Beruflich Dokumente
Kultur Dokumente
First Aid Pharmaco
Hochgeladen von
girCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
First Aid Pharmaco
Hochgeladen von
girCopyright:
Verfügbare Formate
17 6
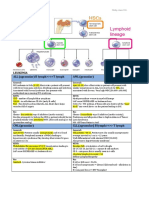
SECTION II
M I C RO B I O LOGY
MIC R O BIO L O G Y - ANTIMIC R O BIA L S
MIC R O BIO L O G Y - ANTIMIC R O BIA L S
Antimicrobial therapy
MECHANISM OF ACTION
DRUGS
0 Block cell wall synthesis by inh ibition of
Penicillin, methicillin, ampicillin, piperacillin,
cephalosporins, aztreonam, i mipenem
peptidoglycan cross-l inking
f) Block peptidoglycan synthesis
Bacitracin, vancomycin
E) Block nucleotide synthesis by inhibiting folic
Sulfonamides, trimethopri m
acid synthesis (involved in methylation)
0 Block DNA topoisomerases
Fluoroqui nolones
0 Block m RNA synthesis
Rifampin
0 Damage DNA
Metronidazole
0 Block protein synthesis at 50S ribosomal
Chloramphenicol, macrol ides, cl indamycin,
streptogramins (qui nupristin , dalfopristin),
l i nezol id
subunit
(l) Block protein synthesis at 30S ribosomal
Ami noglycosides, tetracycl i nes
subunit
E) SMX, TMP
0 - lacta ms
f) Vancomycin
and bacitraci n
Penicillin
Tetracycli nes,
a m i noglycosides
Macrolides, c h lora m p h e n icol,
clindamycin, linezolid, streptogra m i n s
Pen icillin G (IV and I M form), pen icillin V (oral) . Prototype P-lactam antibiotics.
MECHANISM
Bind penicill in-binding proteins (transpeptidases)
Block transpeptidase cross-l inking of peptidoglycan
Activate autolytic enzymes
CLINICAL USE
Mostly used for gram-positive organisms (S. pneumoniae, S. pyogenes, Actinomyces) . Also used for
Neisseria meningitidis, Treponema pallidum, and syphilis. Bactericidal for gram-positive cocc i ,
gram-positive rods, gram-negative cocci, an d spirochetes. N ot pen icillinase resistant.
TOXICITY
Hypersensitivity reactions, hemolytic anem ia.
RESISTANCE
P-lactamases cleave P-lactam ring.
M I C R O B I O LOGY
MIC R O BI O L O G Y -ANTI MI C R O BIA L S
SECTION II
1 77
Oxacillin, nafcillin, dicloxacillin (penicillinase-resistant penicillins)
MECHANISM
Same as pen icil l i n . Narrow spectrum;
pen icillinase resistant because bulky R group
blocks access of -lactamase to -lactam ring.
CliNICAL USE
S. au reus (except MRSA; resistant because of
altered penicill in-binding protein target site) .
TOXICITY
Hypersensitivity reactions, interstitial nephritis.
"Use naf (nafcillin) for staph."
Ampicillin, amoxicillin (aminopenicillins)
MECHANISM
Same as penicillin. Wider spectrum ;
penicill inase sensitive. Also combine with
clavulanic acid to protect against -lactamase.
Am Oxicillin has greater Oral bioavai labil ity
than ampicillin.
AMinoPenicillins are AMPed-up penicill in .
CliNICAL USE
Extended-spectrum penicillin Haemophilus
influenzae, E. coli, Listeria monocytogenes,
Proteus mirabilis, Salmonella, Shigella,
enterococci.
Coverage : ampici l l i n /amoxicillin HELPSS kill
enterococci .
TOXICITY
Hypersensitivity reactions ; ampicillin rash ;
pseudomembranous colitis.
RESISTANCE
-lactamases cleave -lactam ring.
Ticarcillin, piperacillin (antipseudomonals)
MECHANISM
Same as penicillin. Extended spectrum.
CliNICAL USE
Pseudomonas spp. and gram-negative rods; susceptible to penicil l i nase; use with clavulanic acid.
TOXICITY
Hypersensitivity reactions.
-ladamase inhibitors
Include Clavulanic Acid, Sulbactam ,
Tazobacta m . Often added to penici llin
antibiotics to protect the antibiotic from
destruction by -lactamase (pen icill inase) .
CAST.
l 78
SECTION II
MI CROBI OLO G Y
MICROBIOLOGY-ANTIMICROBIALS
Cephalosporins
MECHANISM
-lactam drugs that inhibit cell wall synthesis
but are less susceptible to pen icill inases.
Bactericidal .
Organisms typically not covered by
cephalosporins are LAME : Listeria, Atypicals
(Chlamydia, Mycoplasma) , M RSA, and
Enterococci. Exception : ceftarol ine covers
MRSA.
CliNICAl USE
1 st generation (cefazol in, cephalexin) -grampositive cocci, Proteus mirabilis, E. coli,
Klebsiella pnewnoniae. Cefazol in used prior to
surgery to prevent S. aureus wound infections.
2nd generation (cefoxitin, cefaclor,
cefuroxime) -gram-positive cocci ,
Haemophilus influenzae, Enterobacter
aerogenes, Neisseria spp., Proteus mirabilis,
E. coli, Klebsiella fJneumoniae, Serratia
marcescens.
3rd generation (ceftriaxone, cefotaxime,
ceftazidime) - serious gram-negative infections
resistant to other -lactams.
4th generation (cefepime) - t activity against
Pseudomonas and gram-positive organisms.
1 st generation - PEcK.
TOXICITY
2nd generation - HEN PEcKS.
Ceftriaxone-meningitis and gonorrhea.
Ceftazid i me-Pseudomonas.
Hypersensitivity reactions, vitamin K deficiency.
Low cross-reactivity with pen icillins.
t nephrotoxicity of aminoglycosides.
Aztreonam
MECHANISM
A monobactam resistant to -lactamases. Prevents peptidoglycan cross-l inking by binding to PBP3.
Synergistic with aminoglycosicles. No cross-allergenicity with penicillins.
CliNICAl USE
Gram-negative rods only-No activity against gram-positives or anaerobes. For penicill in-allergic
patients and those with renal insufficiency who cannot tolerate aminoglycosicles.
TOXICITY
Usually nontoxic ; occasional GI upset.
lmipenem/ cilastatin, meropenem
MECHANISM
Im ipenem is a broad-spectrum, -lactamase
resistant carbapenem . Always adm inistered
with cilastatin (inh ibitor of renal
cl e hyclropepticlase I) to ! inactivation of drug
in renal tubules.
CliNICAl USE
Gram-positive cocci, gram-negative rods, and
anaerobes. Wiel e spectru m , but the sign ificant
side effects l i m it use to life-threatening
infections, or after other drugs have failed.
Meropenem, however, has a reduced risk of
seizures and is stable to dehyclropepticlase I.
TOXICITY
GI d istress, ski n rash, and CNS toxicity
(seizures) at high plasma levels.
With im ipenem, " the kill is lastin' with
cilastatin."
ewer carbapenems i nclude ertapenem and
cloripenem.
M I C R O B I O LO G Y
MICRO BIOLO G Y - ANTI MICRO BIAL S
SECTION I I
Vancomycin
MECHANISM
Inhibits cell wall peptidoglycan formation by binding 0-ala 0-ala portion o f cell wall precursors.
Bactericidal .
CLIN ICAL USE
Cram positive only- serious, amulticlru g-resistant organisms, including M RSA, enterococci, and
Clostridium diffi.cile (oral close for pseudomembranous colitis) .
TOXICITY
Nephrotoxicity, Ototoxicity, Thrombophlebitis, diffuse flushing- red m a n syn d ro m e (can largely
prevent by pretreatment with antihistam ines and slow infusion rate ) . Well tolerated in genera l
does N OT have many problems.
RESISTANCE
Occurs with amino acid change of D-ala D-ala to 0-ala 0-lac. " Pay back 2 D-alas (dollars) for
vandalizing (vancomycin) ."
Protein synthesis
inhibitors
Specifically target smaller bacterial ribosome
(70S, made of 30S and 50S subun its), leaving
human ribosome ( 80S) unaffected.
"Buy AT 3 0, CCEL (sell) at 50."
3 05 i n h i b itors
A = Am inoglycosicl e s [bactericidal ]
T Tetracycl i nes [bacteriostatic]
=
5 05 i n h i b itors
C = Chloramphen icol, Clinclamycin [bacteriostatic]
E Erythromycin (macrol icl e s) [bacteriostatic]
L Li nezol icl [variable]
=
l i nezolid
(50S)
mRNA
Z.l
J
I n itiator tRNA
Ribosomal A&P site
,-A---.,
PA
I n itiation
com plex
formation
t-0--
PA
Macrolides (erythromycin ) (505)
aAlso causes misreading of mRNA.
Clindamycin (50S)
A m 1 n og lycos1de s ( 3 0S) a
1 79
1 80
S E CTI O N I I
M I C RO B I O LO G Y
M I C R O B I O LO G Y - A N T I M I C R O B I A L S
Gentamicin, Neomycin, Am ikacin ,
Tobramyci n, Streptomycin.
"Mean" (arninoglyc oside) GNATS caNNOT
kill anaerobes.
MECHANISM
Bactericidal ; inhibit formation of in itiation
complex and cause m isreading of m RNA. Also
block translocation. Require 02 for uptake ;
therefore ineffective against anaerobes.
A " i nitiates" the Alphabet.
CLINICAL USE
Severe gram-negative rod infections. Synergistic
with P-lactam antibiotics.
Neomycin for bowel surgery.
TOXICITY
Nephrotoxicity (especially when used with
cephalosporins ) , Neuromuscular blockade,
Ototoxicity (especially when used with loop
d iuretics) . Teratogen.
RESISTANCE
Transferase enzymes that inactivate the drug by
acetylation , phosphorylation, or adenylation.
Aminoglycosides
Tetracyclines
Tetracycline, doxycycline, demeclocycl ine,
m i nocycl ine.
MECHANISM
Bacteriostatic; bind to 30S and prevent
attachment of aminoacyl-tRNA; lim ited CNS
penetration. Doxycycline is fecally el iminated
and can be used i n patients with renal failure.
Do not take with milk, antacids, or iron
containing preparations because divalent
cations inhibit its absorption in the gut.
CLINICAL USE
Borrelia burgdorferi, M. pnewnoniae. Drug's
abil ity to accumulate intracellularly makes
it very effective against Rickettsia and
Chlamydia .
TOXICITY
Gl distress, discoloration of teeth and inh ibition
Demeclocycl i ne-ADI-1 antagonist; acts as a
Diuretic in SIAD H . Rarely used as antibiotic.
of bone growth in children, photosensitivity.
Contraindicated in pregnancy.
RESISTANCE
Macrolides
! uptake into cells or t efflux out of cell by
plasmid-encoded transport pumps.
Azithromycin, clarithromycin, erythromycin.
MECHANISM
I n h ibit protein synthesis by blocking translocation ( "macroslides" ) ; bind to the 23S rRNA of the
50S ribosomal subunit. Bacteriostatic.
CLINICAL USE
Atypical pneumonias (Mycoplasma, Chlamydia, Legionella) , STDs (for Chla mydia), and gram
positive cocci (streptococcal infections in patients allergic to penici l l i n ) .
TOXICITY
MACRO : Moti lity issues, Arrhythmia caused b y prolonged QT, acute Cholestatic hepatitis, Rash,
eOsinophil ia. I ncreases serum concentration of theophyll ines, oral anticoagulants.
RESISTANCE
Methylation of 23S rRNA binding site.
M I C RO B I O LOGY
M I C R O BIO L O G Y - A N T I MIC R O BIA L S
SECTI O N I I
1 81
Chloramphenicol
MECHANISM
Blocks peptidyltransferase a t 50S ribosomal subunit. Bacteriostatic.
CLINICAL USE
Meningitis (Haemophilus influenzae, Neisseria meningitidis, Streptococcus pneumoniae) .
C onservative use owing to toxicities but often still used in developing countries because of low
cost.
TOXICITY
Anemia (close dependent) , aplastic anem ia (close independent) , gray baby syndrome (in premature
infants because they lack liver UDP-glucuronyl transferase) .
RESISTANCE
Plasmid-encoded acetyltransferase that inactivates drug.
Clindamycin
MECHANISM
Blocks peptide transfer (transpeptidation) at 50S
ribosomal subunit. Bacteriostatic.
CLINICAL USE
Anaerobic infections (e.g., Bacteroides fragilis,
Clostridium perfringens) in aspiration
pneumonia or lung abscesses. Also oral
infections with mouth anaerobes.
TOXICITY
Pseudomembranous colitis ( C . difficile
overgrowth) , fever, diarrhea.
Sulfonamides
Treats anaerobes above the diaphragm vs.
metronidazole (anaerobic infections below
diaphragm) .
Sulfa methoxazole ( SMX), sulfisoxazole, sulfadiazine.
MECHANISM
PABA anti metabol ites inhibit dihydropteroate synthase. Bacteriostatic.
CLINICAL USE
Gram-positive, gram-negative, Nocardia, Chlamydia. Triple sulfas or SMX for simple UTI .
TOXICITY
Hypersensitivity reactions, hemolysis if G6PD deficient, nephrotoxicity (tubulointerstitial
nephritis) , photosensitivity, kernicterus in infants, d isplace other drugs from album i n (e.g.,
warfarin) .
