreement and Issuesrahul vanamaNoch keine Bewertungen
- Affidavit of UndertakingDokument1 SeiteAffidavit of UndertakingRobert Alexander QuinnNoch keine Bewertungen
- Requirements For Cpa AccreditationDokument1 SeiteRequirements For Cpa AccreditationJ'ca EdulanNoch keine Bewertungen
- Republic of The Philippines ManilaDokument9 SeitenRepublic of The Philippines ManilaChaNoch keine Bewertungen
- 1905 (Encs) 2000Dokument4 Seiten1905 (Encs) 2000Loss Pokla100% (1)
- FDA Promissory NoteDokument1 SeiteFDA Promissory NoteRenly BROQUEZANoch keine Bewertungen
- Revenue District Offices - Bureau of Internal RevenueDokument79 SeitenRevenue District Offices - Bureau of Internal RevenueKharylle ConolNoch keine Bewertungen
- GPPB Resolution No 02 2020Dokument6 SeitenGPPB Resolution No 02 2020Marisol RecuerdoNoch keine Bewertungen
- SAMPLE Letter of Intent 1.11.11Dokument1 SeiteSAMPLE Letter of Intent 1.11.11batambintanNoch keine Bewertungen
- Telcon OpolyDokument98 SeitenTelcon OpolyVincient Lee100% (1)
- Partition PaduaDokument3 SeitenPartition Paduabruno12003Noch keine Bewertungen
- PSED Forum 2019: April 11-12, 2019Dokument96 SeitenPSED Forum 2019: April 11-12, 2019Phillip Bernard CapadosaNoch keine Bewertungen
- Kenmed Trading Alltop Trading CorpDokument1 SeiteKenmed Trading Alltop Trading CorpJeffrey MedinaNoch keine Bewertungen
- Frequently Asked Questions - Law Program, University of Asia and The Pacific.Dokument3 SeitenFrequently Asked Questions - Law Program, University of Asia and The Pacific.uapslgNoch keine Bewertungen
- Terms and Conditions: Pre-Employment RequirementsDokument5 SeitenTerms and Conditions: Pre-Employment RequirementsKarl D AntonioNoch keine Bewertungen
- Worksheet - MemorandumDokument3 SeitenWorksheet - MemorandumSoo Zhe mengNoch keine Bewertungen
- Guidelines and Instruction For BIR Form No 1702 RTDokument2 SeitenGuidelines and Instruction For BIR Form No 1702 RTRahrahrahn100% (2)
- 5th Zone 4 Club Draft MOA (Tree Nursery and MRF)Dokument6 Seiten5th Zone 4 Club Draft MOA (Tree Nursery and MRF)Fernando Cresente C. HernandezNoch keine Bewertungen
- Integrated Application Form (97-2003 Compatible)Dokument15 SeitenIntegrated Application Form (97-2003 Compatible)Carmen Yalung Garcia56% (9)
- Integrated Application Form 97 2003 Compatible PDFDokument15 SeitenIntegrated Application Form 97 2003 Compatible PDFdrchughNoch keine Bewertungen
- Legal Forms (2009)Dokument84 SeitenLegal Forms (2009)Sui100% (10)
- Bot Law 7718 Plus Irr PPP ProjectDokument85 SeitenBot Law 7718 Plus Irr PPP ProjectRoby PenalosaNoch keine Bewertungen
- MPOC Intl RoadshowDokument18 SeitenMPOC Intl RoadshowverkieNoch keine Bewertungen
- Basic Legal Citation 1Dokument277 SeitenBasic Legal Citation 1jai_mann1036Noch keine Bewertungen
- Poea Rules and Regulations On Recruitment and EmploymentDokument39 SeitenPoea Rules and Regulations On Recruitment and EmploymentkwinrayNoch keine Bewertungen
- Sample Deed of Absolute SaleDokument4 SeitenSample Deed of Absolute Saleverkie100% (2)
- Do 73-05Dokument10 SeitenDo 73-05verkieNoch keine Bewertungen
- Poea Rules and Regulations On Recruitment and EmploymentDokument39 SeitenPoea Rules and Regulations On Recruitment and EmploymentkwinrayNoch keine Bewertungen
- Illegal Recruitment LawDokument5 SeitenIllegal Recruitment LawJae LopezNoch keine Bewertungen
- Illegal Recruitment LawDokument5 SeitenIllegal Recruitment LawJae LopezNoch keine Bewertungen
- BLOCK D ReviewerDokument235 SeitenBLOCK D Revieweradek_shoppeNoch keine Bewertungen
