Beruflich Dokumente
Kultur Dokumente
CH Phase Diagrams Notes Ws
Hochgeladen von
api-293306937Originalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
CH Phase Diagrams Notes Ws
Hochgeladen von
api-293306937Copyright:
Verfügbare Formate
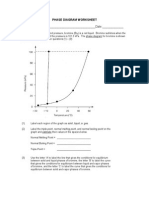
PHASE DIAGRAMS
Temperature and _____________________ control the phase of a substance. A phase diagram is a graph
of pressure versus temperature that shows in which phase a substance exists under different conditions of
temperature and pressure. A phase diagram typically has ______ regions, each representing a different
phase and three curves that ________________________ each phase.
0.0098
Temperature (C)
The points on the curves (lines) indicate conditions under which two phases coexist. The critical point
indicates the critical pressure and the critical temperature above which a substance cannot exist
as a ____________________. The triple point is the point on a phase diagram that represents the
temperature and pressure at which three phases of a substance can __________________________. The
__________________________ slope of the solid-liquid line in the phase diagram for water indicates that
the solid floats on its liquid.
Phase Diagram Questions
1. What phase change occurs for CO2 at 100 C and 1 atm pressure as it is heated to room
temperature?
2. What phase change happens to water at 1 atm as the temperature rises from 15C to 60C?
3. What state of matter is water at 50C and 20 atm?
4. At what temperature does the triple point occur for water?
5. At what temperature does the critical point occur for carbon dioxide?
6. At standard pressure and 78C, what two phase changes can occur for carbon dioxide?
7. What state of matter is carbon dioxide at 80C and 2 atm?
PHASE DIAGRAMS WORKSHEET
____
Name ___________________________
Period
1. What variables are plotted on a phase diagram?
2. How many phases are represented in a phase diagram? What are they?
3. Use the phase diagram for water to complete the following table.
0.00 < T <
_______
P<
_____
4. What phases of water coexist at
point C in waters phase diagram?
6. What is the critical temperature of
water?
5. What two phase changes occur at
point D in the phase diagram for water?
7. What pressure is at waters normal
boiling point?
8. What occurs at the triple point?
9. Look at the phase diagram for carbon dioxide. Above
which pressure and temperature is carbon dioxide unable
to exist as a liquid?
73
50
11.
What two phase changes occur at point E in the
phase diagram for carbon dioxide?
E
24
13.
10.
At which pressure and temperature do the solid,
liquid, and gaseous phases of carbon dioxide coexist?
12.
What phases of water coexist at point G in CO2s
phase diagram?
Use the
phasemoves
diagram
to -78C
the lefttoto24C
answer
14-18.
What phase change occurs as carbon
dioxide
from
at aquestions
pressure of
50 atm?
14. What is the temperature at which the triple point occurs?
15. What 2 phase changes occur at Point A?
16. What phase change does the substance at 100 bars undergo
as the temperature decreases from 250 K to 200 K?
