Beruflich Dokumente
Kultur Dokumente
QOI0809 Alkenes
Hochgeladen von
mtucker17Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
QOI0809 Alkenes
Hochgeladen von
mtucker17Copyright:
Verfügbare Formate
QOI 0809 C=C
Name___________________________________
ESSAY. Write your answer in the space provided or on a separate sheet of paper.
1) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
2) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
3) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
4) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
5) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
6) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
1
7) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
8) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
9) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
10) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
11) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
12) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
13) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
2
14) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
15) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
16) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
17) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
(Z)-3-hexene
1. BH
3
THF
2. H
2
O
2
, -OH
18) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
19) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
20) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
3
21) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
22) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
23) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
24) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
25) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
26) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
27) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
4
28) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
29) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
30) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
31) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
32) Draw the major organic product generated in the reaction below. Pay particular attention to regio- and
stereochemical detail.
33) Humulene is a monocyclic terpene constituent of carnations. When 0.25 mol of humulene is hydrogenated in
the presence of platinum catalyst, 0.75 mol of H
2
reacts with the humulene to produce a monocyclic
hydrocarbon of formula C
15
H
30
. What is the molecular formula of humulene?
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
34) Treatment of cyclopentene with peroxybenzoic acid __________.
A) gives the same product as treatment of cyclopentene with OsO
4
B) results in oxidative cleavage of the ring to produce an acyclic compound
C) yields an equimolar mixture of enantiomeric epoxides
D) yields a meso epoxide
E) none of the above
34)
5
ESSAY. Write your answer in the space provided or on a separate sheet of paper.
35) Complete the following reaction and provide a detailed, step-by-step mechanism for the process.
36) Complete the following reaction and provide a detailed, step-by-step mechanism for the process.
37) Complete the following reaction and provide a detailed, step-by-step mechanism for the process.
38) Provide a detailed, step-by-step mechanism for the reaction shown below.
39) Provide a detailed, step-by-step mechanism for the reaction shown below.
40) When isobutylene [CH
2
C(CH
3
)
2
] is treated with BF
3
, polyisobutylene is formed. Provide a step-by-step
mechanism for this polymerization reaction.
41) Provide the reagents necessary to complete the following transformation.
42) Provide the reagents necessary to complete the following transformation.
6
43) Provide the reagents necessary to complete the following transformation.
44) Provide the reagents necessary to complete the following transformation.
45) Provide the reagents necessary to convert 3-methyl-2-butanol to 2-methyl-2-butanol.
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
46) Both (E)- and (Z)-hex-3-ene can be subjected to a hydroboration-oxidation sequence. How are the
products from these two reactions related to each other?
A) The products of the two isomers are not structurally related.
B) The products of the two isomers are related as constitutional isomers.
C) The (E)- and (Z)-isomers generate the same products in exactly the same amounts.
D) The products of the two isomers are related as diastereomers.
E) The (E)- and (Z)-isomers generate the same products but in differing amounts.
46)
47) Both (E)- and (Z)-hex-3-ene can be treated with D
2
in the presence of a platinum catalyst. How
are the products from these two reactions related to each other?
A) The (E)- and (Z)-isomers generate the same products but in differing amounts.
B) The (E)- and (Z)-isomers generate the same products in exactly the same amounts.
C) The products of the two isomers are related as enantiomers.
D) The products of the two isomers are related as constitutional isomers.
E) The products of the two isomers are related as diastereomers.
47)
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
48) Give the structure of the alkene which would yield the following products upon
ozonolysis-reduction.
CH
3
CH
2
CH
2
CH
2
CHO + CH
2
O
48)
49) Give the structure of the alkene which would yield the following products upon
ozonolysis-reduction.
CH
3
COCH
3
+ CH
3
CH
2
CHO
49)
7
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
50) Addition of Br
2
to (E)-hex-3-ene produces __________.
A) a mixture of enantiomeric dibromides which is optically inactive
B) a meso dibromide
C) (E)-3,4-dibromo-3-hexene
D) (Z)-3,4-dibromo-3-hexene
E) a mixture of enantiomeric dibromides which is optically active
50)
ESSAY. Write your answer in the space provided or on a separate sheet of paper.
51) The following reaction is known to proceed by a free radical chain mechanism. Suggest a reasonable,
step-by-step mechanism for this reaction.
