Beruflich Dokumente
Kultur Dokumente
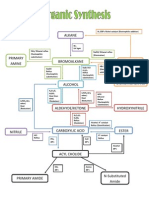
Edexcel A Level (A2) Chemistry Organic Chemistry
Hochgeladen von
AvrinoxOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Edexcel A Level (A2) Chemistry Organic Chemistry
Hochgeladen von
AvrinoxCopyright:
Verfügbare Formate
Reaction
of
Reaction
with
Organic
product
Mechanism/
Type of reaction
Conditions
Carbonyl
compounds
Aldehyde
Aldehyde
Ketone
Hydrogen
cyanide, HCN
Cyanohydrin
(Hydroxynitriles)
Nucleophilic
addition
2,4-DNPH Derivative
(hydrazone)
Condensation
(Addition-elimination)
LiAlH
4
(dry ether)
OR,
NaBH
4
(water/ dry ether
/ethanol)
Primary alcohol
Secondary alcohol
Reduction
K
2
Cr
2
O
7
/H
+
Carboxylic acid Oxidation Heat under reflux
I
2
/ NaOH Iodoform; Sodium
carboxylate
Iodoform reaction Warm if necessary
Nitrile
Water Carboxylic acid Hydrolysis Catalyst --
H
2
SO
4
[Dilute acid (strong)]
Cyanohydrin
Water
Hydroxycarboxylic
acid
Carboxylic
acid
PCl
5
Acyl chloride
Halogenation
LiAlH
4
Primary alcohol Reduction Dry ether
Na
2
CO
3
/
NaHCO
3
/
NaOH
Salt, CO
2
, H
2
O Neutralisation
Alcohol
(Reversible)
Ester Esterification/
Condensation
Strong acid;
Warm;
Add Na
2
CO
3
Acyl chlorides
Water
Carboxylic acid
Alcohol
Ester
S
N
Ammonia (conc.)
1 amide
S
N
Primary amines
2 amide
S
N
Benzene
Nitrating mixture
(Nitric acid)
Nitrobenzene
(& water)
Nitration; S
E
H
2
SO
4
; hur;
Temp-55
o
C
Chloroalkane
Alkyl benzene
FC Alkylation; S
E
Catalyst-
anhydrous AlCl
3
Acyl chloride
Phenyl ketone
FC Acylation; S
E
Catalyst-
anhydrous AlCl
3
Bromine
Bromobenzene
Bromination; S
E
Catalyst-
anhydrous
AlBr
3
/FeBr
3
Oxidation of side
chain
Benzaldehyde;
Benzoic acid
Oxidation
Acidified/Alkaline
KMnO
4
sol
n
; warm
Oxygen CO
2
, H
2
O Combustion
Fuming sulfuric
acid
Benzenesulfonic
acid
Sulfonation
Hydrogen Cyclohexane Free radical
addition
Catalyst - Raney
nickel; 60
o
C
Bromine
1,2,3,4,5,6-
hexabromocyclo
hexane
Free radical
addition
UV radiation
Phenol
NaOH soln
Sodium phenate
(& water)
Ethanoyl
chloride
Phenyl ethanoate
(& HCl)
Bromine
2,4,6-
tribromophenol
(WHITE PPT)
(& HBr)
Nitric acid (dil.)
2,4,6-trinitrophenol
(WHITE PPT)
(& water)
Amine (Prep.)
Halogenoalkanes
Ammonia
1, 2, 3 amines &
4 ammonium salts
Ethanol solvent;
Sealed;
Heat
Nitriles
Alkyl amine (1
o
)
Reduction
Reducing agent--
LiAlH
4
;
Solvent-- Dry ether
Hydrolysis with dil.
HCl
Distil with NaOH
Amides
Alkyl amine (1
o
)
Reduction
Reducing agent--
LiAlH
4
;
Solvent-- Dry ether
Hydrolysis with dil.