RESISTANCE
Altered enzyme (bacterial d ihydropteroate synthase), uptake, or t PABA synthesis.
tI
PABA
Dihydropteroate
synthase
Pteridine
. Su lfo n a m ides I
Dihydropteroic acid
D i hyd rofolic acid
Dihydrofolate
reductase
'----___--'
lir i m e th o p r i m ,
pyrimet h a m i n e
Tetrahydrofolic acid (THF)
N 5 N 1 0 -methylene T H F
/ 1
Pu rines
Thymi d i n e
Methionine
DNA
P rotein
DNA, RNA
(Adapted, with permission, from Katzung BG. Basic and Clinical Pharmacology, 7th ed. Stamford, Cf: Appleton & lange, 1 99 7 : 762.)
1 82
SECTION II
MI C R OBI OLO G Y
MICROBIOLOGY-ANTI MICROBIALS
Trimethoprim
MECHANISM
Inh ibits bacterial dihydrofolate reductase.
Bacteriostatic.
CLI N I CAL USE
Used i n combination with sulfonamides
(trimethoprim-sulfamethoxazole [TMP
SMX] ) , causing sequential block of folate
synthesis. Combination used for UTis,
Shigella, Salmonella, Pneumocystis jirovecii
pneumonia (treatment and prophylaxis).
TOXICITY
Megaloblastic anem ia, leukopenia,
granulocytopenia. ( May alleviate with
supplemental fol inic acid [leucovorin rescue ] . )
Fluoroquinolones
Abbreviated TMP.
TMP: Treats Marrow Poorly.
CiproAoxaci n , norAoxacin, levoAoxacin, oAoxacin, sparAoxaci n , moxiAoxacin, gatiAoxaci n,
enoxacin (Auoroquinolones), nalidixic acid (a quinolone).
MECHAN ISM
Inhibit DNA gyrase (topoisomerase I I ) and
topoisomerase IV Bactericidal. Must not be
taken with antacids.
CLINICAL USE
Gram-negative rods of urinary and GI tracts
(including Pseudomonas), Neisseria, some
gram-positive organisms.
TOXICITY
GI upset, superinfections, skin rashes,
headache, dizziness. Less common ly, can
cause tendon itis, tendon rupture, leg cramps,
and myalgias. Contraind icated in pregnant
women and in children because animal
studies show damage to cartilage. Some may
cause prolonged QT i nterval. May cause
tendon rupture i n people > 60 years old and in
patients taking predn isone.
RESISTANCE
Chromosome-encoded mutation i n DNA
gyrase, plasm id-mediated resistance, efflux
pumps.
Fluoroquinolones hurt attachments to your
bones.
Metronidazole
MECHAN ISM
Forms free radical toxic metabol ites in the
bacterial cell that damage DNA. Bactericidal,
antiprotozoal.
CLIN ICAL U S E
GET GAP on the Metro with metron idazole !
Treats Giardia, Entamoeba, Trichomonas,
Treats
anaerobic infection below the diaphragm
Gardnerella vagina/is, Anaerobes (Bacteroides,
vs. clindamyci n (anaerobic infections above
C. difficile) . Used with a proton pump inhibitor
diaphragm ) .
and clarithromycin for " triple therapy" against
H. Pylori.
TOXICITY
Disulfiram-like reaction with alcohol ; headache,
meta II ic taste.
M I CROBI O L O G Y
MICROBIOLOGY-ANTI MICROBIALS
SE CTI O N I I
1 83
Antimycobaderia l d rugs
BACTERIUM
PROPHYLAXIS
TREATMENT
M. tuberculosis
I son iazid
Rifampin , Ison iazid , Pyrazinamide,
Ethambutol ( R IPE for treatment)
M . avium-intracellulare
Azithromycin
Azithromycin, rifampin, ethambutol ,
streptomycin
M. leprae
N/A
Long-term treatment with dapsone and rifampin
for tuberculoid for m. Add clofazim ine for
lepromatous for m.
l synthesis of mycol ic acids . Bacterial catalase
INH Injures Neurons and Hepatocytes.
Isoniazid (I N H)
MECHAN ISM
peroxidase ( KatG) needed to convert INH to
active metabolite.
CLINICAL USE
Mycobacterium tuberculosis. The only agent
used as solo prophylaxis against TB.
TOXICITY
Neurotoxicity, hepatotoxicity. Pyridoxine
(vitamin B 6 ) can prevent neurotoxicity, lupus.
Different I H half-lives in fast vs. slow
acetyla tors.
Rifampin
MECHANISM
Inh ibits DNA-dependent R A polymerase.
CLINICAL USE
Mycobacterium tuberculosis; delays resistance
to dapsone when used for leprosy. Used
for meningococcal prophylaxis and
chemoprophylaxis in contacts of children with
Haemophilus inf/.uenzae type B .
TOXICITY
M inor hepatotoxicity a n d drug interactions
( t P-4 5 0 ) ; orange body Auids (nonhazardous
side effect) .
Rifampin's 4 R's :
RNA polymerase inh ibitor
Revs up m icrosomal P-45 0
Red /orange body Auids
Rapid resistance i f used alone
Pyrazinamide
MECHAN ISM
Mechanism u ncertain. Thought to acid ify intracel lular environ ment via conversion to pyrazinoic
acid. Effective in acidic pH of phagolysosomes, where TB engulfed by macrophages is found.
CLINICAL U S E
Mycobacterium tuberculosis.
TOXICITY
Hyperuricem ia, hepatotoxicity.
Ethambutol
MECHANISM
l carbohyd rate polymerization of mycobacterium cell wall by blocking arabinosyltransferasc.
CLINICAL USE
Mycobacterium tuberculosis.
TOXICITY
Optic neuropathy (red-green color blindness ) .
1 84
SECTI O N I I
Antimicrobial
prophylaxis
M I C RO B I O LOGY
MIC RO BIO LO G Y - ANTIMIC ROBIA L S
CONDITION
MEDICATION
Meningococcal infection
Ciprofloxacin (drug of choice) , rifampin for
children
Gonorrhea
Ceftriaxone
Syphilis
Benzathine pen icillin G
H istory of recurrent UTis
TMP-SMX
Endocarditis with surgical or dental procedures
Pen icillins
Pregnant woman carrying group B strep
Ampicillin
Prophylaxis of strep pharyngitis in child with
prior rheumatic fever
Oral penicillin
Prevention of postsurgical infection clue to
S. aureus
Cefazol i n
Prevention of gonococcal or chlamydia!
conjunctivitis in newborn
Erythromycin ointment
H IV prophylaxis
PROPHYLAXIS
I N FECTION
CD4 < 200
cel ls/m m3
TMP-SMXa
Pneumocystis pneumon ia
CD4 < 1 00
cel ls/m m3
TMP-SMXa
Pnewnocystis pneumonia and toxoplasmosis
Azithromycin
Mycobacterium aviwn complex
CELL COUNT
C D 4 < 50
a
cel l s/mm3
Aerosolized pentam idine may be used if patient is unable to tolerate TMP-SMX, but this may not prevent toxoplasmosis
infection concurrently.
Treatment of highly
resistant baderia
Antifungal therapy
MRSA-vancomycin.
VRE -linezol icl and streptogramins ( quinupristin/dalfopristin) .
Cell w a ll synthesis
Membrane function
Caspofungin
Anidu lfu n gi n
E rgosterol
synthesis
N ucleic acid
synthesis
5-Fiucytosine
Lanosterol synthes is
Fluconazole
ltraconazole
Voriconazole
Naftifine
Terbi nafine
(Adapted, with permission, from Katzung BG, Trevor AJ . USMLE Rood Mop: Phormocology, l st e d . New York: McGraw-Hill, 2003 : 1 20.)
M I CRO B I O L O G Y
Amphotericin
MIC R O BIO LO G Y - ANTI MIC R O BIA L S
SECT I O N I I
1 85
MECHANISM
Binds ergosterol (unique to fungi) ; forms
membrane pores that allow leakage of
electrolytes.
CLINICAL USE
Serious, systemic mycoses. Cryptococcus
(amphotericin B with/without Aucytosine
for cryptococcal men ingitis) , Blastomyces,
Coccidioides, Histoplasma, Candida,
Mucor. l ntrathecally for fungal men ingitis.
Supplement K and Mg because of altered
renal tubule permeabil ity.
TOXICITY
Fever/chills ( "shake and bake"), hypotension,
nephrotoxicity, arrhythm ias, anemia, IV
phlebitis ( "amphoterrible" ) . Hydration
reduces neph rotoxicity. Liposomal
amphotericin reduces toxicity.
Amphotericin " tears" holes i n the fungal
membrane by forming pores.
Nystatin
MECHANISM
Same as amphotericin B. Topical form because too toxic for systemic use.
CLINICAL USE
" Swish and swallow" for oral candidiasis (thrush); topical for d i aper rash or vaginal candidiasis.
Azoles
Fluconazole, ketoconazole, clotrimazole, m iconazole, itraconazole, voriconazole.
MECHANISM
I n h ibit fungal sterol (ergosterol) synthesis, by inhibiting the P-45 0 enzyme that converts lanosterol
to ergosterol.
CLINICAL USE
Local and less serious systemic mycoses. Fluconazole for chronic suppression of cryptococcal
meningitis in AIDS patients and candidal infections of all types. Itraconazole for Blastomyces,
Coccidioides, Histoplasma. Clotrimazole and miconazole for topical fungal infections.
TOXICITY
Testosterone synthesis inh ibition (gynecomastia, esp. with ketoconazole), l iver dysfunction (inhibits
cytochrome P-4 5 0 ) .
Flucytosine
MECHAN ISM
Inh ibits DNA a n d RNA biosynthesis b y conversion t o 5-Auorouracil b y cytosine deaminase.
CLIN ICAL USE
Used i n systemic fungal infections (esp. men ingitis caused by Cryptococcus) i n combination with
amphotericin B .
TOXICITY
B one marrow suppression.
Caspofungin, micafungin
MECHANISM
Inh ibits cell wall synthesis by inh ibiting synthesis of -glucan.
CLINICAL USE
Invasive aspergillosis, Candida.
TOXICITY
GI upset, Aushing (by histam ine release) .
1 86
SECTION II
MI CROBI OLO G Y
MICROBIOLOGY-ANTI MICROBIALS
Terbinafine
MECHANISM
Inhibits the fungal enzyme squalene epoxidase.
CLINICAL USE
Used to treat dermatophytoses (especially onychomycosis-fungal infection of finger or toe nails).
TOXICITY
Abnormal LFTs, visual d isturbances.
Griseofulvin
MECHANISM
Interferes with m icrotubule function ; disrupts m itosis. Deposits i n keratin-containing tissues (e.g.,
nail s ) .
CLINICAL USE
Oral treatment of superficial infections; inhibits growth of derm atophytes (tinea, ri ngworm) .
TOXICITY
Teratogenic, carcinogenic, confusion , headaches, t P-450 and warfarin metabol ism.
Antiprotoz:oan therapy
Pyrimethamine (toxoplasmosis), suramin and melarsoprol (Trypanosoma brucei) , nifurtimox
(T cruzi), sodium stibogluconate (leishmaniasis) .
Chloroquine
MECHAN ISM
Blocks detoxification of heme into hemozoin. Heme accumulates and is toxic to plasmodia.
CLIN ICAL USE
Treatment of plasmodial species other than P. falciparum (frequency of resistance i n P. falciparwn
is too high) . Resistance due to membrane pump that i ntracellular concentration of d rug. Treat
P. falciparwn with artemether/lumifantrine or atovaquone/proguan i l. For life-threatening malaria,
use quinidine i n U. S . (quinine elsewhere) or artisunate.
TOXICITY
Retinopathy.
Antihelminthic therapy
Mebendazole, pyrantel pamoate, ivermecti n, diethylcarbamazine, praziquantel ; i mmobil ize
helminths. Use praziquantel against flukes (trematodes) such as Schistosoma.
M I C R O B I O LOGY
MIC R O BIO LOGY -ANTI MIC R O BIAL S
SECTI O N I I
1 87
Antiviral therapy
-::l
ati n g
Early p rotei n
synthesis
Blocked by
neuraminidase
i n h ibitors
( i nfluenza)
Mammalian
cell
N u cleic acid
synthesis
Packaging
Late p rotei n
--->.;"'-- and _ synthesis and
assembly
processing
Blocked by purine
and pyri m id i n e
analogs, a n d
reverse transcriptase
i n h ibitors
--r--; Blocked by
p rotease
i n h ibitors
Blocked by f---
rifampin
(vaccinia)
(Adapted, with permission, from Katzung BG, Trevor AJ. USMLE Road Map: Pharmacology, 1 st ed. New York: McGraw-Hill, 2003 : 1 20.)
Zanamivir, oseltamivir
MECHAN ISM
Inhibit influenza neur a m i n idase, decreasing the release of progeny virus.
CLINICAL USE
Treatment and prevention of both influenza A and B.
Ribavirin
MECHAN ISM
Inhibits synthesis of guanine nucleotides by competitively i n h ibiting IMP dehydrogenase.
CLIN ICAL USE
RSV, chronic hepatitis C.
TOXICITY
Hemolytic anemia. Severe teratogen.