CH
3
CHCH
2
+ CHCl
3
ROOR
CH
3
CH
2
CH
2
CCl
3
52) Consider how the ICl bond is polarized and predict the product which results when this mixed halogen adds
to 1-methylcyclohexene.
53) -Ocimene is a natural product with a pleasant odor. Based on the information below, deduce the structure of
-ocimene.
54) Based on the relative stabilities of the intermediates involved, explain the basis for Markovinkov's rule in the
addition of hydrogen halides to alkenes.
55) Explain the regioselectivity observed in the radical addition of HBr to 2-methylpropene.
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
56) The mechanism for the acid-catalyzed hydration of alkenes is simply the reverse of the
mechanism by which alcohols are dehydrated using concentrated acid. This is an
illustration of the principle of __________.
56)
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
57) Which of the following is the best reaction sequence to use if one wants to accomplish a
Markovnikov addition of water to an alkene with minimal skeletal rearrangement?
A) water + dilute acid
B) oxymercuration-demercuration
C) hydroboration-oxidation
D) water + concentrated acid
E) none of the above
57)
8
ESSAY. Write your answer in the space provided or on a separate sheet of paper.
58) Draw the Lewis structure of dibromocarbene.
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
59) Which of the following additions to alkenes occur(s) specifically in an anti fashion?
A) addition of H
2
B) addition of H
2
O in dilute acid
C) addition of Br
2
D) hydroboration-oxidation
E) both A and B
59)
60) Which of the following additions to alkenes occur(s) specifically in an syn fashion?
A) addition of H
2
B) dihydroxylation using OsO
4
, H
2
O
2
C) hydroboration
D) addition of HCl
E) A, B, and C
60)
61) HBr can be added to an alkene in the presence of peroxides (ROOR). What function does the
peroxide serve in this reaction?
A) electrophile
B) acid catalyst
C) radical chain initiator
D) nucleophile
E) solvent
61)
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
62) Name the major product which results when HBr is added to 3-ethyl-3-hexene. 62)
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
63) Which of the following intermediates is thought to occur in the mechanism by which alkenes are
hydrated in the presence of acid?
A) free radical
B) carbene
C) carbocation
D) alkyne
E) carbanion
63)
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
64) Name the major alcohol product which results when 3,3-dimethylbut-1-ene is treated
with dilute acid.
64)
9
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
65) What synthetic goal is achieved by subjecting an alkene to an oxymercuration-demercuration
sequence?
A) anti-Markovnikov addition of H
2
O wherein skeletal rearrangement is prevented
B) Markovnikov addition of H
2
O wherein skeletal rearrangement is promoted
C) anti-Markovnikov addition of H
2
O wherein skeletal rearrangement is promoted
D) syn-hydroxylation
E) Markovnikov addition of H
2
O wherein skeletal rearrangement is prevented
65)
66) When an alkene is subjected to treatment with Hg(OAc)
2
in alcohol followed by reaction with
NaBH
4
, what new class of compound is formed?
A) syn diol B) alkane C) epoxide D) ether E) alkyne
66)
67) Which of the following compounds is (are) appropriate to promote the cationic polymerization of
isobutylene?
A) BF
3
B) NaOH
C) H
2
SO
4
D) ROOR
E) both H
2
SO
4
and BF
3
67)
ESSAY. Write your answer in the space provided or on a separate sheet of paper.
68) Why can methyl acrylate (H
2
CCHCO
2
CH
3
) be polymerized through anionic polymerization?
69) Name the compound PhCO
3
H and give its most common use as a reagent.
70) Provide the structure of the product which results when the alkene below is treated with O
3
followed by
(CH
3
)
2
S.
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
71) Provide the structure of the major organic product of the reaction below. 71)
10
72) Provide the structure of the major organic product of the reaction below. 72)
73) Provide the structure of the major organic product of the reaction below. 73)
74) Provide the structure of the major organic product of the reaction below. 74)
75) Provide the structure of the major organic product of the reaction below. 75)
76) Provide the structure of the major organic product of the reaction below. 76)
77) Provide the structure of the major organic product of the reaction below. 77)
11
78) Provide the structure of the major organic product of the reaction below. 78)
79) Provide the structure of the major organic product of the reaction below. 79)
80) Provide the structure of the major organic product of the reaction below. 80)
81) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
81)
82) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
82)
83) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
83)
12
84) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
84)
85) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
85)
86) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
86)
87) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
87)
88) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
88)
89) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
89)
13
90) Draw the major organic product generated in the reaction below. Pay particular attention
to regio- and stereochemical detail.