17. What is the pressure at which the critical point occurs?
18. What 2 phase changes occur at Point B?
Das könnte Ihnen auch gefallen
- Working Guide to Reservoir Rock Properties and Fluid FlowVon EverandWorking Guide to Reservoir Rock Properties and Fluid FlowBewertung: 3 von 5 Sternen3/5 (1)
- Phase Diagrams: By: Cherides P. MarianoDokument25 SeitenPhase Diagrams: By: Cherides P. MarianoWild RiftNoch keine Bewertungen
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsVon EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsBewertung: 5 von 5 Sternen5/5 (1)
- Phase DiagramsDokument25 SeitenPhase DiagramsRoland Garcia Cadavona33% (3)
- Chapter 2: Pure Substances A) Phase Change, Property Tables and DiagramsDokument7 SeitenChapter 2: Pure Substances A) Phase Change, Property Tables and DiagramsAshesh PradhanNoch keine Bewertungen
- A Modern Course in Statistical PhysicsVon EverandA Modern Course in Statistical PhysicsBewertung: 3.5 von 5 Sternen3.5/5 (2)
- ME231 ProjecttDokument10 SeitenME231 ProjecttHamza AliNoch keine Bewertungen
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationVon EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNoch keine Bewertungen
- G9 Revision NotesDokument74 SeitenG9 Revision Noteskwokrenee827Noch keine Bewertungen
- Assignment 1Dokument3 SeitenAssignment 1IzhharuddinNoch keine Bewertungen
- 25 WaterDokument11 Seiten25 WaterShamir BerrioNoch keine Bewertungen
- Phase Diagram WorksheetDokument6 SeitenPhase Diagram WorksheetIsaiah Pinto100% (1)
- Chem 2 LecDokument4 SeitenChem 2 LecHeidi BardillonNoch keine Bewertungen
- Lecture 1 - Phase EquilibriumDokument73 SeitenLecture 1 - Phase EquilibriumAliah Izzah0% (1)
- Lesson 4 Intermolecular Forces of Liquids and Solids - Phase DiagramsDokument33 SeitenLesson 4 Intermolecular Forces of Liquids and Solids - Phase DiagramsLyndy PantaoNoch keine Bewertungen
- Labsheet SKKC 2721 20162017 - 02Dokument32 SeitenLabsheet SKKC 2721 20162017 - 02HoongNoch keine Bewertungen
- ME 200 Thermodynamics 1 Fall 2017 - Exam 1Dokument8 SeitenME 200 Thermodynamics 1 Fall 2017 - Exam 1ElmaxNoch keine Bewertungen
- UNIT III Properties of Steam and Steam Power CycleDokument8 SeitenUNIT III Properties of Steam and Steam Power CycleamdevaNoch keine Bewertungen
- Phase Diagram WS Long 1Dokument2 SeitenPhase Diagram WS Long 1Jonar MarieNoch keine Bewertungen
- Energy of Phase Diagrams WSDokument5 SeitenEnergy of Phase Diagrams WSalanaNoch keine Bewertungen
- 3 - Phase Diagram of Naphthalene-BiphenylDokument7 Seiten3 - Phase Diagram of Naphthalene-Biphenyldidikkris100% (3)
- 4a - Phase Behaviour of Hydrocarbon, Ideal and Non-Ideal SystemDokument12 Seiten4a - Phase Behaviour of Hydrocarbon, Ideal and Non-Ideal SystemTHE TERMINATORNoch keine Bewertungen
- The Realation Betewwn Press and TemperatureDokument7 SeitenThe Realation Betewwn Press and TemperatureAyad DariNoch keine Bewertungen
- Phase Diagram For Two Partially-Miscible LiquidsDokument6 SeitenPhase Diagram For Two Partially-Miscible LiquidsKojo Eghan67% (6)
- Lesson6 - Phase Diagram of Water and Carbon DioxideDokument12 SeitenLesson6 - Phase Diagram of Water and Carbon DioxideLemonadeNoch keine Bewertungen
- Critical So TemperatureDokument49 SeitenCritical So TemperatureThakur Aditya PratapNoch keine Bewertungen
- Phase EqualibriumDokument16 SeitenPhase EqualibriumRatna BairagiNoch keine Bewertungen
- UNIT-5 Phase EquilibriaDokument13 SeitenUNIT-5 Phase EquilibriaALOK KUMARNoch keine Bewertungen
- A2 Further Practical SkillsDokument8 SeitenA2 Further Practical SkillsFiaz medico0% (1)