- 1st Denr Dao 92-30Dokument11 Seiten1st Denr Dao 92-30Rodney AtibulaNoch keine Bewertungen
- EvictionDokument43 SeitenEvictionverkieNoch keine Bewertungen
- 2011 Outline PDFDokument9 Seiten2011 Outline PDFverkieNoch keine Bewertungen
- Nego Class Policies PDFDokument1 SeiteNego Class Policies PDFverkieNoch keine Bewertungen
- OSH Standards Amended 1989 LatestDokument338 SeitenOSH Standards Amended 1989 Latestverkie100% (1)
- Keeping Filipinos AddictedDokument40 SeitenKeeping Filipinos AddictedverkieNoch keine Bewertungen
- DENR Memo Circ-2010-13 Manual of Land Survey ProceduresDokument155 SeitenDENR Memo Circ-2010-13 Manual of Land Survey Proceduressorbisorbi94% (34)
- Dao 2010 17 - 588 PDFDokument8 SeitenDao 2010 17 - 588 PDFverkieNoch keine Bewertungen
- Directory of Ibp Chapter Presidents 2011-2013Dokument6 SeitenDirectory of Ibp Chapter Presidents 2011-2013mykeruaiiNoch keine Bewertungen
- Tax 1 ReviewerDokument16 SeitenTax 1 ReviewerverkieNoch keine Bewertungen
- Aboitiz V CADokument2 SeitenAboitiz V CAverkieNoch keine Bewertungen
- Landrito and ZamoraDokument3 SeitenLandrito and ZamoraverkieNoch keine Bewertungen
- Letter To PnoyDokument1 SeiteLetter To PnoyverkieNoch keine Bewertungen
- Doj NPS ManualDokument40 SeitenDoj NPS Manualed flores92% (13)
- Corpo Tunnel CrosswordDokument2 SeitenCorpo Tunnel CrosswordverkieNoch keine Bewertungen
- SC Promulgates Special ADR RulesDokument1 SeiteSC Promulgates Special ADR RulesverkieNoch keine Bewertungen
- DebateeeDokument6 SeitenDebateeeverkieNoch keine Bewertungen
- GameFAQs - Shin Megami Tensei - Persona 3 FES (PS2) Persona Fusion List by SionarDokument65 SeitenGameFAQs - Shin Megami Tensei - Persona 3 FES (PS2) Persona Fusion List by SionarverkieNoch keine Bewertungen
- Details About Inspection CommitteeDokument10 SeitenDetails About Inspection CommitteeSree kumar A uNoch keine Bewertungen
- Owner'S Manual: Air - Cooled Diesel Engine Generator SetDokument23 SeitenOwner'S Manual: Air - Cooled Diesel Engine Generator SetJoeNoch keine Bewertungen
- NTE2389 Mosfet N CH, Enhancement Mode High Speed Switch TO220 Type PackageDokument2 SeitenNTE2389 Mosfet N CH, Enhancement Mode High Speed Switch TO220 Type PackagetoroalNoch keine Bewertungen
- Cryptography and Network Security: Seventh Edition by William StallingsDokument34 SeitenCryptography and Network Security: Seventh Edition by William StallingsvanithaNoch keine Bewertungen
- S. Loganathan, P. Balaji-2020Dokument8 SeitenS. Loganathan, P. Balaji-2020Mayang SandyNoch keine Bewertungen
- Build A VMware Home Lab in A Complete WalkthroughDokument1 SeiteBuild A VMware Home Lab in A Complete WalkthroughPhilipNoch keine Bewertungen
- Exercise Property Phase DiagramDokument5 SeitenExercise Property Phase DiagramRizkatul Amriah ANoch keine Bewertungen
- AMM HJH DeGruyter PSDokument2 SeitenAMM HJH DeGruyter PSSt Peter Life Plan ChapelNoch keine Bewertungen
- mxn738 - P01ed - A - B&R - Swiftlock Vacuum FrontloadDokument62 Seitenmxn738 - P01ed - A - B&R - Swiftlock Vacuum FrontloadCaalaa Dabalaa LamuuNoch keine Bewertungen
- JAP234 Handout 2Dokument4 SeitenJAP234 Handout 2studentNoch keine Bewertungen
- Directional (Yaw) Stability AnalysisDokument7 SeitenDirectional (Yaw) Stability AnalysisHarshini AichNoch keine Bewertungen
- ATB Series Integral Throttle Body Actuators: NomenclatureDokument5 SeitenATB Series Integral Throttle Body Actuators: NomenclatureMaxiSanchezNoch keine Bewertungen
- Module1 Prelim Activity&CaseAnalysisDokument8 SeitenModule1 Prelim Activity&CaseAnalysisKirk anthony TripoleNoch keine Bewertungen