90)
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
91) Which of the following alkenes will yield a meso dihalide when reacted with Br
2
/CCl
4
at room
temperature?
A)
B)
C)
D)
E) both Band D
91)
92) Acid catalyzed hydration (H
2
SO
4
/water/) of an unknown compound (A)with a chemical formula
C
6
H
12
, yielded a racemic mixture of product C
6
H
13
OH. Which, if any, of the following
compounds is/are possible structures for the initial compound (A)?
A) compounds 2 and 3
B) compound 2 only
C) compound 1 only
D) compounds 1 and 3
E) none of the above
92)
14
93) A reaction of an unknown alkene with MCPBA in dichloromethane followed by work-up with
H
2
O/H+ yielded, as the major product, a racemic mixture of (2S, 3S) and (2R,
3R)-3-methylpentan-2,3-diol. What is the specific structure of the alkene used in the reaction?
A) (E)-3-methylpent- 2-ene
B) 2-methylpent-2-ene
C) (Z)-3-methylpent-2-ene
D) 2,3-dimethylbut-2-ene
E) none of the above
93)
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
94) When 3,6-dimethylcyclohexene is reacted with dry gaseous HBr, one of the products is
1-bromo-1,4-dimethylcyclohexane. Provide a detailed step-by-step mechanism to
explain the formation of this product
94)
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
95) Treatment of 2-methylpropene with which of the following reaction conditions results in an
anti-Markovnikov addition product?
A) dry gaseous HBr with peroxides present
B) BH
3
-THF, followed by alkaline H
2
O
2
C) aqueous Hg(OAc)
2
, followed by alkaline NaBH
4
D) dilute H
2
SO
4
and heat
E) both A and B
95)
15
96) An unknown compound with empirical formula C
3
H
5
was treated with Br
2
/CCl
4
.
The bromine
solution went from orangish/red to clear immediately at room temperature. Upon treatment with
O
3
followed by work-up with dimethylsulfide the following products were identified. From the
information provided what is/are the most likely structure(s) for this unknown compound.
A)
B)
C)
D)
E) both A and D
96)
16
Answer Key
Testname: UNTITLED1
1)
ID: oc6w 8-1
Diff: 3
2)
ID: oc6w 8-2
Diff: 1
3)
ID: oc6w 8-3
Diff: 1
4)
ID: oc6w 8-4
Diff: 3
5)
ID: oc6w 8-5
Diff: 2
6)
ID: oc6w 8-6
Diff: 3
7)
ID: oc6w 8-7
Diff: 2
17
Answer Key
Testname: UNTITLED1
8)
ID: oc6w 8-8
Diff: 2
9)
ID: oc6w 8-9
Diff: 2
10)
ID: oc6w 8-10
Diff: 1
11)
ID: oc6w 8-11
Diff: 3
12)
ID: oc6w 8-12
Diff: 2
13)
ID: oc6w 8-13
Diff: 2
14)
ID: oc6w 8-14
Diff: 2
18
Answer Key
Testname: UNTITLED1
15)
ID: oc6w 8-15
Diff: 3
16)
ID: oc6w 8-16
Diff: 3
17)
ID: oc6w 8-17
Diff: 3
18)
ID: oc6w 8-18
Diff: 2
19)
ID: oc6w 8-19
Diff: 1
20)
ID: oc6w 8-20
Diff: 3
21)
ID: oc6w 8-21
Diff: 3
22)
ID: oc6w 8-22
Diff: 1
19
Answer Key
Testname: UNTITLED1
23)
ID: oc6w 8-23
Diff: 3
24)
ID: oc6w 8-24
Diff: 3