Acyclovir
MECHANISM
Monophosphorylated by H SV/VZV thymidine kinase. Guanosine analog. Triphosphate formed by
cellular enzymes. Preferentially inh ibits viral DNA polymerase by chain term ination .
CLINICAL USE
H SV and VZV. Weak activity against EBV. No activity against CMV. Used for H SV
induced mucocutaneous and gen ital lesions as wel l as for encephalitis. Prophyl axis in
im munocompromised patients. No effect on latent forms of H SV and VZV. Valacyclovir, a
prodrug of acyclovir, has better oral bioavailability.
For herpes zoster, use a related agent, famciclovir.
TOXICITY
Few serious adverse effects.
MECHANISM OF RESISTANCE
Mutated viral thym idine kinase.
1 88
SECTI O N I I
M I C R O B I O LO G Y
MIC RO BIOLOG Y - A N TI MIC RO BIA L S
Cianciclovir
MECHANISM
5'-monophosphate formed by a CMV viral kinase. Guanosine analog. Triphosphate formed by
cellular kinases. Preferentially inh ibits viral DNA polymerase.
CliNICAL USE
CMV, especially in im munocomprom ised patients. Valganciclovir, a prodrug of ganciclovir, has
better oral bioava ilabil ity.
TOXICITY
Leukopenia, neutropenia, thrombocytopenia, renal toxicity. More toxic to host enzymes than
acyclovir.
MECHANISM OF RESISTANCE
Mutated CMV DNA polymerase or lack of viral kinase.
Foscamet
MECHANISM
Viral DNA polymerase inhibitor that binds to
the pyrophosphate-binding site of the enzyme.
Does not require activation by viral kinase.
CLINICAL USE
CMV retinitis in immunocomprom ised patients
when ganciclovir fa ils; acyclovir-resistant HSV.
TOXICITY
MECHANISM OF RESISTANCE
Foscarnet
pyrofosphate analog.
ephrotoxicity.
Mutated DNA polymerase.
Cidofovir
MECHANISM
Preferentially inhibits viral DNA polymerase. Does not require phosphorylation by viral kinase.
CLINICAL USE
CMV retinitis in immunocomprom ised patients ; acyclovir-resistant H SV. Long half-life.
TOXICITY
Nephrotoxicity (coadminister with probenecid and IV saline to reduce toxicity) .
M I C R O B I O LO G Y
H IV therapy
DRUG
M I C R O B I O LO G Y- A N T I M I C R O B I A L S
S ECTI O N I I
1 89
H ighly active antiretroviral therapy ( HAART) : in itiated when patients present with A I D S -defining
illness, low C D4 cell counts (< 500 cells/m m 3 ) , or h igh viral load. Regimen consists of 3 d ru gs to
prevent resistance :
[2 nucleoside reverse transcriptase inh ibitors ( N RTis)] +
[ l non-nucleoside reverse transcriptase inh ibitor ( N N RTI) OR l protease inh ibitor OR l
i ntegrase i nh ibitor]
M ECHANISM
TOXICITY
Assembly of virions depends on HIV-l protease
(pol gene), wh ich cleaves the polypeptide
products of HIV mR A into their functional
parts. Thus, protease i nh ibitors prevent
maturation of new viruses.
Ritonavir can "boost" other drug concentrations
by inh ibiting cytochrome P-450.
All protease inh ibitors end in -navir.
Navir ( never) tease a protease.
Hyperglycemia, G I i ntolerance (nausea,
diarrhea) , l ipodystrophy.
Neph ropathy, hematuria (indinavir) .
Competitively inhibit nucleotide binding to
reverse transcriptase and terminate the D A
chai n ( lack a 3' OH group) . Tenofovir is a
nucleotide analog and does not have to be
activate d ; the others are nucleoside analogs
and do need to be phosphorylated to be active.
ZDV is used for general prophylaxis and during
pregnancy to reduce risk of fetal transm ission .
Have you dined (vudine) with my nuclear
( nu cleo sides) fam i ly?
Bone marrow suppression (can be reversed
with G-CSF and erythropoieti n ) , peripheral
neuropathy, lactic acidosis (nucleosides ) , rash
(non-nucleosides), anemia (ZDV ) .
Bind to reverse transcriptase at site different
from RTis. Do not require phosphorylation
to be active or compete with nucleotides.
S a m e as N RTJs.
Inh ibits HIV genome integration i nto host cell
chromosome by reversibly inhibiting HTV
integrase.
I-Iypercholesterolem ia.
Protease inhibitors
Lopinavir
Atazanavir
Darunavi r
Fosamprenavir
Saquinavir
R itonavi r
l n d inavir
N RTis
Tenofovir (TDF)
Emtricitabine (FTC)
Abacav i r (ABC)
Lamivudine (3TC)
Zidovudine (ZDV,
formerly AZT)
Didanosine (ddl)
Stavud i n e (d4T)
N N RTis
Nevirapine
Efavi renz
Delavirdine
lntegrase inhibitors
Ra ltegravi r
Interferons
MECHANISM
Glycoproteins synthesized by virus-infected cells ; block repl ication of both RNA and DNA viruses.
CLINICAL USE
I FN-a - chronic hepatitis B and C , Kaposi 's sarcoma. I FN- - M S . I FN-y- NADPH oxidase
deficiency.
TOXICITY
Neutropenia, myopathy.
l 90
SECTION I I
Antibiotics to avoid i n
pregnancy
M I C RO B I O LO G Y
MIC R O BIO L O G Y - ANTI MIC R O BIA L S
ANTIBIOTIC
ADVERSE EFFECT
Sulfonam ides
Kern icterus
Am inoglycosides
Ototoxicity
Fl uoroqui nolones
Cartilage damage
Clarithromycin
Embryotoxic
Tetracyclines
D iscolored teeth, inhibition of bone growth
Ribavirin (antiviral )
Teratogenic
Griseofulvin (antifungal)
Teratogenic
Chloramphenicol
"Gray baby"
--------------------------------------------
SAFe Children Take Really Good Care.
IMMUNOLOGY
IMMUNOLOGY-IMMUNOSUP PRESSANTS
SECTION II
209
IM MUNOLOGY-IM M UNOSUPPRESSANTS
Cyclosporine
MECHANISM
Binds to cycloph il ins. Comp l ex blocks the d i fferentiation and activation of T ce lls by i n h ibiting
ca l cineurin, thus preventing the production of i L-2 and its receptor.
CLINI C AL USE
Suppresses organ rejection after transplantation ; selected autoimmune d isorders.
TOXICITY
Nephrotoxicity, hypertension, hyperlipidemia, hypergl ycemia, tremor, gingiva l hyperplasia,
h i rsutism .
Tacrolimus {FK-506)
MECHANISM
Similar to cyclosporine; binds to FK-binding protein, inhibiting ca l cineur i n and secretion of I L-2
and other cytokines.
CLINICAL USE
Potent immunosuppressive used in organ transplant recipients.
TOXICITY
S i m ilar to cyclosporine except no gingival hyperplasia and h i rsuti sm.
Sirolimus (rapamycin)
MECHANISM
Inh ibits mTOR. Inh ibits T-ce ll prol iferation in response to I L-2 .
CLINI C AL USE
I m munosuppression after kidney transplantation in combination with cyclosporine and
corticosteroids. Also used with drug-e l uting stents.
TOXICITY
Hyperl ipidemia, thrombocytopenia, l eukopen ia.
Azathioprine
MECHANISM
CLINICAL USE
TOXICITY
Antimetabol ite precursor of 6-mercaptopurine that i nterferes with the metabo l ism and synthesis of
nucleic acids. Toxic to prol iferating l ymphocytes.
Kidney transplantation, autoim mune disorders (inc l uding glomeru lonephritis and hemolytic
anemia) .
Bone marrow suppression. Active metabol ite mercaptopurine is metabol ized by xanth ine oxidase ;
thus, toxic effects may be increased by allopurinol.
Muromonab-CD3 {OKT3)
MECHANISM
Monoclona l antibody that binds to CD3 (epsilon chain) on the surface of T ce l ls. Blocks cel l u l ar
i nteraction with C D 3 protein responsibl e for T-ce l l signa l transduction.
CLINICAL USE
I mmunosuppression after kidney transp l antation .
TOXICITY
Cytokine re l ease syndrome, hypersensitivity reaction.
210
SECTION II
I M MUN O LOGY
IMMUNOLOGY-IMMUNOSU P PRESSANTS
N
T
AG
Recombinant cytokines
E
CLI NI C A L U S ES
---------------------------
______
__
__
__
__
________
__
__
__
__
__
and clinical uses
Aldesleukin (interleukin-2 )
Renal cell carcinoma, metastatic melanoma
Epoetin alfa (erythropoietin)
Anem ias (especially in renal fai lure)
Filgrastim (granulocyte colony-stimulating
Recovery of bone marrow
factor)
Sargramostim (granulocyte-macrophage colony
Recovery of bone marrow
stimulating factor)
a-interferon
Hepatitis B and C, Kapos i 's sarcoma, leukemias,
mal ignant melanoma
-interferon
Multiple sclerosis
y-interferon
Chron ic granulomatous d isease
O prelvekin (interleu kin- l l )
Thrombocytopen ia
Throm bopoietin
Thrombocytopenia
TARGET
CL I NI C AL USE
Muromonab-CD3
(OKT3)
CD3
Prevent acute transplant rej ection
Digoxin I m mune Fab
Digoxin
Antidote for d igoxi n i ntoxication
lnfliximab
TNF-a
Crohn's disease, rheumatoid arthritis, psoriatic
arthritis, ankylosing spondyl itis
Adalimumab
TNF-a
Crohn's d isease, rheumatoid arthritis, psoriatic
arthritis
Abciximab
Glycoprotein Jib/Ilia
Prevent card iac ischem ia in unstable angina and
i n patients treated with percutaneous coronary
intervention
Trastuzumab
( Herceptin)
HER2
H E R2-overexpressing breast cancer
Rituximab
CD20
B-cell non-Hodgkin's lymphoma
O malizumab
IgE
Add itional l ine of treatment for severe asthma
Therapeutic antibodies
A GENT
EN DOCRINE
ENDOCRINE-PHA R M ACOLOGY
SECTION Ill
305
ENDOCRINE-PHA R M ACOLOGY
Diabetes drugs
Treatment strategy for type l DM -low-sugar diet, insulin replacement.
Treatment strategy for type 2 DM-dietary modification and exercise for weight loss; oral
hypoglycemics and insulin replacement.
DRUG CLASSES
ACTION
Insulin :
Bind insulin receptor (tyrosine
kinase activity).
Liver: t glucose stored as glycogen.
Muscle : t glycogen and protein
synthesis, K+ uptake.
Fat: aids TG storage.
Lispro (rapid-acting)
Aspart (rapid-acting)
Glulisine (rapid-acting)
Regular (short-acting)
NPH (intermediate)
Glargine (long-acting)
Detemir (long-acting)
Biguanides :
M etformin
Sulfonylurea s :
First generation :
Tol butamide
Chlorpropamide
Second generation:
G lyburide
Glimepiride
G l ipizide
G litazones/
thiazolidinediones :
Piogl itazone
Rosiglitazone
a-glucosidase
inhibitors :
Acarbose
M igl itol
CLINICAL USE
Type l DM, type 2 DM,
gestational diabetes, life
threatening hyperkalemia,
and stress-induced
hyperglycemia.
TOXICITIES
Hypoglycemia, very rarely
hypersensitivity reactions.
Exact mechanism is unknown.
gluconeogenesis, t glycolysis,
t peripheral glucose uptake
(insulin sensitivity).
Oral. First-line therapy in
type 2 DM.
Can be used in patients
without islet function.
GI upset; most serious
adverse effect is
lactic acidosis (thus
contraindicated in renal
failure).
Close K+ channel in -cell
membrane, so cell depolarizes
triggering of insulin release via
t Ca 2+ influx.
Stimulate release of
endogenous insulin in type
2 DM. Require some islet
function, so useless in type
l DM.
First generation : disulfiram
like effects.
Second generation :
hypoglycemia.
Used as monotherapy in type
2 DM or combined with
above agents.
Weight gain, edema.
Hepatotoxicity, heart failure.
Used as monotherapy in type
2 DM or in combination
with above agents.
GI disturbances.
-+
t insulin sensitivity in peripheral
tissue. Binds to PPAR-y nuclear
transcription regulator."
Inhibit intestinal brush-border
a-glucosidases.
Delayed sugar hydrolysis
and glucose absorption
postprandial hyperglycemia.
-+
Amylin analogs:
glucagon.
Type l and type 2 DM.
t insulin, glucagon release.
Type 2 DM.
Nausea, vomiting;
pancreatitis.
t insulin, glucagon release.
Type 2 DM.
Mild urinary or respiratory
infections.
Pra m lintide
GLP-1 analogs :
Exenatide
Liraglutide
DPP-4 inhibitors :
Linagliptin
Saxagliptin
Sitagliptin
Hypoglycemia,
nausea, diarrhea.
"Genes activated by PPAR-y regulate fatty acid storage and glucose metabolism. Activation of PPAR-y t insulin sensitivity and
levels of adiponectin.
30 6
SECTION Il l
ENDOCRINE
ENDOC R I NE- P H A R M ACO LOG Y
Propylthiouracil. methimazole
MECHANISM
Block peroxidase, thereby inhibiting organification of iodide and coupling of thyroid hormone
synthesis. Propylthiouracil also blocks 5 '-deiodinase, which peripheral conversion of T4 to T 3 .
