Beruflich Dokumente
Kultur Dokumente
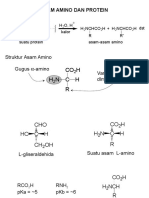
Organic Functional Groups and Nomenclature Chart
Hochgeladen von
Nhi HinOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Organic Functional Groups and Nomenclature Chart
Hochgeladen von
Nhi HinCopyright:
Verfügbare Formate
Organic Functional Groups and Nomenclature
Compound
Functional group
Structural
formula
H H
|
alkane
N/A
CC
|
H H
H
|
alkene
alkene
C=C
|
Condenses to
Example
CH2 CH2
or
CH2CH2
CH3CH2CH3
propane
CH = CH
or
CHCH
CH3CH=CH2
or CH3CHCH2
propene
CC
or
CC
CH3CCH
or CH3CCH
propyne
CH3CH2F
fluoro ethane
CH3CH2Cl chloro ethane
CH3CH2Br bromo ethane
CH3CH2I
iodo ethane
alkyne
CC
alkyl halide
halogen
F
Cl
Br
I
N/A
alcohol
hydroxyl
OH
OH
aldehyde
carbonyl
(at end of chain)
alkyne
ketone
O
||
CH3CH2CHO
propanal
CO
CH3COCH3
propanone
COOH
CH3CH2COOH
propanoic acid
COO-
CH3CH2COOpropanoate ion
COO
CH3COOCH2CH2CH3
propyl ethanoate
NH2
CH3CH2NH2
ethanamine
CONH
CH3CH2CONH2
propanamide
O
||
C
O
carboxylic acid
carboxyl
||
COH
O
carboxylate ion
carboxylate
||
C OO
ester
ester
||
CO
amine
amino
amide
amide
H
|
NH
O
||
CN
ethanol
CHO
CH
carbonyl
(in middle of chain)
CH3CH2OH
A blank space beside a bond line means a carbon chain (alkyl group) of any length is bonded there.*
In an amino group, any H in the structure shown can be replaced with an alkyl group.
Structures are often drawn with bonds on angles, and often use a mixture of condensed and expanded forms.
Condensed forms must be drawn backwards (e.g. H2N and HO ) in some cases to preserve meaning.
*In alkanes, alkenes, alkynes, aldehydes, carboxylic acids, and amides any of these can be also be a H. The bond that is part of the
'oate' in the ester can also be a H.
Das könnte Ihnen auch gefallen
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsVon EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsNoch keine Bewertungen
- Flavonoid Biosynthesis: Nila HudaDokument5 SeitenFlavonoid Biosynthesis: Nila HudaNilaHudaBaqirNoch keine Bewertungen
- Common Functional GroupsDokument1 SeiteCommon Functional Groupszeeshan876Noch keine Bewertungen
- Priority Table of Functional Groups of Organic ChemistryDokument1 SeitePriority Table of Functional Groups of Organic ChemistryArut SelvanNoch keine Bewertungen
- Amino Acid TableDokument1 SeiteAmino Acid TableEugene OkpanteyNoch keine Bewertungen
- Carbanions - C: - The Conjugate Bases of Weak Acids, Strong Bases, Excellent NucleophilesDokument40 SeitenCarbanions - C: - The Conjugate Bases of Weak Acids, Strong Bases, Excellent NucleophilesSiicwek GeminieNoch keine Bewertungen
- Nomenclature, Functional Groups: Family F (X) Group Suffix PrefixDokument3 SeitenNomenclature, Functional Groups: Family F (X) Group Suffix PrefixertyucbNoch keine Bewertungen
- Notes - Chapt.25 Amino Acids and PeptidesDokument51 SeitenNotes - Chapt.25 Amino Acids and PeptidesSiddarth PalletiNoch keine Bewertungen
- Carbanions IDokument40 SeitenCarbanions INurhan KishaliNoch keine Bewertungen
- Lecture 3 PDFDokument16 SeitenLecture 3 PDFNazir KhanNoch keine Bewertungen
- Carboxylic Acids and Esters: Key ConceptsDokument26 SeitenCarboxylic Acids and Esters: Key ConceptsIrsyad KamilNoch keine Bewertungen
- AldehydesDokument19 SeitenAldehydesSeverina MamauagNoch keine Bewertungen
- Amino Acid Abbreviations Molecular Formula Linear FormulaDokument1 SeiteAmino Acid Abbreviations Molecular Formula Linear FormulaBeatrix RossNoch keine Bewertungen