25)
ID: oc6w 8-25
Diff: 3
26)
ID: oc6w 8-26
Diff: 3
27)
ID: oc6w 8-27
Diff: 2
28)
ID: oc6w 8-28
Diff: 2
29)
ID: oc6w 8-29
Diff: 3
20
Answer Key
Testname: UNTITLED1
30)
ID: oc6w 8-30
Diff: 3
31)
ID: oc6w 8-31
Diff: 2
32)
ID: oc6w 8-32
Diff: 2
33) C
15
H
24
ID: oc6w 8-33
Diff: 2
34) D
ID: oc6w 8-34
Diff: 3
35)
ID: oc6w 8-35
Diff: 1
36)
ID: oc6w 8-36
Diff: 2
37)
ID: oc6w 8-37
Diff: 2
21
Answer Key
Testname: UNTITLED1
38)
ID: oc6w 8-38
Diff: 2
39)
ID: oc6w 8-39
Diff: 3
40)
ID: oc6w 8-40
Diff: 2
41) 1. Br
2
, h
2. H
2
O,
or
1. Br
2
, h
2. NaOCH
3
, CH
3
OH
3. H
3
O
+
or Hg(OAc)
2
, H
2
O; NaBH
4
ID: oc6w 8-41
Diff: 3
42) 1. NaOCH
3
, CH
3
OH
2. OsO
4
, H
2
O
2
or cold, dilute KMnO
4
,
-
OH
ID: oc6w 8-42
Diff: 2
43) 1. NaOCH
3
, CH
3
OH
2. MCPBA or CH
3
CO
3
H
3. H
3
O
+
or
-
OH
ID: oc6w 8-43
Diff: 3
22
Answer Key
Testname: UNTITLED1
44) 1. Br
2
, h
2. NaOCH
3
, CH
3
OH
3. CH
2
I
2
, Zn(Cu)
ID: oc6w 8-44
Diff: 3
45) 1. conc. H
2
SO
4
2. H
3
O
+
or Hg(OAc)
2
, H
2
O
or
1. PBr
3
2. NaOCH
3
, CH
3
OH
3. NaBH
4
ID: oc6w 8-45
Diff: 2
46) C
ID: oc6w 8-46
Diff: 2
47) E
ID: oc6w 8-47
Diff: 2
48) CH
3
CH
2
CH
2
CH
2
CHCH
2
ID: oc6w 8-48
Diff: 1
49) (CH
3
)
2
CCHCH
2
CH
3
ID: oc6w 8-49
Diff: 1
50) B
ID: oc6w 8-50
Diff: 2
51) ROOR 2 RO
RO + HCCl
3
ROH + CCl
3
Cl
3
C + CH
2
CHCH
3
Cl
3
CCH
2
CH
CH
3
Cl
3
CCH
2
CH
CH
3
+ HCCl
3
Cl
3
CCH
2
CH
2
CH
3
+ CCl
3
ID: oc6w 8-51
Diff: 3
52)
ID: oc6w 8-52
Diff: 3
23
Answer Key
Testname: UNTITLED1
53)
ID: oc6w 8-53
Diff: 2
54) The rate-determining step in this reaction is the production of a carbocation intermediate. Since this step is
endothermic, Hammond's postulate allows one to gauge the relative stabilites of the transition states by comparing the
relative stabilities of the carbocation intermediates. The reaction pathway which produces the more substituted
carbocation will thus occur more rapidly.
ID: oc6w 8-54
Diff: 2
55) The reaction proceeds via the addition of Br
to the alkene. Two competing pathways are possible, but the transition
state leading to the more substituted alkyl radical is lower in energy. This process ultimately makes the addition
anti-Markovnikov in nature.
ID: oc6w 8-55
Diff: 3
56) microscopic reversibility
ID: oc6w 8-56
Diff: 1
57) B
ID: oc6w 8-57
Diff: 1
58)
ID: oc6w 8-58
Diff: 3
59) C
ID: oc6w 8-59
Diff: 1
60) E
ID: oc6w 8-60
Diff: 1
61) C
ID: oc6w 8-61
Diff: 2
62) 3-bromo-3-ethylhexane
ID: oc6w 8-62
Diff: 2
63) C
ID: oc6w 8-63
Diff: 2
64) 2,3-dimethyl-2-butanol
ID: oc6w 8-64
Diff: 2
65) E
ID: oc6w 8-65
Diff: 2
24
Answer Key
Testname: UNTITLED1
66) D
ID: oc6w 8-66
Diff: 2
67) E
ID: oc6w 8-67
Diff: 2
68) The intermediate carbanion and the transition state leading to it are stabilized by the electron-withdrawing capacity of
the carbonyl group.