CLINICAL USE
Hyperthyroidism.
TOXICITY
Skin rash, agranulocytosis (rare), aplastic anemia, hepatotoxicity (propylthiouracil). Methimazole is
a possible teratogen.
Levothyroxine. triiodothyronine
MECHANISM
Thyroxine replacement.
CLINICAL USE
Hypothyroidism, myxedema.
TOXICITY
Tachycardia, heat intolerance, tremors, arrhythmias.
Hypothalamic/pituitary d rugs
DRUG
CLINICAL USE
GH
GH deficiency, Turner syndrome.
Somatostatin
(octreotide)
Acromegaly, carcinoid, gastrinoma, glucagonoma, esophageal varices.
Oxytocin
Stimulates labor, uterine contractions, milk let-down; controls uterine hemorrhage.
ADH (desmopressin)
Pituitary (central, not nephrogenic) DI.
Demeclocycline
MECHANISM
ADH antagonist (member of the tetracycline family).
CLINICAL USE
SIADH.
TOXICITY
Nephrogenic DI, photosensitivity, abnormalities of bone and teeth.
Glucocorticoids
MECHANISM
Hydrocortisone, prednisone, triamcinolone, dexamethasone, beclomethasone.
the production of leukotrienes and prostaglandins by inhibiting phospholipase A 2 and expression
of COX-2.
CliNICAl USE
Addison's disease, inflammation, immune suppression, asthma.
TOXICITY
Iatrogenic Cushing's syndrome -buffalo hump, moon facies, truncal obesity, muscle wasting, thin
skin, easy bruisability, osteoporosis, adrenocortical atrophy, peptic ulcers, diabetes (if chronic).
Adrenal insufficiency when drug stopped abruptly after chronic use.
CAR D I O VASC U LAR
C A R D I OVA S C U LAR- P H A R M A C O LOGY
SE C T I O N I l l
2 79
C A R D I OVAS C U L A R- P H A R M A COLOGY
Antihypertensive therapy
Essential hypertension
Diuretics, ACE inh ibitors, angiotensin I I
receptor blockers (ARBs) , calcium channel
blockers.
See the Ren a l chapter for more deta ils about
diuretics and ACE inh ibitors/ARBs.
CHF
Diuretics, AC E inh ibitors/ARBs, -blockers
(compensated C H F ) , K+ -sparing d iuretics.
-blockers must be used cautiously in
decompensated C H F, and are contraindicated
in cardiogen ic shock.
Diabetes mellitus
AC E inhibitors/ARBs, calcium channel
blockers, d iuretics, -blockers, a-blockers.
AC E i n h ibitors are protective aga inst diabetic
nephropathy. See the Pharmacology chapter
for more details about a-blockers.
Calcium channel
blockers
Nifedipine, verapamil, diltiazem, amlodipine.
MECHAN ISM
Block voltage-dependent L-type calcium chan nel s of card iac and smooth muscle and thereby
reduce muscle contractil ity.
Vascular smooth muscle -amlodipine = nifed ipine > d iltiazem > verapamil.
Heart-verapamil > diltiazem > amlodipine = nifedipine (verapam il = ventricle) .
CliNICAL USE
Hypertension, angina, arrhythm ias (not nifed ipine) , Prinzmeta l 's angina, Raynaud's.
TOXICITY
Cardiac depression, AV block, peripheral edema, flush ing, d i zziness, and constipation.
Hydralazine
MECHAN ISM
t cGMP
-+
smooth muscle relaxation. Vasodilates arterioles > veins; afterload reduction .
CliNICAL USE
Severe hypertension, C H F. First-l ine therapy for hypertension in pregnancy, with methyldopa.
Frequently coadmin istered with a -blocker to prevent reflex tachycardia.
TOXICITY
Compensatory tachycardia (contra ind icated in angina/CAD ) , fluid retention, nausea, headache,
angina. Lupus-like syndrome.
Malignant
hypertension treatment
Commonly used d rugs include n itroprusside, n icard ipine, clevidipine, labetalol , and fenoldopam.
Nitroprusside
Short acting; t cGMP via direct release of NO. Can cause cyanide toxicity (releases cyanide) .
Fenoldopam
Dopamine D 1 receptor agon ist- coronary, peripheral, renal, and splanchnic vasodilation. BP and
t natriuresis.
280
SECT I O N I l l
CAR D I OVASC U L A R
C A R D I OVASC U L A R- P H A R M A COLOGY
Nitroglycerin, isosorbide dinitrate
MECHANISM
Vasodilate by releasing n itric oxide in smooth muscle, causing t i n cGM P and smooth muscle
rel axation . Dilate veins >> arteries. ! preload.
CLIN ICAL USE
Angina, pulmon ary edema.
TOXICITY
Reflex tachycardia, hypotension , Hushing, headache, " Monday disease" i n industrial exposur e :
development o f tolerance for the vasod ilating action during the work week a n d loss o f tolerance
over the weekend results in tachycardia, dizziness, and headache upon reexposure .
Antianginal therapy
COMPONENT
Goal- reduction o f myocard ial 02 consumption (MV02 ) b y decreasing 1 or more of the
determinants of MV0 2 : end-diastol ic volume, blood pressure, heart rate, contractility, ej ection
time.
N ITRATES (AFFECT PRELOAD)
End-diastolic volume
-BLOCKERS (AFFECT AFTERLOAD)
N ITRATES + -BLOCKERS
No effect or !
Blood pressure
Contractility
t (reflex response)
Heart rate
t (reflex response)
Little/no effect
Ejection time
Little/no effect
MV02
!!
Calcium channel blockers - n ifedipine is similar to nitrates in effect; verapamil is similar to -blockers i n effect.
Pindolol and acebutolol - partial -agonists contraindicated in angina.
CARD I O VASC U LAR
C A R D I OV ASC U L A R - P H A R M A C O LOGY
SECT I O N I l l
28 1
Lipid-lowering agents
EFFECT ON HDl
EFFECT ON lDl
"BAD CHOlESTEROL" "GOOD CHOlESTEROl'
DRUG
EFFECT ON
TRIGlYCERIDE$
HMG -CoA reductase
inhibitors (lovastatin,
pravastatin,
simvastatin,
atorvastatin,
rosuvastatin)
Niacin (vitamin
83)
Bile acid resins
(cholestyramine,
tt
Slightly t
Slightly t
colestipol,
colesevelam)
Cholesterol absorption
blockers (ezetimibe)
Fibrates (gemfibrozil,
clofibrate,
bezafibrate,
fenofibrate)
MECHANISMS O F ACTION
SIDE EFFECTS/PROBlEMS
I n h ibit conversion
of H MG-CoA
to mevalonate, a
cholesterol precursor
Hepatotoxicity
(t LFTs),
rhabdomyolysis
I n h ibits l ipolysi s
in ad ipose tissue ;
reduces hepatic
VLDL secretion i nto
circulation
Reel , flushed face,
which is by aspirin
or long-term use
Hyperglycemia
(acanthosis
n i gricans)
Hyperuricemia
(exacerbates gout)
Prevent i ntestinal
reabsorption of bile
acids; l iver must use
cholesterol to make
more
Patients hate it- tastes
bad and causes
GI discomfort,
absorption of fatsoluble vitam ins
Cholesterol gal l stones
Prevent cholesterol
reabsorption at small
i ntesti n e brush border
Rare t LFTs, diarrhea
Upregulate LPL
t TG clearance
Myositis,
hepatotoxicity
(t LFTs), cholesterol
gallstones
--+
Endothelial
cells
B lood
Gut
Hepatocytes
Ac-CoA
Ezetl mlbe
HMG
oA
HMG-CoA
:J
reductase
bitors
I
/Li)L\
T"'
]'"'
Nla::..
-r
L[
Reslns
""'
e
@
rr-
Gemfibrozil
,------2-.,
/}'
._/
Lipid
oxidation
(Adapted, with permission, from Katzung B G , Trevor AJ. USMLE Rood Map: Pharmacology, I st e d . N e w York: McGraw-Hill, 2003 : 56.)
28 2
SECTI O N I l l
Cardiac glycosides
MECHAN ISM
CAR D I OVASC U LAR
D igoxin-7 5 % bioavailability, 20-40% protein bound, t 1 12
TOXICITY
ANTIDOTE
40 hours , u rinary excretion .
D i rect inh ibition of Na+fK+ ATPase leads to indirect inhibition of Na+fCa2 + exchanger/antiport.
t [Ca 2 +] i positive inotropy. Stimulates vagus nerve ! H R .
-+
CLIN ICAL USE
C A R D I OVASC U L A R - P H A R M A COLOGY
-+
C H F ( t contractil ity) ; atrial fibrillation ( ! conduction a t AV node a n d depression of SA node) .
Chol i nergi c - nausea, vom iting, diarrhea, blurry yel low vision (th ink Van Gogh) .
E C G - t PR, ! QT, ST scooping, T-wave inversion, arrhythm ia, AV block.
Can lead to hyperkalem ia, a poor prognostic indicator.
Factors predisposing to toxicity- renal fai l ur e ( ! excretion), hypokalemia (permissive for digoxin
binding at K+ -binding site on Na+fK+ ATPase), quinidine ( ! digoxin clearance ; d isplaces d igoxin
from tissue-binding sites) .
Slowly normalize K+, l i docaine, cardiac pacer, anti-digoxin Fab fragments, Mg 2 +.
CAR D I O VASC U LAR
Antiarrhythmics
Na+ channel blockers
(class I)
C A R D I OVAS C U L A R- P H A R M A COLOGY
SECT I O N I l l
283
Local anesthetics. Slow or block ( ! ) conduction (especially in depolarized cells). ! slope of phase 0
depolarization and t threshold for firing in abnormal pacemaker cells. Are state dependent
(selectively depress tissue that is frequently depolarized [e.g., tachycardia] ) .
Hyperkalemia causes t toxicity for all class I d rugs.
Class lA
Qu i n idine, Procainam ide, Disopyramide.
t AP duration, t effective refractory period
( ERP) , t QT interval. Affect both atrial and
ventricular arrhythmias, especially reentrant
and ectopic supraventricular and ventricular
tachycard ia.
Toxicity: quinidine (ci nchon ism- headache,
tinn itus) ; procainam ide (reversible SLE-l ike
syndrome) ; d isopyram ide (heart failur e ) ;
thrombocytopen i a ; torsades d e pointes d u e to
t QT interval.
"The Queen Proclai ms D iso's pyramid ."
Class IB
Lidocaine, Mexiletine, Tocainide.
! AP duration . Preferentially affect ischem ic or
depolarized Purkinje and ventricular tissue.
Usefu l i n acute ventricular arrhythmias
(especially post-MI) and in digital is-induced
arrhythmias.
Toxicity: local anesthetic. C S stimulation /
depression, cardiovascular depression .
'T cl
Class IC
Flecai nide, propafenone.
No effect on AP duration. Useful in ventricular
tachycardias that progress to VF and in
intractable SVT. Usually used only as last
resort i n refractory tachyarrhythm ias. For
patients without structural abnormalities.
Toxicity: proarrhythm ic, especially post-MI
(contraind icated) . Significantly prolongs
refractory period in AV node.
IC is C ontra ind icated in structural heart d isease
and post-M I .
Buy Lidy's Mexican Tacos."
Phenytoin can also fal l into the I B category.
IB is B est post- M I .
All class I drugs
O mV
Phase 0
I Na
Phase 3 ( I K)
-85 mV
Phase 4
(Adapted, with permission, from Katzung BG, Trevor AJ. Pharmacology: Examination & Board Review, 5th ed. Stamford, G: Appleton & Lange, 1 99 8 : 1 1 8.)
284
SECT I O N I l l
Antiarrhythmics
CARD I O VASC U LAR
C A R D I OVAS C U LA R -PH A R M A COLO G Y
Metoprolol , propranolol , esmolol , atenolol , timolol.
-blockers (class I I)
MECHANISM
Decreases SA and AV nodal activity by ! cAM P, ! Ca 2 + currents. Suppress abnormal pacemakers by
! slope of phase 4.
AV node particularly sensitive - t PR interval . Esmolol very short acting.
CliN ICAl USE
Ventricular tachycard ia, SVT, slowing ventricular rate during atrial fibrillation and atrial flutter.
TOXICITY
I mpotence, exacerbation of asthma, cardiovascular effects (bradycard ia, AV block, C H F) , CNS
effects (sedation, sleep alterations) . May mask the signs of hypoglycem i a .
Metoprolol c a n cause dysl ipidemia. Treat overdose with glucagon . Propranolol c a n exacerbate
vasospasm i n Prinzmetal 's angina.
Antiarrhythmics
K+ channel blockers
(class I l l)
MECHANISM
TOXICITY
Am iodarone, Ibutil ide, Dofetil ide, Sotalol .
"AIDS."
t AP duration, t ERP. Used when other
antiarrhyth m ics fai l . t QT interval.
Sotalol - torsades de pointes, excessive block;
ibutilide - torsades; am iodarone -pulmonary
fibrosis, hepatotoxicity, hypothyroidism/
hyperthyroidism (am iodarone is 40% iodine by
weight) , corneal deposits, skin deposits (blue/
gray) resulting in photodermatitis, neurologic
effects, constipation, cardiovascular effects
(bradycard ia, heart block, C H F ) .
Amiodarone h a s class I , I I , I I I , a n d IV effects
because it alters the lipid membrane.
