Beruflich Dokumente
Kultur Dokumente
2.2 Exercise 1 - Kinetics
Hochgeladen von
Ronaldo0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
50 Ansichten1 SeiteThis document discusses kinetics and factors that influence reaction rates. It asks the reader to:
1) Explain collision frequency, collision energy, and activation energy and how they can be changed.
2) Describe how increasing temperature affects the Maxwell-Boltzmann distribution and rate of reaction.
3) Define a catalyst and explain how it lowers activation energy and increases reaction rate.
4) List three ways to increase the rate for each of three reactions: increasing temperature, concentration, or using a catalyst.
Originalbeschreibung:
gb
Copyright
© © All Rights Reserved
Verfügbare Formate
DOC, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenThis document discusses kinetics and factors that influence reaction rates. It asks the reader to:
1) Explain collision frequency, collision energy, and activation energy and how they can be changed.
2) Describe how increasing temperature affects the Maxwell-Boltzmann distribution and rate of reaction.
3) Define a catalyst and explain how it lowers activation energy and increases reaction rate.
4) List three ways to increase the rate for each of three reactions: increasing temperature, concentration, or using a catalyst.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
50 Ansichten1 Seite2.2 Exercise 1 - Kinetics
Hochgeladen von
RonaldoThis document discusses kinetics and factors that influence reaction rates. It asks the reader to:
1) Explain collision frequency, collision energy, and activation energy and how they can be changed.
2) Describe how increasing temperature affects the Maxwell-Boltzmann distribution and rate of reaction.
3) Define a catalyst and explain how it lowers activation energy and increases reaction rate.
4) List three ways to increase the rate for each of three reactions: increasing temperature, concentration, or using a catalyst.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 1
2.
2 EXERCISE 1 Kinetics
1.
2.
a)
Explain what is meant by the terms:
i)
collision frequency
ii)
collision energy
iii)
activation energy
b)
How can these be changed in a chemical system?
a)
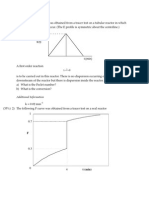
Sketch the Maxwell-Boltzmann distribution of molecular energies for a
low temperature T1 and a higher temperature T2. Explain what happens to
i)
the mean kinetic energy
ii)
the area under the graph
iii)
the number of particles having the most common amount of energy
3.
4.
c)
Hence explain why an increase in temperature has such a large effect on
the rate of reaction.
a)
Explain the meaning of the term catalyst.
b)
Explain how a catalyst lowers the activation energy for a reaction.
c)
Use the Maxwell-Boltzmann distribution of molecular energies to explain
how this leads to an increase in reaction rate.
For each of the following reactions, list three ways in which the rate of the
reaction could be increased:
a)
b)
c)
CaCO3(s) + 2HCl(aq) CaCl2(aq) + CO2(g) + H2O(l)
H2(g) + I2(g) 2HI(g)
2H2O2(aq) 2H2O(l) + O2(g)
Das könnte Ihnen auch gefallen
- Topic 5 Exercise 1 - Rates of ReactionDokument2 SeitenTopic 5 Exercise 1 - Rates of Reactionzakaraya0Noch keine Bewertungen
- Unit One AnswersDokument11 SeitenUnit One AnswersMarko MihokovićNoch keine Bewertungen
- AP Chem Ch12 Practice QuizDokument8 SeitenAP Chem Ch12 Practice QuizlhijeanNoch keine Bewertungen
- ModelQuestionsCh16 AKDokument5 SeitenModelQuestionsCh16 AKYasmeen ElsawafNoch keine Bewertungen
- Chemical Kinetics Problem SetDokument6 SeitenChemical Kinetics Problem Setascd_msvuNoch keine Bewertungen