ID: oc6w 8-68
Diff: 2
69) peroxybenzoic acid; an oxidizing agent which converts alkenes to epoxides
ID: oc6w 8-69
Diff: 1
70)
ID: oc6w 8-70
Diff: 3
71)
ID: oc6w 8-71
Diff: 1
72)
ID: oc6w 8-72
Diff: 2
25
Answer Key
Testname: UNTITLED1
73)
ID: oc6w 8-73
Diff: 3
74)
ID: oc6w 8-74
Diff: 3
75)
ID: oc6w 8-75
Diff: 2
76)
ID: oc6w 8-76
Diff: 2
77)
ID: oc6w 8-77
Diff: 2
26
Answer Key
Testname: UNTITLED1
78)
ID: oc6w 8-78
Diff: 3
79)
ID: oc6w 8-79
Diff: 2
80)
ID: oc6w 8-80
Diff: 3
81)
ID: oc6w 8-81
Diff: 3
82)
ID: oc6w 8-82
Diff: 3
27
Answer Key
Testname: UNTITLED1
83)
ID: oc6w 8-83
Diff: 3
84)
ID: oc6w 8-84
Diff: 3
85)
ID: oc6w 8-85
Diff: 3
86)
ID: oc6w 8-86
Diff: 3
87)
ID: oc6w 8-87
Diff: 3
28
Answer Key
Testname: UNTITLED1
88)
ID: oc6w 8-88
Diff: 3
89)
ID: oc6w 8-89
Diff: 3
90)
ID: oc6w 8-90
Diff: 3
91) B
ID: oc6w 8-91
Diff: 2
92) E
ID: oc6w 8-92
Diff: 2
93) C
ID: oc6w 8-93
Diff: 3
94)
ID: oc6w 8-94
Diff: 3
29
Answer Key
Testname: UNTITLED1
95) E
ID: oc6w 8-95
Diff: 1
96) A
ID: oc6w 8-96
Diff: 2
30
Das könnte Ihnen auch gefallen
- Alkenes SeatworkDokument5 SeitenAlkenes SeatworkJhefNoch keine Bewertungen
- Practice Problems On Addition Reactions To Alkenes With AnswersDokument4 SeitenPractice Problems On Addition Reactions To Alkenes With AnswersSangetha ChelladoraiNoch keine Bewertungen
- Organic Chemistry II Practice Exam #3A Answer KeyDokument8 SeitenOrganic Chemistry II Practice Exam #3A Answer Keyhiep237Noch keine Bewertungen
- Reactions of Alcohols: Organic Chemistry, 7Dokument53 SeitenReactions of Alcohols: Organic Chemistry, 7haha_le12100% (1)
- Chap. 5 Reactive Intermediates: Energy SurfaceDokument20 SeitenChap. 5 Reactive Intermediates: Energy SurfaceAnil KumarNoch keine Bewertungen
- 2007Dokument9 Seiten2007Anil KumarNoch keine Bewertungen
- Welcome To Chem 206: Fall Term, 2005, David A. EvansDokument22 SeitenWelcome To Chem 206: Fall Term, 2005, David A. EvanseraborNoch keine Bewertungen
- Resonance and Inductive Effects PresentationDokument36 SeitenResonance and Inductive Effects Presentationeagl33yeNoch keine Bewertungen
- Stereochemistry by Qari Abdullah SiddiqueDokument48 SeitenStereochemistry by Qari Abdullah SiddiqueABDULLAH0% (1)
- Aromatic Cmpds AnskeyDokument6 SeitenAromatic Cmpds AnskeyAaron LeeNoch keine Bewertungen
- Ch1b Ps3 Key SerDokument7 SeitenCh1b Ps3 Key SerRichard ZhuNoch keine Bewertungen
- Structure and Synthesis of Alcohols: Organic Chemistry, 7Dokument52 SeitenStructure and Synthesis of Alcohols: Organic Chemistry, 7haha_le12100% (1)
- 1 Alkene Practice Problems MOC PDFDokument8 Seiten1 Alkene Practice Problems MOC PDFsamkarthik47Noch keine Bewertungen
- Organic Chemistry Midterm 1 Dir+eff++keyDokument1 SeiteOrganic Chemistry Midterm 1 Dir+eff++keyNorma Leticia RamosNoch keine Bewertungen
- Chapter 5 Elimination Reaction - 2016Dokument19 SeitenChapter 5 Elimination Reaction - 2016Syuhadah NoordinNoch keine Bewertungen
- Organic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The SDokument9 SeitenOrganic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The Ssweta KushwahaNoch keine Bewertungen