Remember to check PFTs, LFTs, and TFTs
when using am iodarone.
C lass I l l act ion
(Adapted, with permission, from Katzung BG, Trevor AJ. Pharmacology: Examination & Boord Review, 5th ed. Stamford, a: Appleton & Lange, 1 99 8 : 1 20.)
Antiarrhythmics
Verapam i l , diltiazem.
Cal+ channel blockers
(class IV)
MECHANISM
TOXICITY
! conduction velocity, t ERP, t PR interval. Used in prevention of nodal arrhythmias (e.g., SVT) .
Constipation , flushing, edema, CV effects ( C H F, AV block, sinus node depression ) .
Other antiarrhythmics
Adenosine
t K + out o f cells --+ hyperpolarizing t h e cell + !
lea D r u g o f choice in d iagnosing/abol ishing
supraventricular tachycard ia. Very short acting ( 1 5 sec) . Toxicity includes flushing, hypotension,
chest pai n . Effects blocked by theophylline and caffeine.
Effective in torsades de pointes and digoxin toxicity.
53 8
SECTION Ill
R EPRODUCTIVE-PHARMACOLOGY
REPRODUCTIVE
REPRODUCTIVE-PHARMACOLOGY
Control of reproductive
Hypothalamus
hormones
Hypothalamus
$
H
Anterior
---0- GnRH antagonists
---0- 0ral
contraceptives,
danazol
pituitary
-&-- GnR H a ntagonists
+--G)- GnRH agonists
Ovary
GnRH agonists
Pituitary
gonadotrophs
Testis
Progesterone
(luteal phase)
'-7------.r'
------e-- Ketoconazole,
danazol
A ndrostenedione
Estrone -- Estriol
'
Expression in estrogen-responsive cells
Control of female hormones
+-0--- Finasteride
Flutamide,
cyproterone,
spironolactone
Androgen-receptor complex
:.---=--cB- SEAMs
Estrogen
response
element
Sareductase
Dihydrotestostero ne
Fulvestrant
m {/\\ rf\
, W W
Ketoconazole,
spironolactone
Testosterone
Testosterone
Estradiol
l --0-
Androgen
response
element
Expression of appropriate
genes in androge n-responsive cells
Control of androgen secretion
(Adapted, with permission, from Katzung BG. Basic & Clinical
(Adapted, with permission, from Katzung BG. Basic & Clinical
Pharmacology, I Oth ed. New York: McGraw,Hill, 2006, Fig. 40-5.)
Pharmacology, I Oth ed. New York: McGraw-Hill, 2006, Fig. 40-6.)
REPRODUCTIVE
REPRODUCTIVE-PHARMACOLOGY
SECTION Ill
53 9
Leuprolide
MECHANISM
GnRH analog with agon ist properties
when used i n pulsatile fashion ; antagon ist
properties when used in continuous fashion
(downregulates GnRH receptor in pituitary
F S H /L H ) .
Leuprol ide can be used i n lieu of G n R H .
....
CliNICAl USE
Infertil ity (pulsatile) , prostate cancer
(continuous- use with flutamide), uterine
fibroids (continuous), precocious puberty
(continuous) .
TOXICITY
Antiandrogen, nausea, vom iting.
Testosterone, methyltestosterone
MECHANISM
CliNICAl USE
TOXICITY
Antiandrogens
Agon ist at androgen receptors.
Treats hypogonadism and promotes development of zo sex characteristics ; stimulation of anabol ism
to promote recovery after burn or injur y.
Causes masculinization in females; reduces intratesticular testosterone in males by inh ibiting
release of LH (via negative feedback) , leading to gonadal atrophy. Premature closure of epiphyseal
plates. t LDL, H DL .
Testosterone
) a-reductase
DHT (more potent) .
Finasteride
A 5 a-reductase inh ibitor ( ! conversion of
testosterone to DHT). Useful in BPH . Also
promotes hair growth - used to treat male
pattern baldness.
Flutamide
A nonsteroidal competitive inh ibitor of
androgens at the testosterone receptor. Used in
prostate carcinoma.
Ketoconazole
I n h ibits steroid synthesis (inhibits
1 7, 20-desmolase ) .
Spironolactone
I n h ibits steroid binding.
To prevent male-pattern hair loss, give a drug
that will encourage female breast growth .
Ketoconazole and spironolactone are used in
the treatment of polycystic ovarian syndrome
to prevent h i rsutism. B oth h ave side effects of
gynecomastia and amenorrhea.
Estrogens (ethinyl estradiol, DES, mestranol)
MECHANISM
Bind estrogen receptors.
CliNICAl USE
Hypogonadism or ovarian failure, menstru al abnormal ities, H RT in postmenopausal women ; use
in men with androgen-dependent prostate cancer.
TOXICITY
t risk of endometrial cancer, bleeding in postmenopausal women, clear cell adenocarcinoma of
vagina in females exposed to DES in utero, t risk of thrombi . Contraind ications - E R-positive
breast cancer, history of DVTs.
540
SECTION Ill
REPRODUCTIVE
REPRODUCTIVE-PHARMACOLOGY
Selective estrogen receptor modulators-SERMs
Clomiphene
Partial agonist at estrogen receptors in hypothalamus. Prevents normal feedback inh ibition and
t release of LH and FSH from pituitary, which stimulates ovulation. Used to treat infertil ity and
polycystic ovarian syndrome. May cause hot flashes, ovarian enlargement, multiple simultaneous
pregnancies, and visual disturbances.
Tamoxifen
Antagonist on breast tissue ; used to treat and prevent recurrence of E R-positive breast cancer.
Raloxifene
Agon ist on bone ; reduces resorption of bone ; used to treat osteoporosis.
Hormone replacement
therapy
Anastrozole/
Used for rel ief or prevention of menopausal symptoms (e.g., hot flashes, vaginal atrophy) and
osteoporosis (t estrogen, osteoclast activity) .
Unopposed estrogen replacement therapy (ERT) t the risk of endometrial cancer, so progesterone
is added. Possible t CV risk.
Aromatase i n hibitors used in postmenopausal women with breast cancer.
exemestane
Progestins
MECHANISM
CLINICAL USE
Bind progesterone receptors, reduce growth and t vasculari zation of endometrium.
Used in oral contraceptives and in the treatment of endometrial cancer and abnormal uterine
bleeding.
Mifepristone (RU-486)
MECHANISM
Competitive inh ibitor of progestins at progesterone receptors.
CLINICAl USE
Termination of pregnancy. Administered with m isoprostol ( PGE 1 ) .
TOXICITY
Heavy bleed ing, G I effects (nausea, vom iting, anorexia) , abdom inal pa i n .
Oral contraception
(synthetic progestins.
estrogen)
Estrogen a n d progestins inhibit LH/FSH a n d thus prevent estrogen surge. o estrogen surge no
LH surge
no ovulation.
Progestins cause th ickening of the cervical mucus, thereby l i m iting access of sperm to uterus.
Progestins also inhibit endometrial prol iferation , thus making endometrium less suitable for the
implantation of an embryo.
Contra ind ications- smokers > 35 years of age (t risk of cardiovascular events ) , patients with h istory
of thromboembol ism and stroke or history of estrogen-dependent tumor.
-+
-+
Terbutaline
Tagonist that relaxes the uteru s ; reduces premature uterine contractions.
Tamsulosin
a 1 -antagonist used to treat BPH by inhibiting smooth muscle contraction. Selective for a 1A,D
receptors (found on prostate) vs. vascular a 1 B receptors.
REPRODUCTIVE
REPRODUCTIV E-PHARMACOLOGY
SECTION Ill
Sildenafil, vardenafil
MECHANISM
Inh ibit phosphodiesterase 5, causing t cGM P,
smooth muscle relaxation in the corpus
cavernosu m , t blood flow, and penile erection .
CLINICAL USE
Treatment of erectile dysfunction .
TOXICITY
Headache, flushing, dyspepsia, impaired blue
green color vision. Risk of l i fe-threatening
hypotension i n patients taking nitrates.
Sildenafil and vardenafil fill the pen is.
"Hot and sweaty," but then Headache,
Heartburn, Hypotension .
Danazol
MECHANISM
Synthetic androgen that acts as partial agon ist at androgen receptors.
CLINICAL USE
Endometriosis and hereditary angioedema.
TOXICITY
Weight gai n , edema, acne, hirsuti sm, masculinization, ! HDL levels, hepatotoxicity.
54 1
RENAL
RENAL-PHARM ACOLOGY
SECTION Ill
RENAL-PHARM ACOLOGY
Diuretics: site of adion
Acetazolam ide
D i stal convoluted
Th iazides
ca 2 +
(+ PTH )
tubule
Potassi u m-spa r i n g
d i u retics
NaCI
Cortex
(+aldoste ron e)
Outer med u l l a
M a n n itol
t
I n n e r med u l l a
ADH
antagon i sts
C o l l ecti n g
d uct
(Adapted, with permission, from Katzung BG. Basic and Clinical Pharmacology, 7th ed. Stamford, G: Appleton & Lange, 1 997: 243.)
499
500
SECTION Ill
R ENAL
RENAL-PHARM ACOLOGY
Mannitol
MECHANISM
Osmotic diuretic, t tubular fluid osmolarity,
producing t urine flow, * intracranial/
intraocular pressure.
CliNICAl USE
Drug overdose, elevated intracranial/intraocular
pressure.
TOXICITY
Pulmonary edema, dehydration.
Contraindicated in anuria, CHF.
Acetazolamide
MECHANISM
Carbonic anhydrase inhibitor. Causes self
limited NaHC0 3 diuresis and reduction in
total-body HC0 3 - stores.
CliNICA l USE
Glaucoma, urinary alkalinization, metabolic
alkalosis, altitude sickness, pseudotumor
cerebri.
TOXICITY
Hyperchloremic metabolic acidosis,
paresthesias, NH 3 toxicity, sulfa allergy.
"ACID"azolamide causes ACIDosis.
Loop diuretics
Furosemide
MECHANISM
Sulfonamide loop diuretic. Inhibits cotransport
system (Na+, K+, 2 CJ-) of thick ascending
limb of loop of Henle. Abolishes hypertonicity
of medulla, preventing concentration of urine.
Stimulates PGE release (vasodilatory effect
on afferent arteriole); inhibited by NSAIDs.
t Ca2 + excretion. Loops Lose calcium.
CliNICAl USE
Edematous states (CHF, cirrhosis, nephrotic
syndrome, pulmonary edema), hypertension,
hypercalcemia.
TOXICITY
Ototoxicity, Hypokalemia, Dehydration, Allergy
(sulfa), Nephritis (interstitial), Gout.
Ethacrynic acid
MECHANISM
Phenoxyacetic acid derivative (not a
sulfonamide). Essentially same action as
furosemide.
CliNICAl USE
Diuresis in patients allergic to sulfa drugs.
TOXICITY
Similar to furosemide; can cause
hyperuricemia; never use to treat gout.
OH DANG!
RENAL
RENAL - PHARM ACOLOGY
SECTION Ill
50 1
Hydrochlorothiazide
MECHANISM
Thiazide diuretic. Inhibits aCl reabsorption in
early distal tubule, reducing diluting capacity
of the nephron. ! Ca2+ excretion.
CLINICAL USE
Hypertension, CHF, idiopathic hypercalciuria,
nephrogenic diabetes insipidus.
TOXICITY
Hypokalemic metabolic alkalosis,
hyponatremia, hyperGlycemia,
hyperLipidemia, hyperUricemia, and
hyperCalcemia. Sulfa allergy.
HyperGLUC.
Spironolactone and eplerenone; Triamterene,
and Amiloride.
T he K+ STAys.
K+sparing diuretics
MECHANISM
Spironolactone and eplerenone are competitive
aldosterone receptor antagonists in the cortical
collecting tubule. Triamterene and amiloride
act at the same part of the tubule by blocking
Na+ channels in the CCT.
CLINICAL USE
Hyperaldosteronism, K+ depletion, CHF.
TOXICITY
Hyperkalemia (can lead to arrhythmias),
endocrine effects with spironolactone (e.g.,
gynecomastia, antiandrogen effects).
Diuretics: electrolyte changes
Urine NaCI
t (all diuretics). Serum NaCl may ! as a result.
Urine K+
t (all except K+ -sparing diuretics). Serum K+ may ! as a result.
Blood pH
! (acidemia) : carbonic anhydrase inhibitors- ! HC0 3 - reabsorption. K+ sparing-aldosterone
blockade prevents K+ secretion and H+ secretion. Additionally, hyperkalemia leads to K+ entering
all cells (via H+fK+ exchanger) in exchange for J-I+ exiting cells.
t (alkalemia) : loop diuretics and thiazides cause alkalemia through several mechanisms:
Volume contraction -+ t AT II .... t Na+fJ-I+ exchange in proximal tubule
t HC0 3 reabsorption ("contraction alkalosis")
K+ loss leads to K+ exiting all cells (via J-I+fK+ exchanger) in exchange for f-I+ entering cells
In low K+ state, f-I+ (rather than K+) is exchanged for Na+ in cortical collecting tubule, leading
to alkalosis and "paradoxical aciduria"
t with loop diuretics: ! paracellular Ca2+ reabsorption hypocalcemia.
! with thiazides : Enhanced paracellular Ca2+ reabsorption in proximal tubule and loop of Henle.
....