- 2016 Mid Term ExamDokument3 Seiten2016 Mid Term ExamMahmoud AsemNoch keine Bewertungen
- Physical Characteristics of ThermistorDokument6 SeitenPhysical Characteristics of ThermistorSohi KulwinderNoch keine Bewertungen
- Refining Technology Workbook Experiment 01Dokument4 SeitenRefining Technology Workbook Experiment 01Muhammad MohtashimNoch keine Bewertungen
- AC Lab 4 Molecular Weight Freezing Point DepressionDokument10 SeitenAC Lab 4 Molecular Weight Freezing Point DepressionSohamDixitNoch keine Bewertungen
- ME 200 Thermodynamics FinalDokument14 SeitenME 200 Thermodynamics FinalElmaxNoch keine Bewertungen
- Handout 4Dokument26 SeitenHandout 4coppernitrateNoch keine Bewertungen
- NOTES Genchem 2 Lesson 6 Phase Diagram of Water and Carbon DioxideDokument8 SeitenNOTES Genchem 2 Lesson 6 Phase Diagram of Water and Carbon DioxidestephniedayaoNoch keine Bewertungen
- Chemistry 123 Problem Set #1Dokument58 SeitenChemistry 123 Problem Set #1tedhungNoch keine Bewertungen
- Equation of State - GuideDokument4 SeitenEquation of State - GuideAbd Elrahman HamdyNoch keine Bewertungen
- 02-Week 3Dokument31 Seiten02-Week 3JL S. HornillaNoch keine Bewertungen
- Calibration of The Liquid in Glass, Gas (Vapor) Pressure and Bi-Metal DevicesDokument6 SeitenCalibration of The Liquid in Glass, Gas (Vapor) Pressure and Bi-Metal Devicesميسرة50% (4)
- ,1752'8&7,21723 (5621$/&20387 (56) 25&+ (0,&$/ (1, 1 ( (56 &+$37 (5 &203/ ( &216 (&87,9 (&$/&8/$7,216 0Rughfkdl6KdfkdpDokument20 Seiten,1752'8&7,21723 (5621$/&20387 (56) 25&+ (0,&$/ (1, 1 ( (56 &+$37 (5 &203/ ( &216 (&87,9 (&$/&8/$7,216 0Rughfkdl6KdfkdpÖzlem YılmazNoch keine Bewertungen
- S 1 JTP / STDP S P S N T: When Converting From K To C, Take Away 273.15Dokument3 SeitenS 1 JTP / STDP S P S N T: When Converting From K To C, Take Away 273.15Harish PrabhuNoch keine Bewertungen
- How To Use A Two-Column Pressure-Temperature ChartDokument2 SeitenHow To Use A Two-Column Pressure-Temperature ChartzhyhhNoch keine Bewertungen
- Name(s) : Fernando Chilig: T - Could - OutDokument6 SeitenName(s) : Fernando Chilig: T - Could - OutFernando ChiligNoch keine Bewertungen
- Chemistry SPM Trial Paper 3Dokument7 SeitenChemistry SPM Trial Paper 3Kaneson IyarooNoch keine Bewertungen
- Belvia Huo Material Science - Lab 8 Construction of A One-Component Phase Diagram 4/8/14Dokument4 SeitenBelvia Huo Material Science - Lab 8 Construction of A One-Component Phase Diagram 4/8/14Belvia HuoNoch keine Bewertungen
- BSL2Dokument7 SeitenBSL2Kevin CruzNoch keine Bewertungen
- PVT Properties of Crude OilsDokument121 SeitenPVT Properties of Crude OilsRavi Shankar PatelNoch keine Bewertungen
- Labsheet 3Dokument5 SeitenLabsheet 3raidahNoch keine Bewertungen
- GasCalc V 1.3Dokument4 SeitenGasCalc V 1.3George Paul Goycochea SandovalNoch keine Bewertungen
- Suite Chapter 1 Phase TransformationsDokument3 SeitenSuite Chapter 1 Phase TransformationsKhellaf SarraNoch keine Bewertungen
- Notes Ws Phase Diagram Vapor Pressure KeyDokument4 SeitenNotes Ws Phase Diagram Vapor Pressure KeyVanessa JabagatNoch keine Bewertungen
- RefrigerationDokument15 SeitenRefrigerationRiki MandolNoch keine Bewertungen
- Psycho Metric ChartDokument3 SeitenPsycho Metric ChartAparajita MalhotraNoch keine Bewertungen
- CHE 325 (3 Units) : Dr. F. B. ElehinafeDokument27 SeitenCHE 325 (3 Units) : Dr. F. B. ElehinafeGlory UsoroNoch keine Bewertungen
- NOTES Genchem 2 Lesson 6 Phase Diagram of Water and Carbon DioxideDokument8 SeitenNOTES Genchem 2 Lesson 6 Phase Diagram of Water and Carbon DioxidestephniedayaoNoch keine Bewertungen