- TEST 2 GOC & POC Tough by S.K.sinha See Chemistry Animations atDokument3 SeitenTEST 2 GOC & POC Tough by S.K.sinha See Chemistry Animations atmyiitchemistryNoch keine Bewertungen
- Chapter 11 - Reactions of Alcohol (Compatibility Mode)Dokument13 SeitenChapter 11 - Reactions of Alcohol (Compatibility Mode)Billy HoNoch keine Bewertungen
- Chapter 2 Acid and BaseDokument8 SeitenChapter 2 Acid and BaseKelsi Kyla PeraltaNoch keine Bewertungen
- Alcohol Ether and ExpoksideDokument64 SeitenAlcohol Ether and ExpoksideAhmadBadruzzamanShuib100% (1)
- Lecture 1Dokument11 SeitenLecture 1Fang GaoNoch keine Bewertungen
- Problems On Named ReactionsDokument103 SeitenProblems On Named ReactionsBapu ThoratNoch keine Bewertungen
- Midterm II Key Chem 2312-003 F '12Dokument7 SeitenMidterm II Key Chem 2312-003 F '12acb4039Noch keine Bewertungen
- Organic Chemistry, Second Edition Janice Gorzynski Smith, ch1Dokument40 SeitenOrganic Chemistry, Second Edition Janice Gorzynski Smith, ch1sungyeon heoNoch keine Bewertungen
- Hyperconjugation, The Anomeric Effect, and More: Chem 206 D. A. EvansDokument12 SeitenHyperconjugation, The Anomeric Effect, and More: Chem 206 D. A. Evansomkar9996767Noch keine Bewertungen
- EnolateansDokument1 SeiteEnolateanskevinamyNoch keine Bewertungen
- Asymmetric SynthesisDokument55 SeitenAsymmetric Synthesisevsgoud_goud0% (1)
- Lecture Notes Chapter-12-Aldehydes, Ketones & Carboxylic AcidsDokument26 SeitenLecture Notes Chapter-12-Aldehydes, Ketones & Carboxylic AcidsSHUBHAMNoch keine Bewertungen
- Organic Chemistry 231 Final ExamDokument19 SeitenOrganic Chemistry 231 Final ExamAlex Rose100% (1)
- Qoii0708 CO 17 TIFDokument34 SeitenQoii0708 CO 17 TIFLovely Joysweet100% (2)
- Aromaticity 2019Dokument65 SeitenAromaticity 2019Shreya PrakashNoch keine Bewertungen
- HW1 Solns KineticsDokument10 SeitenHW1 Solns Kineticsapb91781Noch keine Bewertungen
- Chapter 21 II PDFDokument23 SeitenChapter 21 II PDFadelNoch keine Bewertungen
- Hetero-Cyclic CompoundsDokument69 SeitenHetero-Cyclic CompoundsNaveed SajidNoch keine Bewertungen
- Electron Delocalization and ResonanceDokument9 SeitenElectron Delocalization and ResonanceMariana LizethNoch keine Bewertungen
- Atropisomerism PDFDokument22 SeitenAtropisomerism PDFAnil KumarNoch keine Bewertungen
- Test 3Dokument1 SeiteTest 3Windellea WongNoch keine Bewertungen
- Vollhardt 6e Lecture PowerPoints - Chapter 11Dokument58 SeitenVollhardt 6e Lecture PowerPoints - Chapter 11superfr3shmNoch keine Bewertungen
- Module8 PDFDokument40 SeitenModule8 PDFFaizan AhmadNoch keine Bewertungen
- Organometallic CompoundsDokument66 SeitenOrganometallic CompoundsJon Ho100% (1)
- CH 44 Organic Reactions - Supp Ex 1 (Updated)Dokument4 SeitenCH 44 Organic Reactions - Supp Ex 1 (Updated)伊貝P-Noch keine Bewertungen
- 11 Chemistry Exemplar Chapter 4Dokument12 Seiten11 Chemistry Exemplar Chapter 4adarshNoch keine Bewertungen
- Kuliah MG 9 Racemix Mixture and ResolutionDokument184 SeitenKuliah MG 9 Racemix Mixture and ResolutionBowo Aank ApriantoNoch keine Bewertungen
- Chem 212 Alkyl Halide Problems 3Dokument1 SeiteChem 212 Alkyl Halide Problems 3kevinamyNoch keine Bewertungen