Urine Cal+
-+
502
SECTION Ill
ACE inhibitors
MECHANISM
RENAL
RENAL-PHARM ACOLOGY
Captopril, enalapril, lisinopril.
Inhibit angiotensin-converting enzyme (ACE)
-+ angiotensin II GF R by preventing
constriction of efferent arterioles. Levels
of renin t as a result of loss of feedback
inhibition. Inhibition of ACE also prevents
inactivation of bradykinin, a potent vasodilator.
-
CLINICAL USE
Hypertension, CHF, proteinuria, diabetic renal
disease. Prevent unfavorable heart remodeling
as a result of chronic hypertension.
TOXICITY
Cough, Angioedema, Teratogen (fetal renal
malformations), Creatinine increase U GF R),
Hyperkalemia, and Hypotension. Avoid in
bilateral renal artery stenosis, because ACE
inhibitors will further GF R -+ renal failure.
Angiotensin II receptor blockers (-sartans) have
effects similar to ACE inhibitors but do not
t bradykinin -+ no cough or angioedema.
Captopril's CATCHH.
47 2
SECTION I l l
Alcoholism
Wernicke- Korsakoff
syn d rome
Mallory-Weiss
synd rome
Delirium tremens (DTs)
PSYCHI ATRY
PSYCHIATRY-PHAR MACOLOGY
Physiologic tolerance and dependence with symptoms of withdrawal (tremor, tachycard ia,
hypertension, malaise, nausea, DTs) when intake is interrupted.
Complication s : alcohol ic cirrhosis, hepatitis, pancreatitis, peripheral neuropathy, testicular atrophy.
Treatment: disulfiram (to condition the patient to abstai n from alcohol use), supportive care.
Alcoholics Anonymous and other peer support groups are helpful i n sustaining abstinence.
Caused by th iamine deficiency. Triad of confusion, ophthal moplegia, and ataxia (Wernicke's
encephalopathy) . May progress to irreversible memory loss, confabulation, personal ity change
( Korsakoff's psychosis) . Associated with periventricular hemorrhage/necrosis of mamm illary
bodies. Treatment: IV vitamin B1 (thiamine).
Longitudinal lacerations at the gastroesophageal junction caused by excessive vom iting. Often
presents with hematemesis. Associated with pain (vs. esophageal varices).
Life-threatening alcohol withdrawal syndrome that peaks 2-5 clays after last drink.
Symptoms in order of appearance : autonom ic system hyperactivity (tachycardia, tremors, anx iety,
seizures), psychotic symptoms (halluci nations, delusions), confusion .
Treatment: benzodiazepines.
PSYCHIATRY-PHAR MACOLOGY
Treatment for selected
psychiatric condit ions
CNS
stimulants
PSYCHIATRIC CONDI TION
PREFERRED DRUGS
Alcoho l withdr awal
Benzocliazepines
Anxiety
SSRis, SNRis, buspirone
ADHD
Methylphen idate, ampheta m i nes
Bipolar d isorder
"Mood stabilizers" (e.g., l ithium, valproic acid,
carbamazepine), atypical antipsychotics
Bulimia
SSRis
Depression
SSRis, SNRis, TCAs, buspi rone, m irtazapine
(especially with insomnia)
Obsessive-compulsive d isorder
SSRis, clomipra m i ne
Panic disorder
SSRis, venlafaxine, benzodiazepines
PTSD
SSRis
Sch izophrenia
Antipsychotics
Social phobias
SSRis
Tourette's syndrome
Antipsychotics (e.g., haloperidol, rispericlone)
-------
Methylphenidate, dextroamphetamine, methamphetamine.
MECHANISM
t catecholamines at the synaptic cleft, especially NE and dopamine.
CliNICAL USE
ADHD, narcolepsy, appetite control.
PSYCHIATRY-PHAR MACOLOGY
PSYCHIATRY
Antipsychotics
(neuroleptics)
All typical antipsychotics block dopamine D 2
receptors ( t [cAMP]).
C LINICAL USE
Schizophren ia (primarily positive symptoms),
psychosis, acute man ia, Tourette's syndrome.
OTHER TOXICITIES
H ighly lipid soluble and stored in body fat; thus,
very slow to be removed from body.
Extrapyram idal system (EPS) side effects (e.g.,
clyski nesias).
Endocrine side effects (e.g., dopamine receptor
antagonism .... hyperprolactinem ia ....
galactorrhea).
Side effects arising from blocking muscarinic
(dry mouth, constipation), a 1 (hypotension),
and h ista m i ne (sedation) receptors.
N e u roleptic m a l ignant syndrome ( N M S )
rigid ity, myoglobinuria, autonomic instability,
hyperpyrexia. Treatment: clantrolene, 0 2
agonists (e.g., bromocriptine).
stereotypic oral
facial movements as a result of long-term
antipsychotic use. Often irreversible.
Ta rdive dysk i nesia
Atypical antipsychotics
4 73
Haloperidol, trifluoperazine, fluphenazine, thioriclazine, chlorpromazine (haloperidol + "-azines").
MECHANISM
TOXICITY
SECTION I l l
Olanzapine, clozapine, quetiapine ris per icl o ne,
aripiprazole, ziprasiclone.
,
MECHANISM
Not completely u nderstood. Varied effects on
5-HT 2 , dopami ne, and a- and H 1 -receptors.
C LINICAL USE
Sch izophrenia- both positive and negative
symptoms. Also used for bipolar disorder,
OCD, anxiety disorder, depression, man ia,
Tourette's syndrome.
TOXICITY
Fewer extrapyram idal and antichol inergic
side effects than trad itional antipsychotics.
Olanzapine/clozapine may cause significant
weight gai n . Clozapine may cause
agranulocytosis (requ ires weekly WBC
mon itoring) and seizu re. Ziprasiclone may
prolong the QT i nterval.
potency: Trifluoperazine, Fluphenazine,
Haloperidol (Try to Fly H igh) -neurologic
side effects (extrapyram idal symptoms).
High
Low potency: Chlorpromazine, Thioridazine
(Cheating Thieves are low) -non-neurologic
side effects (antichol inergic, antihistami ne,
and a 1 -blockacle effects).
Chlorpromazine- C orneal deposits ;
Th ioriclazine - reT inal deposits ; halopericl o l
N M S, tard ive dyskinesia.
Evolution of EPS side effects :
4 hr acute dystonia (muscle spasm, stiffness,
oculogyric crisis)
4 clay akath isia (restlessness)
4 wk bradykinesia (parki nsonism)
4 mo tard ive dyskinesia
For N M S, think FEVER:
Fever
Encephalopathy
Vitals unstable
Elevated enzymes
Rigidity of muscles
It's atypical for old closets to quietly risper from
A to Z .
Must watch clozapine clozely !
47 4
SECTION I l l
P SYCHI ATRY
PSYCHIATRY-PHAR MACOLOGY
Lithium
MECHANISM
Not establ ished ; possibly related to inh ibition of
phosphoinositol cascade.
C LINICAl USE
Mood stabil izer for bipolar disorder; blocks
rel apse and acute manic events . Also SIAD H .
TOXICITY
Tremor, sedation, edema, heart block,
hypothyroidism, polyuria (ADH antagon ist
causing nephrogenic diabetes insipidus),
teratogenesis. Fetal cardiac defects include
Ebstein anomaly and malformation of the
great vessels. Narrow therapeutic window
requires close monitoring of serum levels.
Almost exclusively excreted by the kidneys ;
most is reabsorbed at the proximal convoluted
tubules fol lowing Na+ reabsorption.
LMNOP:
Lith ium side effects
Movement (tremor)
Neph rogenic diabetes i nsipidus
HypOthyroid ism
Pregnancy problems
Buspirone
MECHANISM
Stimulates 5-HT 1 A receptors.
C LINICAl USE
General ized anxiety disorder. Does not cause
sedation, addiction, or tolerance. Takes 1-2
weeks to take effect. Does not interact with
alcohol (vs. barbiturates, benzodiazepines).
I 'm always anxious if the bus will be on ti me, so
I take buspirone.
Antidepressants
Norad renergic
neuron
Serotonergic
n e u ron
receptor
receptor
Postsynaptic
neuron
(Adapted, with permission, from Katzung B G , Trevor A J . USMLE Road Map: Pharmacology, 2nd ed. N e w York: McGraw-Hill, 2006: Fig. 5-7.)
PSYCHIATRY
PSYCHIATRY-PHAR MACOLOGY
SECTION Il l
47 5
Fluoxetine, paroxetine, sertraline, citalopram.
Flashbacks paralyze senior citizens .
MECHANISM
Serotonin-specific reuptake inh ibitors.
C LINICAL USE
Depression, general ized anxiety disorder, panic
disorder, O C D, bulim ia, social phobias,
PTSD.
It normally takes 4-8 weeks for antidepressants
to have an effect.
TOXICITY
Fewer than TCAs . CI distress, sexual
dysfunction (anorgasm ia and ! l ibido) .
Serotonin syndrome with any drug that t
seroton i n (e.g., MAO inhibitors, SNRis,
TCAs) - hypertherm ia, confusion, myoclonus,
cardiovascular collapse, Hushing, di arrhea,
seizures. Treatment: cyproheptadine (5 -HT 2
receptor antagon ist) .
SSRis
SNRis
Venlafaxine, cluloxetine.
MECHANISM
I n h ibit seroton i n and NE reuptake.
C LINICAL USE
Depression. Venlafaxine is also used in general ized anxiety and panic d isorders ; cluloxetine is also
ind icated for diabetic peripheral neuropathy. Duloxetine has greater effect on N E .
TOXICI TY
Tricyclic
antidepressants
t B P most common ; also stimulant effects, sedation, nausea.
Am itriptyl i ne, nortriptyl ine, im ipramine, desipramine, clomipra m ine, cloxepin, amoxapine (all
TCAs end i n -iptyl ine or -ipramine except cloxepin and amoxapine ) .
MECHANISM
Block reuptake o f NE a n d seroton in.
C LINICAL USE
Major depression, becl wetting (im ipramine), OCD (clomipram ine), fibromyalgia.
TOXICI TY
Sedation, a 1 -blocki ng effects including postural hypotension, and atropi ne-like (antichol inergic)
side effects (tachycardia, urinary retention, dry mouth ) . 3 TCAs (am itriptyl ine) have more
antichol i nergic effects than 2 TCAs (nortriptyl ine) have. Desipramine is less sedating and has
higher seizure threshold.
Tri-C 's : C onvulsions, Coma, Carcliotoxicity (arrhythm ias); also respiratory depression,
hyperpyrexia. Confusion and hallucinations in elderly clue to anticholinergic side effects (use
nortriptyl ine) . Treatment: NaHC03 for card iovascular toxicity.
Monoamine oxidase
(MAO) inhibitors
Tranylcyprom ine, Phenel zine, Isocarboxazicl, Selegi line (selective MAO-B inh ibitor) .
(MAO Takes Pride In Shanghai) .
MECHANISM
Nonselective MAO inh ibition t levels of amine neurotransm itters (NE, seroton in, dopamine) .
C LINICAL USE
Atypical depression, anxiety, hypochondriasis.
TOXICITY
Hypertensive crisis (most notably with ingestion of tyram i ne, which is found i n many foods such
as wine and cheese); CNS stimulation . Contraind icated with SSRis, TCAs, St. John's Wort,
meperidine, and clextromethorphan (to prevent seroton i n syndrome) .
47 6
SECTION Ill
PSYCHI ATRY
PSYCHIATRY-PHAR MACOLOGY
Atypical antidepressants
B upropion
Also used for smoking cessation . t N E and
dopa m i ne via unknown mechanism. Toxicity:
stimulant effects (tachycardia, insomnia),
headache, seizure in bulimic patients. No
sexual side effects.
M i rtazapine
a2-antagon ist ( t release of N E and seroton in)
and potent 5-HT2 and 5-HT 3 receptor
antagonist. Toxicity: sedation (wh ich may be
desirable in depressed patients with insom nia),
t appetite, weight ga in (wh ich may be
desirable i n elderly or anorexic patients), dry
mouth .
Maprotiline
Blocks N E reuptake. Toxicity: sedation,
orthostatic hypotension.
Trazodone
Primarily inh ibits seroton in reuptake. Used
primarily for insomn ia, as high doses are
needed for antidepressant effects. Toxicity:
sedation, nausea, priapism, postural
hypotension.
Called trazobone due to male-specific side
effects.
N E U ROLOGY
N E U ROLOGY- PHA RMACOLOGY
SECTI O N I l l
44 9
NE U ROLOGY- PHA RMACOLOGY
Glaucoma drugs
l intraocular pressure via l amount of aqueous humor ( i n h ibit synthesis/secretion or increase
drainage ) .
DRUG
MECHANISM
SIDE EFFECTS
a-agonists
Epinephrine
Brimonidine ()
l aqueous humor synthesis via vasoconstriction
l aqueous humor synthesis
Mydriasis; do not use i n closed-angle glaucoma
Blurry vision, ocular hyperem ia, foreign body
sensation , ocular allergic reactions, ocular
pruritus
l aqueous humor synthesis
No pupillary or vision changes
l aqueous humor synthesis via inh ibition of
No pupillary or vision changes
-blockers
Timolol, betaxolol,
carteolol
D i uretics
Acetazolamide
carbonic anhydrase
Cholinomimetics
Direct (pilocarpine,
carbachol)
Indirect
(physostigmine,
echothiophate)
t outflow of aqueous humor via contraction
of cil iary muscle and open ing of trabecular
meshwork
Use pilocarpine in emergencies-very effective
at opening meshwork into canal of Schlemm
M iosis and cyclospasm (contraction of cili ary
muscle)
Prostaglandin
Latanoprost (PG F2a)
Opioid analgesics
MECHANISM
t outflow of aqueous humor
Darkens color of iris ( brow n ing)
Morphine, fentanyl, codeine, heroin, methadone, meperidine, dextromethorphan, d iphenoxylate.