- Flushless Cartridge Seal: Performance Capabilities Product DescriptionDokument2 SeitenFlushless Cartridge Seal: Performance Capabilities Product DescriptiontestNoch keine Bewertungen
- Artigo Sons Respira o Edvan InglesDokument6 SeitenArtigo Sons Respira o Edvan InglesBruno SantosNoch keine Bewertungen
- Morse CodeDokument5 SeitenMorse CodeAndu CampuNoch keine Bewertungen
- Buoyant FoundationDokument2 SeitenBuoyant FoundationKaushal MoreNoch keine Bewertungen
- Haier HRF-661FF ASSDokument15 SeitenHaier HRF-661FF ASSyayayalNoch keine Bewertungen
- Ev Charging Station Based On Phc-Dab ConverterDokument28 SeitenEv Charging Station Based On Phc-Dab ConverterAkshay SNoch keine Bewertungen
- Form 4Dokument2 SeitenForm 4Tanmoy DebnathNoch keine Bewertungen
- Recruitment AE Poster SoCtronicsDokument1 SeiteRecruitment AE Poster SoCtronicsSaivenkat BingiNoch keine Bewertungen
- Point - Io 1734-AENTR UMDokument118 SeitenPoint - Io 1734-AENTR UMgeorge stanley paceteNoch keine Bewertungen
- Python Module Wise Important QuestionsDokument4 SeitenPython Module Wise Important QuestionsAryan Ameen100% (1)
- AgreementDokument7 SeitenAgreementVOJE DIFNoch keine Bewertungen
- Assignment 1Dokument4 SeitenAssignment 1FAGUN SONINoch keine Bewertungen
- 2ND Ia QP 2022Dokument6 Seiten2ND Ia QP 2022chandrashekar hiregoudarNoch keine Bewertungen
- P O W E R Learning Strategies For Success in College and Life 9Th Edition Robert Feldman Full Chapter PDF ScribdDokument67 SeitenP O W E R Learning Strategies For Success in College and Life 9Th Edition Robert Feldman Full Chapter PDF Scribdsteven.hendrix700100% (6)
- DP PaperDokument31 SeitenDP Paperaymane.oubellaNoch keine Bewertungen
- Hanna COD Test Tube Heater HI - 839800 - 2008Dokument2 SeitenHanna COD Test Tube Heater HI - 839800 - 2008B Azam MalikNoch keine Bewertungen
- Machine Learning For Natural Language Processing: Hidden Markov ModelsDokument33 SeitenMachine Learning For Natural Language Processing: Hidden Markov ModelsHưngNoch keine Bewertungen
- Algorithms to Live By: The Computer Science of Human DecisionsVon EverandAlgorithms to Live By: The Computer Science of Human DecisionsBewertung: 4.5 von 5 Sternen4.5/5 (722)
- Defensive Cyber Mastery: Expert Strategies for Unbeatable Personal and Business SecurityVon EverandDefensive Cyber Mastery: Expert Strategies for Unbeatable Personal and Business SecurityBewertung: 5 von 5 Sternen5/5 (1)
- Scary Smart: The Future of Artificial Intelligence and How You Can Save Our WorldVon EverandScary Smart: The Future of Artificial Intelligence and How You Can Save Our WorldBewertung: 4.5 von 5 Sternen4.5/5 (55)
- Chaos Monkeys: Obscene Fortune and Random Failure in Silicon ValleyVon EverandChaos Monkeys: Obscene Fortune and Random Failure in Silicon ValleyBewertung: 3.5 von 5 Sternen3.5/5 (111)
- Digital Gold: Bitcoin and the Inside Story of the Misfits and Millionaires Trying to Reinvent MoneyVon EverandDigital Gold: Bitcoin and the Inside Story of the Misfits and Millionaires Trying to Reinvent MoneyBewertung: 4 von 5 Sternen4/5 (51)
- Cyber War: The Next Threat to National Security and What to Do About ItVon EverandCyber War: The Next Threat to National Security and What to Do About ItBewertung: 3.5 von 5 Sternen3.5/5 (66)
- Generative AI: The Insights You Need from Harvard Business ReviewVon EverandGenerative AI: The Insights You Need from Harvard Business ReviewBewertung: 4.5 von 5 Sternen4.5/5 (2)