HCl
Distil with NaOH
Primary
amines
Aqueous
hydrogen ions
Alkyl/Phenyl
ammonium ions
Aqueous copper
(ii) ions
Tetraaminediaqua
copper (ii) ion
(& water)
Acyl chloride 2
o
amide
Nitrobenzene
Phenyl amine
Reduction
Tin and conc. HCl
Phenyl amine
Nitrous acid
Benzenediazonium
ion
Temp-
0
o
C -10
o
C
NaNO
2
& HCl
Benzene
diazonium ion
Phenol Azo-dye dil. NaOH soln
Das könnte Ihnen auch gefallen
- Organic SynthesisDokument1 SeiteOrganic Synthesiszozoxo0% (1)
- AQA A Level Chemistry Unit 4 NotesDokument29 SeitenAQA A Level Chemistry Unit 4 NotesMuadh Chati100% (2)
- Hem Actsheet: Organic Chemistry 4: Carbonyl CompoundsDokument3 SeitenHem Actsheet: Organic Chemistry 4: Carbonyl CompoundsDaniel C. WalshNoch keine Bewertungen
- As Chemistry Unit 2 NotesDokument26 SeitenAs Chemistry Unit 2 NotesFaisal AR92% (12)
- 2022 January Chemistry Unit 1 - SolvedDokument24 Seiten2022 January Chemistry Unit 1 - SolvedSidra SaifNoch keine Bewertungen
- A2 Chemistry Unit 4 NotesDokument27 SeitenA2 Chemistry Unit 4 NotesRebecca78% (9)
- Questions&AnswersDokument25 SeitenQuestions&AnswersSenthiaathavan90% (10)
- IAL Edexcel Biology Unit 4 Sorted by TopicDokument22 SeitenIAL Edexcel Biology Unit 4 Sorted by TopicWillie Wong50% (2)
- A-level Sciences Revision Boxset: Cheeky Revision ShortcutsVon EverandA-level Sciences Revision Boxset: Cheeky Revision ShortcutsBewertung: 3 von 5 Sternen3/5 (2)
- Unit 6 NotesDokument6 SeitenUnit 6 Noteseeshvari50% (4)
- O Level Chemistry Structured Practice Papers 9Von EverandO Level Chemistry Structured Practice Papers 9Bewertung: 5 von 5 Sternen5/5 (1)
- A2 Student Unit Guide - Edexcel Biology Unit 5Dokument95 SeitenA2 Student Unit Guide - Edexcel Biology Unit 5claimstudent3515100% (12)
- A2 Chemistry Revision NotesDokument13 SeitenA2 Chemistry Revision NotesJobe Bryer50% (4)
- Biology Experiments Unit 36 PDFDokument38 SeitenBiology Experiments Unit 36 PDFMehwish Arif100% (1)
- Unit 1 Chemistry IAL EDEXCEL 2024 Jan PaperDokument20 SeitenUnit 1 Chemistry IAL EDEXCEL 2024 Jan Papersamehsamdi100% (1)
- IAL Edexcel Biology Unit 5 Frequently Asked Questions Sorted by TopicDokument15 SeitenIAL Edexcel Biology Unit 5 Frequently Asked Questions Sorted by TopicWillie Wong100% (2)
- New Index PDFDokument2 SeitenNew Index PDFYingss ChiamNoch keine Bewertungen
- Edexcel Hodder Chemistry A2 Review Question AnswerDokument4 SeitenEdexcel Hodder Chemistry A2 Review Question AnswerDhulanjalieeh Joseph100% (1)
- O Level Biology Practice Questions And Answers: Coordination And ResponseVon EverandO Level Biology Practice Questions And Answers: Coordination And ResponseNoch keine Bewertungen
- PDF PDFDokument80 SeitenPDF PDFAnirudh Makhana100% (1)
- Edexcel Biology Unit 1 NotesDokument74 SeitenEdexcel Biology Unit 1 NotesBene Bin100% (2)
- Unit-5 Topic-7 Astrophysics and Cosmology Answers (End-Of-Chapter & Examzone)Dokument8 SeitenUnit-5 Topic-7 Astrophysics and Cosmology Answers (End-Of-Chapter & Examzone)Avrinox100% (3)
- Chemistry Unit 4 PDFDokument60 SeitenChemistry Unit 4 PDFsammam mahdi samiNoch keine Bewertungen
- GCSE Sciences Revision Boxset: Cheeky Revision ShortcutsVon EverandGCSE Sciences Revision Boxset: Cheeky Revision ShortcutsNoch keine Bewertungen
- Edexcel A Level A2 Chemistry Organic ChemistryDokument3 SeitenEdexcel A Level A2 Chemistry Organic ChemistrymohdburhantalatNoch keine Bewertungen
- Chemistry Unit 1 Edexcel Notes (AS Level)Dokument1 SeiteChemistry Unit 1 Edexcel Notes (AS Level)--------100% (1)
- Important Reagents For Organic ChemistryDokument2 SeitenImportant Reagents For Organic ChemistryRohan NewaskarNoch keine Bewertungen