- Phase Diagrams: Equilibrium Diagrams or Constitutional DiagramsDokument7 SeitenPhase Diagrams: Equilibrium Diagrams or Constitutional Diagramsshekhar singhNoch keine Bewertungen
- Hess' Law Enthalpy of Formation of Solid NH CL: PrelabDokument8 SeitenHess' Law Enthalpy of Formation of Solid NH CL: PrelabMuhammad Nurul HidayatNoch keine Bewertungen
- UntitledDokument11 SeitenUntitledTural EmirliNoch keine Bewertungen
- 2007 AtomsDokument67 Seiten2007 Atomsapi-293306937Noch keine Bewertungen
- Pogil - Basic Skills Supplement - Converting Units With Dimensional AnalysisDokument4 SeitenPogil - Basic Skills Supplement - Converting Units With Dimensional Analysisapi-293306937Noch keine Bewertungen
- 2007 Electrons in AtomsDokument123 Seiten2007 Electrons in Atomsapi-293306937Noch keine Bewertungen
- 2013 Reaction EnergyDokument62 Seiten2013 Reaction Energyapi-293306937Noch keine Bewertungen
- Pogil - Basic Skills Supplement - The Mole-1Dokument4 SeitenPogil - Basic Skills Supplement - The Mole-1api-293306937100% (1)
- Seminar Evaluation RubricDokument2 SeitenSeminar Evaluation Rubricapi-293306937100% (1)
- CH Gas Laws NotesDokument6 SeitenCH Gas Laws Notesapi-293306937Noch keine Bewertungen
- IbchkineticsDokument16 SeitenIbchkineticsapi-293306937Noch keine Bewertungen
- IbchequilibriumDokument16 SeitenIbchequilibriumapi-293306937Noch keine Bewertungen
- 2013 Phase ChangesDokument52 Seiten2013 Phase Changesapi-293306937Noch keine Bewertungen
- IB Chemistry ABS - IntroductionDokument20 SeitenIB Chemistry ABS - Introductionapi-293306937Noch keine Bewertungen
- IbchorganicDokument35 SeitenIbchorganicapi-293306937100% (1)
- 21 Ale 21 Ideal Gases KM f09Dokument6 Seiten21 Ale 21 Ideal Gases KM f09api-2933069370% (1)
- Ibsolutions and GasesDokument16 SeitenIbsolutions and Gasesapi-293306937Noch keine Bewertungen
- IbchatomicDokument13 SeitenIbchatomicapi-293306937Noch keine Bewertungen
- IbchstoichDokument11 SeitenIbchstoichapi-293306937Noch keine Bewertungen
- Ib Chem Bonding NotesDokument19 SeitenIb Chem Bonding Notesapi-293306937100% (1)
- IbchintroDokument17 SeitenIbchintroapi-293306937Noch keine Bewertungen
- Gas Law StationsDokument3 SeitenGas Law Stationsapi-293306937Noch keine Bewertungen
- Pogil - Changes of PhaseDokument4 SeitenPogil - Changes of Phaseapi-293306937Noch keine Bewertungen
- Vapor-Pressure OriginalDokument5 SeitenVapor-Pressure Originalapi-293306937Noch keine Bewertungen
- Heating Curve WorksheetDokument1 SeiteHeating Curve Worksheetapi-293306937Noch keine Bewertungen
- Phase-Changes OriginalDokument4 SeitenPhase-Changes Originalapi-293306937Noch keine Bewertungen
- Phase Change Virtual LabDokument2 SeitenPhase Change Virtual Labapi-293306937Noch keine Bewertungen
- Student Handout - States of MatterDokument3 SeitenStudent Handout - States of Matterapi-293306937Noch keine Bewertungen
- MAOH600 Ropu 48 Presentation Script and ReferencesDokument10 SeitenMAOH600 Ropu 48 Presentation Script and ReferencesFano AsiataNoch keine Bewertungen
- Standerdised Tools of EducationDokument25 SeitenStanderdised Tools of Educationeskays30100% (11)
- Packed Bed Reactor Slides (B)Dokument32 SeitenPacked Bed Reactor Slides (B)Meireza Ajeng PratiwiNoch keine Bewertungen
- BKM 10e Ch07 Two Security ModelDokument2 SeitenBKM 10e Ch07 Two Security ModelJoe IammarinoNoch keine Bewertungen
- Mdp36 The EndDokument42 SeitenMdp36 The Endnanog36Noch keine Bewertungen
- General Session Two - Work Life BalanceDokument35 SeitenGeneral Session Two - Work Life BalanceHiba AfandiNoch keine Bewertungen
- 99 AutomaticDokument6 Seiten99 AutomaticDustin BrownNoch keine Bewertungen