- SN1 Vs SN2 ReactionsDokument23 SeitenSN1 Vs SN2 Reactionssamnas100Noch keine Bewertungen
- 12B Alcohol 2Dokument11 Seiten12B Alcohol 2Kasun RatnayakeNoch keine Bewertungen
- Pericyclics-2014 Handout PDFDokument79 SeitenPericyclics-2014 Handout PDFnavchemNoch keine Bewertungen
- Brown 5e Ch07Dokument33 SeitenBrown 5e Ch07Li LizNoch keine Bewertungen
- Chapter 4Dokument38 SeitenChapter 4Rebecca Campbell100% (1)
- January 2008 Heterocyclic Chemistry: Exam Questions and Model AnswersDokument17 SeitenJanuary 2008 Heterocyclic Chemistry: Exam Questions and Model AnswersPablo de TarsoNoch keine Bewertungen
- SCH 504 Enolates in Organic SynthesisDokument19 SeitenSCH 504 Enolates in Organic SynthesisRaditya AdipratamaNoch keine Bewertungen
- Intro To Organic ChemDokument91 SeitenIntro To Organic ChemMiguel Marquez GelacioNoch keine Bewertungen
- NMR Organic ChemistryDokument17 SeitenNMR Organic Chemistrysallymoon34Noch keine Bewertungen
- Practices Exam - Organic Chemistry To 2nd PartialDokument10 SeitenPractices Exam - Organic Chemistry To 2nd PartialShary MosqueraNoch keine Bewertungen
- 01 StereochemistryDokument6 Seiten01 StereochemistryGundum Bodyz100% (1)
- CHM 2210 Practice Exam 1Dokument12 SeitenCHM 2210 Practice Exam 1Shaima MossamatNoch keine Bewertungen
- PMR Spectroscopy: Solved Problems Volume : IIVon EverandPMR Spectroscopy: Solved Problems Volume : IIBewertung: 5 von 5 Sternen5/5 (3)
- Electronic Absorption Spectra and Geometry of Organic Molecules: An Application of Molecular Orbital TheoryVon EverandElectronic Absorption Spectra and Geometry of Organic Molecules: An Application of Molecular Orbital TheoryBewertung: 5 von 5 Sternen5/5 (1)
- Lit Rev 2Dokument27 SeitenLit Rev 2mtucker17Noch keine Bewertungen
- Monte Carlo SimulationDokument70 SeitenMonte Carlo SimulationJano Lima100% (2)
- NeoshoRver GravelDokument43 SeitenNeoshoRver Gravelmtucker17Noch keine Bewertungen
- Bio FiltersDokument56 SeitenBio FiltersarchitetoreNoch keine Bewertungen
- Ambio 1989 100-107Dokument8 SeitenAmbio 1989 100-107mtucker17Noch keine Bewertungen
- Aquatic Plants For Domestic Wastewater Treatment: Lotus (Nelumbo Nucifera) and Hydrilla (HydrillaDokument8 SeitenAquatic Plants For Domestic Wastewater Treatment: Lotus (Nelumbo Nucifera) and Hydrilla (Hydrillamtucker17Noch keine Bewertungen
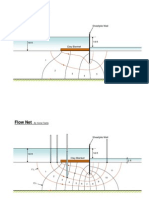
- Seepage Around DamDokument4 SeitenSeepage Around Dammtucker17Noch keine Bewertungen
- Air Quality HomeworkDokument3 SeitenAir Quality Homeworkmtucker17Noch keine Bewertungen
- Ion Exchange For Dummies RHDokument9 SeitenIon Exchange For Dummies RHDaverrrNoch keine Bewertungen
- Urgent Request Erosion Control LongmontDamRd 2 PDFDokument4 SeitenUrgent Request Erosion Control LongmontDamRd 2 PDFmtucker17Noch keine Bewertungen
- Armadio Presentation-2019Dokument45 SeitenArmadio Presentation-2019Subhash Singh TomarNoch keine Bewertungen
- Tips For A Healthy PregnancyDokument2 SeitenTips For A Healthy PregnancyLizaNoch keine Bewertungen
- The History of AstrologyDokument36 SeitenThe History of AstrologyDharani Dharendra DasNoch keine Bewertungen