- Chemistry P3 Practical TipsDokument4 SeitenChemistry P3 Practical TipsSashank Aryal83% (6)
- AS Level Chemistry Practical Paper 3: TitrationDokument8 SeitenAS Level Chemistry Practical Paper 3: TitrationNabindra RuwaliNoch keine Bewertungen
- Reaction List v002Dokument5 SeitenReaction List v002cecil3414Noch keine Bewertungen
- Hasan Sayginel: Edexcel A Level Organic ChemistryDokument41 SeitenHasan Sayginel: Edexcel A Level Organic ChemistryDEEBANNoch keine Bewertungen
- A2 Biology Core Practical SummaryDokument3 SeitenA2 Biology Core Practical SummarySQ100% (2)
- Edexcel Biology Answers - Combined - FINAL PDFDokument50 SeitenEdexcel Biology Answers - Combined - FINAL PDFNikhil DasNoch keine Bewertungen
- IAL Edexcel Biology Unit 3Dokument12 SeitenIAL Edexcel Biology Unit 3Willie WongNoch keine Bewertungen
- Model Answers in Organic Chemistry: For 'A' Level and Ordinary National Certificate StudentsVon EverandModel Answers in Organic Chemistry: For 'A' Level and Ordinary National Certificate StudentsNoch keine Bewertungen
- 9701 Chemistry Paper 5 NotesDokument4 Seiten9701 Chemistry Paper 5 NotesTanvir Ahmed Mazumder75% (4)
- A2 Chemistry Answer BookDokument85 SeitenA2 Chemistry Answer BookHarrys Oustapasidis100% (3)
- Chem Unit 3 NotesDokument12 SeitenChem Unit 3 NotesMena Hashem100% (3)
- Chemistry Unit 4 Part 3 ReallyacademicsDokument35 SeitenChemistry Unit 4 Part 3 ReallyacademicsWill AndyNoch keine Bewertungen
- Edexcel IAL Chemistry Unit 6 October 2021 Question PaperDokument16 SeitenEdexcel IAL Chemistry Unit 6 October 2021 Question PaperEffendi Jabid KamalNoch keine Bewertungen
- IAL Chemistry Data Booklet Issue 3Dokument35 SeitenIAL Chemistry Data Booklet Issue 3jeeshan sayed0% (1)
- Mod 4 Revision Guide 10 Synthetic RoutesDokument2 SeitenMod 4 Revision Guide 10 Synthetic RoutesdufraiscNoch keine Bewertungen
- Organic Chem ReactionsDokument7 SeitenOrganic Chem ReactionsTeo Jia Ming NickolasNoch keine Bewertungen
- Unit 4 Organic Chemistry ReactionsDokument6 SeitenUnit 4 Organic Chemistry ReactionsRobbing_Hood100% (1)
- 634566746179743750Dokument6 Seiten634566746179743750Abhijit SinghNoch keine Bewertungen
- OrganicDokument15 SeitenOrganicI am madNoch keine Bewertungen
- Converssion TipsDokument4 SeitenConverssion TipsJleodennis RajNoch keine Bewertungen
- Done By: Kaijie, Elias, Chenxi, Ashwini, Sahana, Kelly From 0901 and 0914 Compiled From 2007 and 2008 H2 Chemistry Prelim PapersDokument10 SeitenDone By: Kaijie, Elias, Chenxi, Ashwini, Sahana, Kelly From 0901 and 0914 Compiled From 2007 and 2008 H2 Chemistry Prelim Papersdiejunqs sNoch keine Bewertungen
- Alcohols Phenols and EthersDokument81 SeitenAlcohols Phenols and Ethersjjprakash82chemNoch keine Bewertungen
- Aldehyde Ketone and AcidDokument15 SeitenAldehyde Ketone and AcidAbir DuttaNoch keine Bewertungen
- Carbonyl Compounds Aldehydes KetonesDokument58 SeitenCarbonyl Compounds Aldehydes KetonesNur Aliyah Abdul RazakNoch keine Bewertungen
- Chem 332 Exam 1 ReviewDokument6 SeitenChem 332 Exam 1 ReviewhddriNoch keine Bewertungen
- Nucleophilic Subs: AlcoholDokument11 SeitenNucleophilic Subs: AlcoholZhiro Ming HanNoch keine Bewertungen
- Organic Chem Reactions: 1. AlkanesDokument6 SeitenOrganic Chem Reactions: 1. AlkanesFatema KhatunNoch keine Bewertungen
- CARBON COMPOUND (Asid Carboxylic)Dokument24 SeitenCARBON COMPOUND (Asid Carboxylic)Shirley SimonNoch keine Bewertungen
- Carbonyl Compounds: Carboxylic Acids & EsterDokument28 SeitenCarbonyl Compounds: Carboxylic Acids & Esterrustam effendyNoch keine Bewertungen