- 5 5+Collision+Model+StudentDokument4 Seiten5 5+Collision+Model+StudentJannah ElmaghrabyNoch keine Bewertungen
- Note 28 Oct 2023Dokument21 SeitenNote 28 Oct 2023Jihan Abou GhaddaraNoch keine Bewertungen
- U12 Rev Ws - 10 - No Ice or KSPDokument3 SeitenU12 Rev Ws - 10 - No Ice or KSPetud3clNoch keine Bewertungen
- Delhi Public School: Nacharam/ Mahendra Hills/ NadergulDokument3 SeitenDelhi Public School: Nacharam/ Mahendra Hills/ Naderguleeshwar saagarNoch keine Bewertungen
- AP Ch. 12-13 Kinetics & Equilibrium Review AnswersDokument35 SeitenAP Ch. 12-13 Kinetics & Equilibrium Review AnswersRucar Rad0% (1)
- Chemistry (Mains) - 2008 Paper 1Dokument2 SeitenChemistry (Mains) - 2008 Paper 1RituChoudharyNoch keine Bewertungen
- Kinetics Homework 3Dokument4 SeitenKinetics Homework 3RizkiNoch keine Bewertungen
- Chemistry 12 - Reaction KineticsDokument16 SeitenChemistry 12 - Reaction Kineticscharanbagh6402Noch keine Bewertungen
- Big Idea 4 AnswersDokument4 SeitenBig Idea 4 AnswersSreeyaNoch keine Bewertungen
- Chemical Kinetics FinalDokument7 SeitenChemical Kinetics Finalaxiliya6Noch keine Bewertungen
- 163Ch11 13Dokument7 Seiten163Ch11 13Aaron BautistaNoch keine Bewertungen
- Echmtb2 Supp PDFDokument4 SeitenEchmtb2 Supp PDFONNDWELA RAMALAMULANoch keine Bewertungen
- Rate and Extent of Chemical Reactions Revision Test 2Dokument3 SeitenRate and Extent of Chemical Reactions Revision Test 2KiaNoch keine Bewertungen
- CHP 4101Dokument7 SeitenCHP 4101DEBBROTA KUMAR BISWASNoch keine Bewertungen
- 3 - QP - Chemical KineticsDokument5 Seiten3 - QP - Chemical Kineticsssheeladevi84Noch keine Bewertungen
- Experiment Molarity (Mol DM) Rates (Mol DM / Min) : Chy2018 Tutorial 3 (Kinetics)Dokument2 SeitenExperiment Molarity (Mol DM) Rates (Mol DM / Min) : Chy2018 Tutorial 3 (Kinetics)DubistWhiteNoch keine Bewertungen
- 3 QP Chemical KineticsDokument4 Seiten3 QP Chemical KineticsSnehit RajNoch keine Bewertungen
- Q: 2 Attempt Any Three of The Following Question (12) : InstructionsDokument2 SeitenQ: 2 Attempt Any Three of The Following Question (12) : InstructionsSmruthi SuvarnaNoch keine Bewertungen
- Microsoft Word - C18 PS1aDokument6 SeitenMicrosoft Word - C18 PS1aabcdelololNoch keine Bewertungen
- 12th Revision Test Chap. 1,2&3Dokument4 Seiten12th Revision Test Chap. 1,2&3Bloody DemonNoch keine Bewertungen
- X I I Past Papers (From 1993 - 2012)Dokument96 SeitenX I I Past Papers (From 1993 - 2012)asif_zehravi804888% (16)
- Questions On Collision TheoryDokument2 SeitenQuestions On Collision TheoryVincent Tiara100% (1)
- Chapter 13-ChemicalKineticsDokument4 SeitenChapter 13-ChemicalKineticsKhurram KhanNoch keine Bewertungen
- Ki KBR H C Ki BR H C: Oducts B ADokument2 SeitenKi KBR H C Ki BR H C: Oducts B AnaverfallNoch keine Bewertungen
- XII - Revision Sheet - 2 - ChemistryDokument3 SeitenXII - Revision Sheet - 2 - ChemistryVipin VNoch keine Bewertungen
- Chem. Assig.Dokument8 SeitenChem. Assig.aryan asliaNoch keine Bewertungen
- 3.chemical KineticsDokument2 Seiten3.chemical KineticsAnshumyNoch keine Bewertungen
- Tutorial 2 StudentDokument6 SeitenTutorial 2 StudentIrsyad KamilNoch keine Bewertungen
- Kinetics Mc1Dokument6 SeitenKinetics Mc1hylee102594Noch keine Bewertungen
- DQE January 2001: Additional InformationDokument12 SeitenDQE January 2001: Additional InformationryezhuNoch keine Bewertungen
- SuggestedAnswers 46 E ReprintDokument13 SeitenSuggestedAnswers 46 E ReprintLauriceWongNoch keine Bewertungen
- Chemical Kinetics Question BankDokument5 SeitenChemical Kinetics Question BankShivam kumarNoch keine Bewertungen