- Veronte Autopilot Kit DatasheetDokument2 SeitenVeronte Autopilot Kit DatasheetEkmedzicNoch keine Bewertungen
- Presentation AcetanilideDokument22 SeitenPresentation AcetanilideNovitasarii JufriNoch keine Bewertungen
- Dharmakirti39s Commentary On ChakrasamvaraDokument15 SeitenDharmakirti39s Commentary On ChakrasamvaraThiago AlbuquerqueNoch keine Bewertungen
- Frye LGD As A Function of The Default Rate 091013 PDFDokument13 SeitenFrye LGD As A Function of The Default Rate 091013 PDFSushant SinghNoch keine Bewertungen
- DP November 2017 Examination Schedule en PDFDokument4 SeitenDP November 2017 Examination Schedule en PDFSuperlucidoNoch keine Bewertungen
- 2nd APJ Abdul Kalam Essay Writing CompetitionDokument2 Seiten2nd APJ Abdul Kalam Essay Writing CompetitionANURAG SINGHNoch keine Bewertungen
- Book Index The Art of Heavy TransportDokument6 SeitenBook Index The Art of Heavy TransportHermon Pakpahan50% (2)
- Nomenclatura SKFDokument1 SeiteNomenclatura SKFJuan José MeroNoch keine Bewertungen
- Filipino Construction TermsDokument6 SeitenFilipino Construction TermsAdrian Perez75% (4)
- Bagpipe LV 1-5Dokument228 SeitenBagpipe LV 1-5Sathia Kdms100% (2)
- Preview: Proquest Dissertations and Theses 2002 Proquest Dissertations & Theses Full TextDokument24 SeitenPreview: Proquest Dissertations and Theses 2002 Proquest Dissertations & Theses Full TextFelipe AguilarNoch keine Bewertungen
- Optical Scattering of Gold NanosphereDokument24 SeitenOptical Scattering of Gold NanosphereParas KumarNoch keine Bewertungen
- 3 Curvilinear MotionDokument50 Seiten3 Curvilinear Motiongarhgelh100% (1)
- c270 KW NTA855G2 60 HZDokument31 Seitenc270 KW NTA855G2 60 HZAhmad El KhatibNoch keine Bewertungen
- AppearancesDokument4 SeitenAppearancesReme TrujilloNoch keine Bewertungen
- Adaptive Reuse Architecture Documentation and Analysis 2168 9717 1000172Dokument9 SeitenAdaptive Reuse Architecture Documentation and Analysis 2168 9717 1000172Komal HundiaNoch keine Bewertungen
- Comparative Study On Serial and Parallel Manipulators - ReviewDokument23 SeitenComparative Study On Serial and Parallel Manipulators - ReviewShaik Himam SahebNoch keine Bewertungen
- Automatic Train OperationDokument6 SeitenAutomatic Train OperationAnupam KhandelwalNoch keine Bewertungen
- Child DevelopmentDokument15 SeitenChild Development4AndreeaNoch keine Bewertungen
- MMW ReviewerDokument3 SeitenMMW ReviewerMarcSaloj NeryNoch keine Bewertungen
- Crma Unit 1 Crma RolesDokument34 SeitenCrma Unit 1 Crma Rolesumop3plsdn0% (1)
- Integration ConceptDokument34 SeitenIntegration ConceptJANELLA ALVAREZNoch keine Bewertungen
- Etoricoxib - Martindale 39thDokument2 SeitenEtoricoxib - Martindale 39thCachimbo PrintNoch keine Bewertungen
- Quartile1 PDFDokument2 SeitenQuartile1 PDFHanifah Edres DalumaNoch keine Bewertungen
- 5 Contracting Activity and Technical Staff RequirementsDokument2 Seiten5 Contracting Activity and Technical Staff RequirementsDaniyar KussainovNoch keine Bewertungen
- Yoga SadhguruDokument6 SeitenYoga Sadhgurucosti.sorescuNoch keine Bewertungen
- Orbitol Motor TMTHWDokument20 SeitenOrbitol Motor TMTHWRodolfo ErenoNoch keine Bewertungen