- Chip War: The Quest to Dominate the World's Most Critical TechnologyVon EverandChip War: The Quest to Dominate the World's Most Critical TechnologyBewertung: 4.5 von 5 Sternen4.5/5 (228)
- AI Superpowers: China, Silicon Valley, and the New World OrderVon EverandAI Superpowers: China, Silicon Valley, and the New World OrderBewertung: 4.5 von 5 Sternen4.5/5 (398)
- System Error: Where Big Tech Went Wrong and How We Can RebootVon EverandSystem Error: Where Big Tech Went Wrong and How We Can RebootNoch keine Bewertungen
- The Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyVon EverandThe Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyNoch keine Bewertungen
- The Master Algorithm: How the Quest for the Ultimate Learning Machine Will Remake Our WorldVon EverandThe Master Algorithm: How the Quest for the Ultimate Learning Machine Will Remake Our WorldBewertung: 4.5 von 5 Sternen4.5/5 (107)
- The Future of Geography: How the Competition in Space Will Change Our WorldVon EverandThe Future of Geography: How the Competition in Space Will Change Our WorldBewertung: 4 von 5 Sternen4/5 (6)
- ChatGPT Side Hustles 2024 - Unlock the Digital Goldmine and Get AI Working for You Fast with More Than 85 Side Hustle Ideas to Boost Passive Income, Create New Cash Flow, and Get Ahead of the CurveVon EverandChatGPT Side Hustles 2024 - Unlock the Digital Goldmine and Get AI Working for You Fast with More Than 85 Side Hustle Ideas to Boost Passive Income, Create New Cash Flow, and Get Ahead of the CurveNoch keine Bewertungen
- Reality+: Virtual Worlds and the Problems of PhilosophyVon EverandReality+: Virtual Worlds and the Problems of PhilosophyBewertung: 4 von 5 Sternen4/5 (24)
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindVon EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindNoch keine Bewertungen
- ChatGPT Millionaire 2024 - Bot-Driven Side Hustles, Prompt Engineering Shortcut Secrets, and Automated Income Streams that Print Money While You Sleep. The Ultimate Beginner’s Guide for AI BusinessVon EverandChatGPT Millionaire 2024 - Bot-Driven Side Hustles, Prompt Engineering Shortcut Secrets, and Automated Income Streams that Print Money While You Sleep. The Ultimate Beginner’s Guide for AI BusinessNoch keine Bewertungen
- The Infinite Machine: How an Army of Crypto-Hackers Is Building the Next Internet with EthereumVon EverandThe Infinite Machine: How an Army of Crypto-Hackers Is Building the Next Internet with EthereumBewertung: 3 von 5 Sternen3/5 (12)
- The Manager's Path: A Guide for Tech Leaders Navigating Growth and ChangeVon EverandThe Manager's Path: A Guide for Tech Leaders Navigating Growth and ChangeBewertung: 4.5 von 5 Sternen4.5/5 (99)
- Broken Money: Why Our Financial System is Failing Us and How We Can Make it BetterVon EverandBroken Money: Why Our Financial System is Failing Us and How We Can Make it BetterBewertung: 5 von 5 Sternen5/5 (3)
- Solutions Architect's Handbook: Kick-start your career as a solutions architect by learning architecture design principles and strategiesVon EverandSolutions Architect's Handbook: Kick-start your career as a solutions architect by learning architecture design principles and strategiesNoch keine Bewertungen
- The E-Myth Revisited: Why Most Small Businesses Don't Work andVon EverandThe E-Myth Revisited: Why Most Small Businesses Don't Work andBewertung: 4.5 von 5 Sternen4.5/5 (709)
- Four Battlegrounds: Power in the Age of Artificial IntelligenceVon EverandFour Battlegrounds: Power in the Age of Artificial IntelligenceBewertung: 5 von 5 Sternen5/5 (5)