- Chapter 1: Rate of Reaction: Larning Task 1.2 Problem SolvingDokument29 SeitenChapter 1: Rate of Reaction: Larning Task 1.2 Problem Solvingamin_zamanNoch keine Bewertungen
- Reaction KineticsDokument37 SeitenReaction KineticsDaisyNoch keine Bewertungen
- Module 1 Rates of ReactionDokument15 SeitenModule 1 Rates of ReactionWinndell DupresNoch keine Bewertungen
- 5 Sets Model Questions of PhysicsDokument27 Seiten5 Sets Model Questions of Physicsdast DonNoch keine Bewertungen
- t11 Reaction Kinetics 19-26Dokument7 Seitent11 Reaction Kinetics 19-26lorraine_cuaNoch keine Bewertungen
- West Bengal State University: B.Sc./Part-I/Hons./CEMA-II/2017Dokument4 SeitenWest Bengal State University: B.Sc./Part-I/Hons./CEMA-II/2017SwwwwwNoch keine Bewertungen
- Extra Thermodynamics HomeworkDokument6 SeitenExtra Thermodynamics HomeworkSelenaYeliNoch keine Bewertungen
- Factors Affecting Rates of Reaction: Chemguide - QuestionsDokument2 SeitenFactors Affecting Rates of Reaction: Chemguide - QuestionsFatma MoustafaNoch keine Bewertungen
- إمتحانات 4 كيمياء علوم طنطا 2010Dokument42 Seitenإمتحانات 4 كيمياء علوم طنطا 2010ambe123456Noch keine Bewertungen
- Kinetics ReviewDokument5 SeitenKinetics ReviewbrittanypriyaNoch keine Bewertungen
- Rates of Reaction TestDokument10 SeitenRates of Reaction TestSaya MenangNoch keine Bewertungen
- Chemes PY QDokument15 SeitenChemes PY QSanthiiya RevindranathNoch keine Bewertungen
- GZB - Xii - WS-8 - Chemistry - Chemical Kinetics - OctoberDokument3 SeitenGZB - Xii - WS-8 - Chemistry - Chemical Kinetics - OctoberSaman PanwarNoch keine Bewertungen
- Tutorial 3Dokument3 SeitenTutorial 3Mirnal MungraNoch keine Bewertungen
- C CC CDokument9 SeitenC CC CAkhil KhannaNoch keine Bewertungen
- Question Paper Applied Physics, Sem-1, BS-105Dokument5 SeitenQuestion Paper Applied Physics, Sem-1, BS-105Kartik AgrawalNoch keine Bewertungen
- Previous Hse Questions and Answers of The Chapter "States of Matter"Dokument10 SeitenPrevious Hse Questions and Answers of The Chapter "States of Matter"arshaNoch keine Bewertungen
- Statistical Mechanics I: Section ADokument2 SeitenStatistical Mechanics I: Section ABashar Al ZoobaidiNoch keine Bewertungen
- Chemistry Matriculation Note SK025 by Vinarti MahmudDokument47 SeitenChemistry Matriculation Note SK025 by Vinarti MahmudNurun NajwaNoch keine Bewertungen
- A Modern Course in Statistical PhysicsVon EverandA Modern Course in Statistical PhysicsBewertung: 3.5 von 5 Sternen3.5/5 (2)
- Physical Chemistry of Polyelectrolyte SolutionsVon EverandPhysical Chemistry of Polyelectrolyte SolutionsMitsuru NagasawaNoch keine Bewertungen
- Thermodynamic Degradation Science: Physics of Failure, Accelerated Testing, Fatigue, and Reliability ApplicationsVon EverandThermodynamic Degradation Science: Physics of Failure, Accelerated Testing, Fatigue, and Reliability ApplicationsNoch keine Bewertungen
- Music Composition (Kaddy)Dokument7 SeitenMusic Composition (Kaddy)RonaldoNoch keine Bewertungen
- FIGURE 3.1 - Elements in Group Four (IV)Dokument11 SeitenFIGURE 3.1 - Elements in Group Four (IV)RonaldoNoch keine Bewertungen
- 1.4 Exercise 1 - Trends in Period 3Dokument1 Seite1.4 Exercise 1 - Trends in Period 3tttttttttutNoch keine Bewertungen
- Table 5.2 TemperatureDokument2 SeitenTable 5.2 TemperatureRonaldoNoch keine Bewertungen
- WaterPotential (1) HVBDokument16 SeitenWaterPotential (1) HVBRonaldoNoch keine Bewertungen
- Cape Maths SchemeDokument2 SeitenCape Maths SchemeRonaldoNoch keine Bewertungen
- Markscheme UnitC2 (6664) Paper1R Tfjune2014Dokument20 SeitenMarkscheme UnitC2 (6664) Paper1R Tfjune2014RonaldoNoch keine Bewertungen
- ' A - ' W) V 8 - J C/: $ & M K4 J KDokument11 Seiten' A - ' W) V 8 - J C/: $ & M K4 J KRonaldoNoch keine Bewertungen