- Alcohol Phenol & EtherDokument13 SeitenAlcohol Phenol & EtherAbir DuttaNoch keine Bewertungen
- Carbonyl Compounds Xi Xii Study MaterialsDokument171 SeitenCarbonyl Compounds Xi Xii Study MaterialsCristiano Hamdiansyah SempadianNoch keine Bewertungen
- Important biological functional groupsDokument1 SeiteImportant biological functional groupsKevin YeNoch keine Bewertungen
- ch10 Reactions Worksheet and Key 05 7 09Dokument13 Seitench10 Reactions Worksheet and Key 05 7 09api-304182646Noch keine Bewertungen
- SEO-Optimized Title for ASAM SHIKIMAT DocumentDokument11 SeitenSEO-Optimized Title for ASAM SHIKIMAT DocumentLong Ayu100% (1)
- Carboxylic AcidDokument33 SeitenCarboxylic AcidRika Yulliyani RNoch keine Bewertungen
- Table of Common Functional GroupsDokument10 SeitenTable of Common Functional GroupsAngelica Mae Lasam100% (1)
- OrganicDokument15 SeitenOrganicI am madNoch keine Bewertungen
- Organic Chemistry 2 - Chem - f3 - v1Dokument66 SeitenOrganic Chemistry 2 - Chem - f3 - v1Lubanga N JamesNoch keine Bewertungen
- Important Functional Groups and Their PropertiesDokument3 SeitenImportant Functional Groups and Their Propertiesrache1505Noch keine Bewertungen
- Common Functional GroupsDokument2 SeitenCommon Functional GroupsHRiffs GunsNoch keine Bewertungen
- Inborn Errors ChartDokument1 SeiteInborn Errors ChartGrausamvsNoch keine Bewertungen
- Bab 19. Asam AminoDokument58 SeitenBab 19. Asam AminoSisca Ayu VerawatiNoch keine Bewertungen
- Non Polar-Hydrophobic-Buried in Protein Core-AliphaticDokument4 SeitenNon Polar-Hydrophobic-Buried in Protein Core-AliphaticRob FranciscoNoch keine Bewertungen
- SolventDokument1 SeiteSolventlycheatnstealNoch keine Bewertungen
- 08 - Chapter 1Dokument54 Seiten08 - Chapter 1melissaisostdNoch keine Bewertungen
- Proteins Part I: Amino Acids and PeptidesDokument62 SeitenProteins Part I: Amino Acids and PeptidesDaniele Joseph HizonNoch keine Bewertungen
- Substante Organice Pentru Admitere MedicinaDokument21 SeitenSubstante Organice Pentru Admitere MedicinaDragotaNoch keine Bewertungen
- AldehydesDokument21 SeitenAldehydesNoor Farrah Wahida MuradNoch keine Bewertungen
- Denumire Substanta Formula Chimica Clorura de Vinil CH2 CH-CLDokument21 SeitenDenumire Substanta Formula Chimica Clorura de Vinil CH2 CH-CLLayla NicoNoch keine Bewertungen
- Naming AlcoholsDokument9 SeitenNaming AlcoholsMary Margaret "MM" A. Avena0% (1)
- Conversions (ORGANIC)Dokument8 SeitenConversions (ORGANIC)Abir Dutta80% (5)
- Aldehydes, Ketones and Carboxylic AcidsDokument17 SeitenAldehydes, Ketones and Carboxylic AcidsMoshe Cohen'sNoch keine Bewertungen
- Organic ChemistryDokument31 SeitenOrganic ChemistrySameep Kumar SinghNoch keine Bewertungen
- Amino Acid BIOCHEMDokument3 SeitenAmino Acid BIOCHEMCayla Jean T. NavascaNoch keine Bewertungen
- 487 - Concept, Sources, Nomenclature and Isomerism in Alkanoate (Esters) .Dokument4 Seiten487 - Concept, Sources, Nomenclature and Isomerism in Alkanoate (Esters) .emmanuelirem805Noch keine Bewertungen
- Notes Functional GroupsDokument5 SeitenNotes Functional GroupsFrank GaoNoch keine Bewertungen
- Carboxylic Acids and EstersDokument22 SeitenCarboxylic Acids and Estersapi-3706290100% (2)
- C4 OH Cu Carbonilici Monozaharide 1Dokument18 SeitenC4 OH Cu Carbonilici Monozaharide 1deedyannaNoch keine Bewertungen
- Chemistry - Classification and NomenclatureDokument20 SeitenChemistry - Classification and NomenclatureDevesh AgrawalNoch keine Bewertungen