Act as agonists at opioid receptors (mu = morph ine, delta = en kephalin, kappa = dynorph in)
to modulate synaptic transmission- open K+ channels, close C a 2 + channels
l synaptic
transmission. I n h ibit release of ACh, NE, 5-HT, glutamate, substance P.
--+
CliNICAL USE
Pain, cough suppression (dextromethorphan), diarrhea ( lopera m ide and d iphenoxylate ) , acute
pulmonary edema, maintenance programs for add icts (methadone ) .
TOXICITY
Addiction , respi ratory depression , constipation , m iosis (pinpoint pupils), add itive CNS depression
with other drugs. Tolerance does not develop to miosis and constipation . Toxicity treated with
naloxone or naltrexone (opioid receptor antagon ist) .
Butorphanol
MECHANISM
Mu-opioid receptor partial agon ist and kappa-opioid receptor agon ist; produces analgesia.
CliNICAL USE
Severe pain (migraine, labor, etc . ) . Causes less respiratory depression than full opioid agon ists.
TOXICITY
Can cause opioid withdrawal symptoms if patient is also taking full opioid agonist (competition for
opioid receptors ) . Overdose not easily reversed with naloxone.
4 50
SE C T IO N I l l
N E U R O LO G Y
NEUROLOGY- PHARMACOLOGY
Tramadol
MECHANISM
Very weak opioid agonist; also inh ibits seroton in and NE reuptake (works on mu ltiple
neurotransm itters-" tram it all " in with tramadol) .
CliNICAL USE
Chron ic pa i n .
TOXICITY
Similar t o opioids. Decreases seizur e threshold.
NEUROLOGY- PHARMACO LOGY
N E U R O LO G Y
SECTION Ill
45 1
Epilepsy drugs
GENERALIZED
PARTIAL (FOCAL)
STATUS
SIMPLE
Phenytoin
Carbamazepine
./
l st line
COMPLEX
./
l st l i ne
TONIC-CLONIC
ABSENCE
lst l ine
EPILEPTICUS
MECHANISM
NOTES
l st l ine for
prophylaxis
t Na+ channel
Fosphenytoin for
parenteral use
in activation
t Na+ channel
l st l ine
inactivation
./
Lamotrigine
Gabapentin
./
./
Topiramate
./
./
Phenobarbital
./
Valproic acid
./
./
lst line for
trigeminal
neuralgia
Blocks voltagegated Na+
channels
Designed as GABA
analog, but
primarily i n h ibits
h igh-voltageactivated C a 2 +
channels
Also used for
peripheral
neuropathy,
postherpetic
neuralgia,
m tgrame
prophylaxis,
bipolar d isorder
./
Blocks a+
channels,
t GABA action
Also used for
mtgrame
prevention
./
./
l st l i ne i n children
./
lst l ine
t GABA A action
t Na+ channel
./
inactivation,
t GABA
concentration
Blocks thalamic
T-type C a 2 +
channels
l st l ine
Ethosuximide
l st l ine for
acute
Benzodiazepines
(diazepam or
lorazepam)
t GABA A action
Tiagabine
./
./
Inh ibits GABA
reuptake
Vigabatrin
./
./
Irreversibly
inh ibits GABA
transaminase
..... t GABA
Levetiracetam
./
./
Also used for
myoclon ic
setzures
Unknow n ; may
modulate GABA
and glutamate
release
Also used for
seizures of
eclampsia ( l st
l ine is MgS0 4 )
4 52
SECTION I l l
N E U R O LOGY
NEURO LOGY- PHARM ACO LOGY
Epilepsy drug toxicities
Benzodiazepines
Sedation, tolerance, dependence.
Carbamazepine
Diplopia, ataxia, blood dyscrasias
(agranulocytosis, aplastic anem ia) , l iver
toxicity, teratogenesis, i nduction of cytochrome
P-450, SIADH , Stevens-Johnson syndrome.
Ethosuximide
GI d istress, fati gue, headache, urticaria,
Stevens-Johnson syndrome.
Phenobarbital
Sedation, tolerance, dependence, i nduction of
cytochrome P-450.
Phenytoin
Nystagmus, d iplopia, ataxia, sedation , gingival
hyperplasia, h i rsutism, megaloblastic anem ia,
teratogenesis (fetal hydantoin syndrome) , SLE
l ike syndrome, i nduction of cytochrome P-450,
lymphadenopathy, Stevens-Johnson syndrome,
osteopenia.
Valproic acid
GI d istress, rare but fatal hepatotoxicity
(measure 1FT's), neural tube defects in
fetus (spina bifida), tremor, weight ga in.
Contraindicated i n pregnancy.
Lamotrigine
Stevens-Johnson syndrome.
Gabapentin
Sedation, ataxi a .
Topiramate
Sedation , mental dulling, kidney stones, weight
loss.
Stevens-Johnson syndrome -prodrome of
malaise and fever followed by rapid onset of
erythematous/purpuric macules (oral, ocular,
gen ital ) . Skin lesions progress to epidermal
necrosis and sloughing.
EFGH - Ethosuxim ide, Fatigue, GI , Headache.
Phenytoin
MECHANISM
Use-dependent blockade of Na+ channels; inh ibition of glutamate release from excitatory
presynaptic neuron.
CLINICAL USE
Ton ic-clon ic seizures. Also a class I B antiarrhythm ic .
TOXICITY
Nystagmus, ataxia, d iplopia, sedation, SLE-l ike syndrome, i nduction o f cytochrome P-4 5 0 . Chronic
use produces gingival hyperplasia in children, peripheral neuropathy, h i rsutism, megaloblastic
anem ia U folate absorption) . Teratogen ic (fetal hydantoin syndrome) .
Barbiturates
MECHANISM
Phenobarbital, pentobarbital, thiopental , secobarbital.
Facil itate GABAA action by t duration of CJ- chan nel open ing, thus ! neuron firing (barbidurates
t duration) . Contraindicated in porphyria.
CLINICAL USE
Sedative for anxiety, seizures, insomnia, i nduction of anesthesia (th iopental ) .
TOXICITY
Respiratory and card iovascular depression (can b e fatal ) ; CNS depression (can b e exacerbated by
EtOH use) ; dependence; drug interactions (i nduces P-4 5 0 ) .
Overdose treatment is supportive (assist respiration a n d mainta i n BP) .
N E U R O LOGY
Benzodiazepines
MECHANISM
NE UROLOGY- PHARMACOLOGY
4 53
Diazepam, lorazepam, triazolam, temazepam , oxazepam , m idazolam, chlord iazepoxide,
alprazolam.
Facilitate GABA A action by t frequency of
Cl- chan nel opening. ! REM sleep. Most
have long half-l ives and active metabol ites
(exception s : triazolam, oxazepam, and
m idazolam are short acting ....higher
.
addictive
potential ) .
CLINICAL USE
Anxiety, spasticity, status epilepticus (lorazepam
and d iazepam) , detoxification (especially
alcohol withd rawal-DTs), n ight terrors,
sleepwalking, general anesthetic (amnesia,
muscle relaxation ) , hypnotic (insomn ia) .
TOXICITY
Dependence, add itive CNS depression effects
with alcohol . Less risk of respiratory depression
and coma than with barbiturates.
Treat overdose with flumazenil (competitive
antagonist at GABA benzodiazepine receptor) .
Nonbenzodiazepine
S E C TI O N I l l
Frenzodiazepines t frequency.
Benzos, barbs, and EtOH all bind the
GABA A receptor, which is a l igand-gated
chloride channel .
Zolpidem (Ambien) , zaleplon, eszopiclone.
hypnotics
MECHANISM
Act via the B Z l subtype of the GABA receptor. Effects reversed by flumazen i l .
CLINICAL USE
Insomnia.
TOXICITY
Ataxia, headaches, confusion. Short duration because of rapid metabol ism by l iver enzymes. Unlike
older sedative-hypnotics, cause only modest day-after psychomotor depression and few am nestic
effects. Lower dependence risk than benzodiazepines.
Anesthetics-general
principles
CNS drugs must be l ipid soluble (cross the blood-brain barrier) or be actively transported.
Drugs with ! solubility in blood = rapid induction and recovery times.
Drugs with t solubi l ity i n l ipids = t potency =
_
_
MAC
MAC = m i n imal alveolar concentration at which 5 0 % of the population is anesthetized. Varies
with age .
Examples: N 2 0 has ! blood and l ipid solubility, and thus fast induction and low potency.
Halothane, in contrast, has t lipid and blood solubi lity, and thus h i gh potency and slow induction.
Inhaled anesthetics
Halothane, enflurane, isoflurane, sevoflurane, methoxyflurane, n itrous oxide.
MECHANISM
Mechanism unknown .
EFFECTS
Myocard ial depression, respiratory depression , nausea /emesis, t cerebral blood flow ( ! cerebral
metabol ic demand) .
TOXICITY
Hepatotoxicity (halothane), neph rotoxicity (methoxyflurane), proconvu lsant ( enflurane) , mal ignant
hyperthermia (all but nitrous oxide; rare, life-threatening, inherited susceptibility) , expansion of
trapped gas in a body cavity (n itrous oxide ) .
4 54
SE C T IO N I l l
N E U R O LO G Y
NEU ROLOGY- PHARMACOLOGY
Intravenous anesthetics
Barbiturates
Th iopental-h igh potency, high l ipid solubility,
rapid entry into brain. Used for induction
of anesthesia and short surgical procedures.
Effect term inated by rapid redi stribution into
tissue (i.e., skeletal muscle) and fat. ! cerebral
blood Aow.
Benzodiazepines
Midazolam most com mon dru g used for
endoscopy; used adjunctively with gaseous
anesthetics and narcotics. May cause severe
postoperative respiratory depression, ! BP (treat
overdose with Aumazen il), and amnesia.
Arylcyclohexyla mines
(Ketamine)
PCP analogs that act as dissociative anesthetics.
Block M DA receptors. Cardiovascular
stimulants. Cause disorientation ,
hallucination , and bad dreams. t cerebral
blood Aow.
Opioids
Morphine, fentanyl used with other CNS
depressants during general anesthesia.
Propofol
Used for sedation in ICU, rapid anesthesia
induction, and short procedures. Less
postoperative nausea than thiopental.
Potentiates GABA A '
Local anesthetics
B . B. Ki ng on OPIOIDS PROPOses
FOOLishly.
Not recommended for home use by pop stars.
Esters -procaine, cocai ne, tetracaine.
Amides -l ldocalne, meplvacalne, buplvacalne (amldes have 2 I's in name) .
MECHANISM
Block a+ channels by binding to specific receptors on inner portion of channel. Preferentially
bind to activated Na+ channels, so most effective i n rapidly firing neurons. 3 amine local
anesthetics penetrate membrane in uncharged form, then bind to ion channels as charged form.
PRINCIPLE
Can be given with vasoconstrictors (usually epi nephrine) to enhance local action- ! bleedi ng,
t anesthesia by ! systemic concentration.
In infected (acidic) tissue, alkaline anesthetics are charged and cannot penetrate membrane
effectively need more anesthetic.
Order of nerve blockade: small-diameter fibers > large diameter. Myel inated fibers > un myel inated
fibers. Overall, size factor predominates over myel ination such that small myel inated fibers
> small unmyel inated fibers > large myel inated fibers > large unmyel inated fibers.
Order of loss : ( l ) pain, (2) temperature, ( 3 ) touch, (4) pressure.
--+
CliNICAL USE
M inor surgical procedures, spinal anesthesia. If allergic to esters, give am ides.
TOXICITY
CNS excitation, severe card iovascular toxicity (bupivacaine), hypertension, hypotension, and
arrhythm ias (cocaine) .
N E U R O LO G Y
Neuromuscular
blocking drugs
NEUROLOGY- PHARMACOLOGY
SECTION I l l
455
Used for muscle paralysis in surgery or mechanical ventilation. Selective for motor (vs . autonomic)
nicotinic receptor.
Depo l arizing
Succinylcholine- strong ACh receptor agonist; produces sustained depolarization and prevents
muscle contraction .
Reversal of blockade:
Phase I (prolonged depolari zation) -no antidote. Block potentiated by chol inesterase inh ibitors.
Phase I I (repolarized but blocked; ACh receptors are ava ilable, but desensitized) -antidote
consists of chol inesterase inhibitors (e.g., neostigm ine) .
Complications include hypercalcemia, hyperkalemia, and malignant hyperthermia.
Nondepo l arizing
Tubocurarine, atracurium, mivacurium, pancuronium, vecuronium, rocuronium. C ompetitive
antagonists - compete with ACh for receptors.
Reversal of blockade-neostigmine, ecl r ophon ium, and other cholinesterase inh ibitors.
Dantrolene
MECHANISM
CLINICAL USE
Parkinson's disease
Prevents the release of Ca 2 + from the sarcoplasmic reticulum of skeletal muscle.