- NTJN, Full Conference Program - FINALDokument60 SeitenNTJN, Full Conference Program - FINALtjprogramsNoch keine Bewertungen
- Power Divider and Combiner: EE403-Microwave Engineering MTC, EE Dep., Electromagnetic Waves GroupDokument52 SeitenPower Divider and Combiner: EE403-Microwave Engineering MTC, EE Dep., Electromagnetic Waves GroupHabibat El Rahman AshrafNoch keine Bewertungen
- Uttarakhand District Factbook: Almora DistrictDokument33 SeitenUttarakhand District Factbook: Almora DistrictDatanet IndiaNoch keine Bewertungen
- ECC83/12AX7: Quick Reference DataDokument4 SeitenECC83/12AX7: Quick Reference DataLuisNoch keine Bewertungen
- Pressure Classes: Ductile Iron PipeDokument4 SeitenPressure Classes: Ductile Iron PipesmithNoch keine Bewertungen
- Unit Weight of Soil in Quezon CityDokument2 SeitenUnit Weight of Soil in Quezon CityClarence Noel CorpuzNoch keine Bewertungen
- STR Mpa-MpmDokument8 SeitenSTR Mpa-MpmBANGGANoch keine Bewertungen
- Literary Portraiture & Modern Spain: Dr. Rebecca M. Bender (Dokument6 SeitenLiterary Portraiture & Modern Spain: Dr. Rebecca M. Bender (Pedro PorbénNoch keine Bewertungen
- AA-036322-001 - Anchor Bolt DetailsDokument1 SeiteAA-036322-001 - Anchor Bolt DetailsGaurav BedseNoch keine Bewertungen
- What Has The Government and The Department of Health Done To Address To The Issues of Reproductive and Sexual Health?Dokument5 SeitenWhat Has The Government and The Department of Health Done To Address To The Issues of Reproductive and Sexual Health?Rica machells DaydaNoch keine Bewertungen
- Unknown Facts About Physicians Email List - AverickMediaDokument13 SeitenUnknown Facts About Physicians Email List - AverickMediaJames AndersonNoch keine Bewertungen
- Aeroskills DiplomaDokument6 SeitenAeroskills DiplomaDadir AliNoch keine Bewertungen
- Dissertation Topics Forensic BiologyDokument7 SeitenDissertation Topics Forensic BiologyHelpMeWriteMyPaperPortSaintLucie100% (1)
- Method Statement For Installation of Chilled Water Pump & Condenser Water PumpDokument14 SeitenMethod Statement For Installation of Chilled Water Pump & Condenser Water Pump721917114 47Noch keine Bewertungen
- Hubungan Body Image Dengan Pola Konsumsi Dan Status Gizi Remaja Putri Di SMPN 12 SemarangDokument7 SeitenHubungan Body Image Dengan Pola Konsumsi Dan Status Gizi Remaja Putri Di SMPN 12 SemarangNanda MaisyuriNoch keine Bewertungen
- CP 1Dokument22 SeitenCP 1api-3757791100% (1)
- Lesson 49Dokument2 SeitenLesson 49Андрій ХомишакNoch keine Bewertungen
- SA01 GENXXX SDIN BSDS 0001 B04 A - Instrumentation Design Basis Sazeh CommentedDokument31 SeitenSA01 GENXXX SDIN BSDS 0001 B04 A - Instrumentation Design Basis Sazeh Commentedamini_mohiNoch keine Bewertungen
- Manual of GardeningDokument812 SeitenManual of GardeningPrakash PatelNoch keine Bewertungen
- c3175492 Pavan Kumarvasudha Signed OfferletterDokument6 Seitenc3175492 Pavan Kumarvasudha Signed OfferletterPavan Kumar Vasudha100% (1)
- Grand Hyatt Manila In-Room Dining MenuDokument14 SeitenGrand Hyatt Manila In-Room Dining MenuMetroStaycation100% (1)
- Đề ANH chuyên 5Dokument7 SeitenĐề ANH chuyên 5Phạm Ngô Hiền MaiNoch keine Bewertungen
- Fundamental Molecular Biology: GenomesDokument45 SeitenFundamental Molecular Biology: GenomesMoonHoLeeNoch keine Bewertungen
- Process Plant Equipment: Operation, Control, and ReliabilityVon EverandProcess Plant Equipment: Operation, Control, and ReliabilityBewertung: 5 von 5 Sternen5/5 (1)
- Guidelines for Chemical Process Quantitative Risk AnalysisVon EverandGuidelines for Chemical Process Quantitative Risk AnalysisBewertung: 5 von 5 Sternen5/5 (1)