- Alcohols: Chemistry Unit 2 C. Bailey PolackDokument24 SeitenAlcohols: Chemistry Unit 2 C. Bailey PolackBritney PattersonNoch keine Bewertungen
- Edexcel IAL Chemistry June 2014 Unit-5 Question PaperDokument32 SeitenEdexcel IAL Chemistry June 2014 Unit-5 Question PaperAvrinox100% (1)
- Unit-5 Topic-6 Oscillations Answers (End-Of-Chapter & Examzone)Dokument5 SeitenUnit-5 Topic-6 Oscillations Answers (End-Of-Chapter & Examzone)AvrinoxNoch keine Bewertungen
- Edexcel IAL Biology October 2017 Unit 1 Question PaperDokument24 SeitenEdexcel IAL Biology October 2017 Unit 1 Question PaperAvrinoxNoch keine Bewertungen
- 6BI05 June 2011Dokument24 Seiten6BI05 June 2011areyouthere92Noch keine Bewertungen
- Edexcel GCE Chemistry Unit-5 June 2014 Question PaperDokument28 SeitenEdexcel GCE Chemistry Unit-5 June 2014 Question PaperAvrinoxNoch keine Bewertungen
- Edexcel GCE Chemistry Unit-5 June 2013 Question Paper (R)Dokument28 SeitenEdexcel GCE Chemistry Unit-5 June 2013 Question Paper (R)Avrinox100% (1)
- Edexcel IAL Biology Unit-5 June 2014 Mark SchemeDokument27 SeitenEdexcel IAL Biology Unit-5 June 2014 Mark SchemeAvrinoxNoch keine Bewertungen
- Edexcel GCE Chemistry Unit-5 June 2014 Question Paper (R)Dokument28 SeitenEdexcel GCE Chemistry Unit-5 June 2014 Question Paper (R)AvrinoxNoch keine Bewertungen
- Chemistry A Level Edexcel June 2013Dokument32 SeitenChemistry A Level Edexcel June 2013NavoditteNoch keine Bewertungen
- Edexcel GCE Chemistry Unit-4 June 2014 Question Paper (R)Dokument24 SeitenEdexcel GCE Chemistry Unit-4 June 2014 Question Paper (R)AvrinoxNoch keine Bewertungen
- Edexcel GCE Chemistry Unit-4 June 2014 Question PaperDokument24 SeitenEdexcel GCE Chemistry Unit-4 June 2014 Question PaperAvrinoxNoch keine Bewertungen
- 6CH04 01R Que 20130612Dokument24 Seiten6CH04 01R Que 20130612Fuzzbuzz95Noch keine Bewertungen
- Edexcel IAL Chemistry January 2014 U4 Question PaperDokument24 SeitenEdexcel IAL Chemistry January 2014 U4 Question PaperAvrinoxNoch keine Bewertungen
- EDEXCEL A2 CHEMISTRY UNIT 4 January 2011Dokument24 SeitenEDEXCEL A2 CHEMISTRY UNIT 4 January 2011Ghaleb W. MihyarNoch keine Bewertungen
- Edexcel IAL Biology Unit-5 June 2014 Question PaperDokument28 SeitenEdexcel IAL Biology Unit-5 June 2014 Question PaperAvrinoxNoch keine Bewertungen
- Edexcel IAL Biology Unit-5 January 2014 Mark SchemeDokument22 SeitenEdexcel IAL Biology Unit-5 January 2014 Mark SchemeAvrinoxNoch keine Bewertungen
- 6BI05 01 Que 20120307Dokument24 Seiten6BI05 01 Que 20120307epenium14Noch keine Bewertungen
- Edexcel GCE Biology Unit-4 June 2014 Question PaperDokument24 SeitenEdexcel GCE Biology Unit-4 June 2014 Question PaperAvrinoxNoch keine Bewertungen
- Unit 5 Jan 2011Dokument20 SeitenUnit 5 Jan 2011Izzat Azmeer AhmadNoch keine Bewertungen
- Edexcel IAL Biology Unit-5 January 2014 Question PaperDokument28 SeitenEdexcel IAL Biology Unit-5 January 2014 Question PaperAvrinoxNoch keine Bewertungen
- Edexcel GCE Biology Unit-5 June 2013 Question PaperDokument36 SeitenEdexcel GCE Biology Unit-5 June 2013 Question PaperAvrinoxNoch keine Bewertungen
- Edexcel GCE Biology Unit-4 (R) June 2014 Question PaperDokument28 SeitenEdexcel GCE Biology Unit-4 (R) June 2014 Question PaperAvrinoxNoch keine Bewertungen
- Edexcel GCE Biology Unit-5 June 2014 Question PaperDokument24 SeitenEdexcel GCE Biology Unit-5 June 2014 Question PaperAvrinox0% (1)
- Edexcel GCE Biology Unit-5 June 2014 Question Paper (R)Dokument24 SeitenEdexcel GCE Biology Unit-5 June 2014 Question Paper (R)AvrinoxNoch keine Bewertungen