- Nombre Grupo Funcional Formula Desarrollada Semi-Desarrollada EjemploDokument1 SeiteNombre Grupo Funcional Formula Desarrollada Semi-Desarrollada EjemploGerardo Salas SánchezNoch keine Bewertungen
- Aldehydes Ketones Carboxylic AcidsDokument40 SeitenAldehydes Ketones Carboxylic AcidsAlexandrea WynnoevahNoch keine Bewertungen
- LEARN ABOUT ALCOHOLSDokument42 SeitenLEARN ABOUT ALCOHOLSThe Mini KitchenNoch keine Bewertungen
- Basic Hydrocarbons:: RH CC R R R R CC R R R R R R R RDokument2 SeitenBasic Hydrocarbons:: RH CC R R R R CC R R R R R R R RyoonnyungleeNoch keine Bewertungen
- Mareial Complementar Unidade 4 Quimica Organica Gupos FuncionaisDokument10 SeitenMareial Complementar Unidade 4 Quimica Organica Gupos FuncionaisepambarbaNoch keine Bewertungen
- DerivativesDokument58 SeitenDerivativesravi_balaskarNoch keine Bewertungen
- Inter Conversions of Functional GroupsDokument9 SeitenInter Conversions of Functional Groupssaqib sulmanNoch keine Bewertungen
- Masteranswersorganic 4 UDokument9 SeitenMasteranswersorganic 4 Ukriss WongNoch keine Bewertungen
- Functional Groups 4Dokument12 SeitenFunctional Groups 4isel melianiNoch keine Bewertungen
- Functional Groups: Organic Chemistry EssentialsDokument8 SeitenFunctional Groups: Organic Chemistry EssentialsJeremiah Paul Gotia HumiwatNoch keine Bewertungen
- Organic Chemistry Carboxilc Acids and EstersDokument6 SeitenOrganic Chemistry Carboxilc Acids and EstersKasun WekasingheNoch keine Bewertungen
- Aldehydes and Ketones - 1-MergedDokument94 SeitenAldehydes and Ketones - 1-MergedseNoch keine Bewertungen
- Amino AcidsDokument26 SeitenAmino Acidsrumela1504Noch keine Bewertungen
- Assembly Instructions for Polypeptide Models: Academic Press/Molecular Design Inc. Precision Molecular ModelsVon EverandAssembly Instructions for Polypeptide Models: Academic Press/Molecular Design Inc. Precision Molecular ModelsNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersVon EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNoch keine Bewertungen
- SACE2 Chem Sample Chapter From Essentials TextbookDokument15 SeitenSACE2 Chem Sample Chapter From Essentials TextbookNhi HinNoch keine Bewertungen
- Revision Biology Midyear Exam 2011 - Stage 1 SACEDokument2 SeitenRevision Biology Midyear Exam 2011 - Stage 1 SACENhi HinNoch keine Bewertungen
- SACE2 Chemistry Test Exam Pack Sample PagesDokument6 SeitenSACE2 Chemistry Test Exam Pack Sample PagesNhi HinNoch keine Bewertungen
- Chemistry Pre Test - Before Starting Yr 12Dokument8 SeitenChemistry Pre Test - Before Starting Yr 12Nhi HinNoch keine Bewertungen
- 2012 Cancer Assignment Stage 1 BiologyDokument2 Seiten2012 Cancer Assignment Stage 1 BiologyNhi HinNoch keine Bewertungen
- SACE Stage 1 - Psychology Revision NotesDokument9 SeitenSACE Stage 1 - Psychology Revision NotesNhi Hin100% (1)
- Core Knowledge Chemistry 2012 SAMPLEDokument6 SeitenCore Knowledge Chemistry 2012 SAMPLENhi HinNoch keine Bewertungen
- UMATE 2012 Sample UMAT QuestionsDokument10 SeitenUMATE 2012 Sample UMAT QuestionsNhi HinNoch keine Bewertungen
- Microscope Skills Year 11 WorksheetDokument1 SeiteMicroscope Skills Year 11 WorksheetNhi HinNoch keine Bewertungen
- SACE Stage 1 Bonding and Structure Chemistry NotesDokument3 SeitenSACE Stage 1 Bonding and Structure Chemistry NotesNhi HinNoch keine Bewertungen
- SACE Stage 1 Biology Test Revision/Summary QuestionsDokument1 SeiteSACE Stage 1 Biology Test Revision/Summary QuestionsNhi HinNoch keine Bewertungen
- Quadratic and cubic equations from graphsDokument7 SeitenQuadratic and cubic equations from graphsNhi HinNoch keine Bewertungen