- Sodium Bicarbonate: Nature's Unique First Aid RemedyVon EverandSodium Bicarbonate: Nature's Unique First Aid RemedyBewertung: 5 von 5 Sternen5/5 (21)
- Well Control for Completions and InterventionsVon EverandWell Control for Completions and InterventionsBewertung: 4 von 5 Sternen4/5 (10)
- Lees' Process Safety Essentials: Hazard Identification, Assessment and ControlVon EverandLees' Process Safety Essentials: Hazard Identification, Assessment and ControlBewertung: 4 von 5 Sternen4/5 (4)
- Well Integrity for Workovers and RecompletionsVon EverandWell Integrity for Workovers and RecompletionsBewertung: 5 von 5 Sternen5/5 (3)
- An Applied Guide to Water and Effluent Treatment Plant DesignVon EverandAn Applied Guide to Water and Effluent Treatment Plant DesignBewertung: 5 von 5 Sternen5/5 (4)
- Water-Based Paint Formulations, Vol. 3Von EverandWater-Based Paint Formulations, Vol. 3Bewertung: 4.5 von 5 Sternen4.5/5 (6)
- Asphaltene Deposition Control by Chemical Inhibitors: Theoretical and Practical ProspectsVon EverandAsphaltene Deposition Control by Chemical Inhibitors: Theoretical and Practical ProspectsNoch keine Bewertungen
- Process Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersVon EverandProcess Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersNoch keine Bewertungen
- Machinery Lubrication Technician (MLT) I and II Certification Exam GuideVon EverandMachinery Lubrication Technician (MLT) I and II Certification Exam GuideBewertung: 2 von 5 Sternen2/5 (1)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsVon EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNoch keine Bewertungen
- Enhanced Oil Recovery Handout: A Focus on WaterfloodingVon EverandEnhanced Oil Recovery Handout: A Focus on WaterfloodingNoch keine Bewertungen
- Advanced Production Decline Analysis and ApplicationVon EverandAdvanced Production Decline Analysis and ApplicationBewertung: 3.5 von 5 Sternen3.5/5 (4)
- Flow Analysis for Hydrocarbon Pipeline EngineeringVon EverandFlow Analysis for Hydrocarbon Pipeline EngineeringNoch keine Bewertungen
- Chemical Engineering Design: Principles, Practice and Economics of Plant and Process DesignVon EverandChemical Engineering Design: Principles, Practice and Economics of Plant and Process DesignBewertung: 4 von 5 Sternen4/5 (16)
- Fun Facts about Hydrogen : Chemistry for Kids The Element Series | Children's Chemistry BooksVon EverandFun Facts about Hydrogen : Chemistry for Kids The Element Series | Children's Chemistry BooksNoch keine Bewertungen
- Asset Integrity Management for Offshore and Onshore StructuresVon EverandAsset Integrity Management for Offshore and Onshore StructuresNoch keine Bewertungen
- Internal Combustion: How Corporations and Governments Addicted the World to Oil and Subverted the AlternativesVon EverandInternal Combustion: How Corporations and Governments Addicted the World to Oil and Subverted the AlternativesBewertung: 4 von 5 Sternen4/5 (2)
- The Perfumed Pages of History: A Textbook on Fragrance CreationVon EverandThe Perfumed Pages of History: A Textbook on Fragrance CreationBewertung: 4 von 5 Sternen4/5 (1)
- Pulp and Paper Industry: Emerging Waste Water Treatment TechnologiesVon EverandPulp and Paper Industry: Emerging Waste Water Treatment TechnologiesBewertung: 5 von 5 Sternen5/5 (1)
- Distillation Design and Control Using Aspen SimulationVon EverandDistillation Design and Control Using Aspen SimulationBewertung: 5 von 5 Sternen5/5 (2)
- Principles and Case Studies of Simultaneous DesignVon EverandPrinciples and Case Studies of Simultaneous DesignNoch keine Bewertungen