Used in the treatment of mal ignant hyperthermia, a rare but l i fe-threatening side effect of
inhalation anesthetics (except N 2 0) and succinylchol ine. Also used to treat neuroleptic mal ignant
syndrome (a toxicity of antipsychotic drugs).
Parkinsonism is clue to loss of cloparninergic neurons and excess chol inergic activity.
drugs
STRATEGY
AGENTS
Dopamine agonists
Bromocriptine (ergot) , pram ipexole, ropinirole
(non-ergot) ; non-ergots are preferred
dopamine
Amantadine (may t dopamine release) ; also
used as an antiviral against influenza A and
rubella; toxicity = ataxia
L-clopa/carbidopa (converted to dopamine in
CNS)
Prevent dopamine
breakdown
Selegiline (selective MAO type B inh ibitor) ;
entacapone, tolcapone (COMT inh ibitors
prevent L-clopa clegraclation , thereby increasing
dopamine availabil ity)
Curb excess chol inergic
activity
Benztropine (Antimuscarinic ; improves
tremor and rigidity but has l ittle effect on
bradykinesia)
BALSA :
Bromocriptine
Am antad ine
Levocl o pa (with carbiclopa)
Selegiline (and COMT inh ibitors)
Antimuscarin ics
For essential or fam i l ial tremors, use a -blocker
(e.g., propranolol ) .
Park your Mercedes-Benz.
456
SECTI O N I l l
N E UROLOGY
NEUROLOGY- PHARMACOLOGY
Ldopa (levodopa)/ carbidopa
MECHANISM
t level of dopamine in brain. Unl ike dopamine, L-clopa can cross blood-brai n barrier and is
converted by dopa decarboxylase in the CNS to dopamine. Carbiclopa, a peripheral decarboxylase
inhibitor, is given with L-clopa to t the bioavailabil ity of L-clopa i n the bra i n and to l i m it peripheral
s i cl e effects.
CliNICAL USE
Parkinson's disease.
TOXICITY
Arrhythmias from increased peripheral formation of catecholamines. Long-term use can lead
to dyskinesia following administration, akinesia between clo ses.
Selegiline
MECHANISM
Selectively inh ibits MAO-B, which preferentially metabol izes dopamine over N E and 5-HT,
thereby increasing the availability of dopamine.
CLINICAL USE
Adjunctive agent to L-clopa in treatment of Parkinson's disease.
TOXICITY
May enhance adverse effects of L-cl o pa .
Alzheimer's drugs
Memantine
MECHANISM
N M DA receptor antagon ist; helps prevent excitotoxicity (mediated by Ca 2 +) .
TOXICITY
Dizziness, confusion, hallucinations.
Donepezil, galantam ine, rivastigmine
MECHANISM
Acetylchol inesterase inh ibitors.
TOXICITY
Nausea, dizziness, insomnia.
Huntington's drugs
Neurotransmitter changes in Huntington's d isease : GABA, ACh, t dopamine.
Treatments :
Tetrabenazine and reserpine - inhibit VMAT; l i m it dopamine vesicle packaging and release.
H aloperidol - dopa m i ne receptor antagonist.
Sumatriptan
MECHANISM
5-HT J B/ID agonist. Inh ibits trigem inal nerve
activation ; prevents vasoactive peptide release ;
i nduces vasoconstriction. Half-life < 2 hours.
C liNICAL USE
Acute m igraine, cluster headache attacks.
TOXICITY
Coronary vasospasm (contraindicated in
patients with CAD or Prinzmetal's angina),
mild tingling.
A SUMo wrestler TRIPs ANd falls on your
head.
404
MUSCULOSKELETAL, SKIN, AND CONNECTIVE T ISSUE
SECTION Ill
PHAR M ACOLOGY
M U S C U LOS K EL E TAL, S K I N , AN D C O N N E C T I V E TI S S U E - PHAR M ACOLOGY
Lipoxygenase pathway yields Leukotrienes.
LT B 4 is a neutrophil chemotactic agent.
LTC 4 , 0 4 , and E4 function in
bronchoconstriction, vasoconstriction ,
contraction of smooth muscle, and t vascular
permeabil ity.
PGI2 inhibits platelet aggregation and promotes
vasod ilation.
Arachidonic acid
products
L for Lipoxygenase and Leukotriene.
Neutrophils arrive " B4" others.
Platelet-Gathering Inhibitor.
Membrane lipid (e.g . , phosphatidylinositol)
N SAI DS, aspirin,
acetaminophen,
Endoperoxides COX-2 inhibitors
(PGG2, PGH2)
Hydroperoxides
(HPET Es)
Leu ko enes
(LTC4 . LT D4)
(LT B4)
=!
.)
It Bronchial tone I
.
Prostacycli
(PG I 2)
t Platelet aggregation
t Vascular tone
t Bronchial tone
t Uterine tone
{Ada pted, with permission, from Katzung BG, Trevor
CT: Appleton & Lange, 1 998: 1 5 0 )
AJ.
omboxane
A
t Uterine tone
(TX 2)
Prostaglandins
(PGE2, PGF2a)
t Vascular tone
t Bronchial tone
t Platelet aggregation
t Vascular tone
t Bronchial tone
----
Pharmacology: Examination & Board Review, 5th ed. Sta mford,
Aspirin
MECHANISM
Irreversibly inh ibits cyclooxygenase (both COX- I and C OX-2 ) by acetylation, which synthesis of
both thromboxane A 2 (TXA 2 ) and prostaglandins. t bleed ing time. No effect on PT, PTT. A type
of NSAID.
CLINICAL USE
Low close (< 300 mg/clay) : platelet aggregation. Intermediate close ( 3 0 0 -240 0 mg/clay) : antipyretic
and analgesic. H igh close (2400-4000 mg/clay) : anti-inflammatory.
TOXICITY
Gastric ulceration, tinn itus (CN V I I I ) . Chron ic use can lead to acute renal fa i lure, interstitial
nephritis, and upper GI bleeding. Risk of Reye's syndrome i n children treated with aspirin for
viral infection . Also stimulates respiratory centers, causing hyperventilation and respiratory
alkalosis.
M USCULOSKELETAL, SKI N, AND CONNECTIVE TISS U E
NSAI Ds
PHA R M ACOLO G Y
SECT ION Ill
40 5
I buprofen, naproxen, indomethacin, ketorolac, diclofenac.
MECHANISM
Reversibly inhibit cyclooxygenase (both COX- 1 and C OX-2 ) . Block prostaglandin (PC) synthesis.
CLINICAL USE
Antipyretic, analgesic, anti-inflam matory. Indomethacin is used to close a PDA.
TOXICITY
I nterstitial neph ritis, gastric ulcer ( PGs protect gastric mucosa ) , renal ischemia ( PGs vasodilate
afferent arteriole) .
COX-2 inhibitors (celecoxib)
MECHANISM
Reversibly inhibit specifically the cyclooxygenase (COX) isoform 2 , which is found in inflammatory
cells and vascular endothelium and med iates inflammation and pain; spares COX- 1 , which helps
mainta i n the gastric mucosa. Thus, should not have the corrosive effects of other NSAIDs on the
GI l i n ing. Spares platelet function as TXA 2 production is dependent on COX- I .
CLINICAL USE
Rheumatoid arthritis and osteoarthritis; patients with gastritis o r u lcers.
TOXICITY
t risk of thrombosis. Sulfa allergy.
Acetaminophen
MECHANISM
Reversibly inh ibits cyclooxygenase, mostly in C N S . Inactivated peripherally.
CLINICAL USE
Antipyretic, a nalgesic, but not anti-inflammatory. Used i nstead of aspirin to avoid Reye's syndrome
i n children with viral infection .
TOXICITY
Overdose produces hepatic necrosis; acetami nophen metabol ite depletes glutathione and forms
toxic tissue adducts i n l iver. N-acetylcysteine is antidote - regenerates glutath ione.
Bisphosphonates
Alendronate, other -d ronates.
MECHANISM
Pyrophosphate analogs; bind hydroxyapatite in bone, inh ibiti ng osteoclast activity.
CLINICAL USE
Osteoporosis, hypercalcemia, Paget's d isease of bone.
TOXICITY
C orrosive esophagitis, osteonecrosis of the jaw.
4 () b
S E CT I O N I l l
PHAR M ACOLOG Y
M USCULOSK E L E TAL, S K I N, AND C ONN E C T I V E T I SSUE
Ciout drugs
Chronic gout drugs
Allopurinol
I n h ibits xanthine oxidase, conversion of
xanth ine to uric acid. Also used in lymphoma
and leukemia to prevent tumor lysis
associated urate nephropathy. t concentrations
of azathioprine and 6-MP (both normally
metabolized by xanth ine oxidase) .
Do not give sal icylates; all but the highest
closes depress uric acid clearance. Even h igh
closes ( 5 -6 g/clay) have only m inor uricosuric
activity.
Febuxostat
Inh ibits xanthine oxidase.
Probenecid
Inh ibits reabsorption of uric acid in PCT (also
inh ibits secretion of penicillin) .
Colchicine
Diet --- Purines -- Nucleic acids
Hypoxanthine
l
Xanthine
Xanthine
oxidase
>
Allopurinol
Plasma --- Urate crystals --- Gout
uric acid
deposited
in j oints
T
Probenecid and
Binds and stabil izes tubulin to inhibit
polymerization, impa iring leukocyte
chemotaxis and degranulation .
GI side effects, especially if given orally.
high-dose salicylates
----=,.------
+-\
T
Diuretics and
Tubular
reabsorption
Tubular
secretion
low-dose salicylates
Urine
Acute gout drugs
NSAI Ds
Naproxen , indomethacin.
Cilucocorticoids
Oral or i ntraarticular.
TN F-a inhibitors
Xanthine
oxidase
All TNF-a inhibitors predispose to infection including reactivation of latent TB since TNF
blockade prevents activation of macrophages and destruction of phagocytosed m icrobes.
DRUG
MECHANISM
CLINICAL USE
Etanercept
Fusion protein (receptor for TNF-a + IgG 1 Fe) ,
produced b y recombinant DNA.
Etanercept is a TNF decoy receptor.
Rheumatoid arthritis, psoriasis, ankylosing
spondyl itis
lnfliximab,
adalimumab
Anti-TNF-a monoclonal antibody
Crohn's disease, rheumatoid arthritis, ankylosing
spondyl itis, psoriasis
HEMATOLOGY AND ONCOLO GY
H E M AT O L O G Y A N D O N C O LO G Y-P H A R M A C O LO G Y
SECTION Ill
3 67
H E M AT O L O G Y A N D O N C O L O G Y-P H A R M A C O L O G Y
Heparin
MECHAN ISM
Cofactor for the activation of antithrombin, ! thrombin, and ! factor Xa. Short half-l i fe .
CliNICAl USE
Immediate anticoagulation for pulmonary embol ism, acute coronary syndrome, M I , DVT. Used
during pregnancy (does not cross placenta ) . Follow PTT.
TOXICITY
Bleeding, thrombocytopenia ( H IT), osteoporosis, drug-dru g i nteractions. For rapid reversal
(antidote), use protamine sulfate (positively charged molecule that binds negatively charged
hepari n ) .
NOTES
Low-molecular-weight heparins (e.g., enoxaparin, dalteparin) a c t more on factor X a , h ave better
bioavailabil ity and 2-4 times longer half-l ife. Can be adm i n i stered subcutaneously and without
laboratory mon itoring. Not easily reversible.
Heparin-induced thrombocytopenia ( H IT) - development of IgC antibodies against heparin
bound to platelet factor 4 ( PF4 ) . Antibody-heparin-PF4 complex activates platelets -+ thrombosis
and thrombocytopenia.
Lepirudin, bivalirudin
Derivatives of hirudin, the anticoagulant used by leeches ; i n h ibit thrombin. Used as an alternative
to heparin for anticoagulating patients with H IT.
Warfarin (Coumadin)
MECHAN ISM
Interferes with normal synthesis and
y-carboxylation of vitamin K-dependent
clotting factors I I , V I I , IX, and X and proteins
C and S . Metabol ized by the cytochrome
P-450 pathway. In laboratory assay, has effect
on EXtrinsic pathway and t PT. Long halflife.
CliNICAl USE
Chronic anticoagulation (after STE M I , venous
thromboembolism prophylaxis, and prevention
of stroke i n atrial fibrillation ) . Not used in
pregnant women (because warfarin, unl ike
heparin, can cross the placenta ) . Follow PT/
I N R values.
TOXICITY
Bleeding, teratogenic, skin/tissue necrosis, drug
drug interactions.
The EX-PresidenT went to war (farin) .
For reversal of warfarin overdose, give vitamin
K. For rapid reversal of severe warfarin
overdose, give fresh frozen plasma.
368
SECTI O N Ill
HEMATOLOGY AND ONC OLOGY
H E M AT O L O G Y A N D O N C O L O G Y-P H A R M A C O L O G Y
Heparin vs. warfarin
Heparin
Warfarin
STRUCTURE
Large anionic, acidic polymer
Small l ipid-soluble molecule
ROUTE O F ADMIN ISTRAT I O N
Parenteral (IV, S C )
Oral
S ITE O F ACTION
Blood
Liver
ONSET OF ACTION
Rapid (seconds)
Slow, l i m ited by half-l ives of normal clotting
factors
Activates antithrombin, which the action of
I la (thrombin) and factor Xa
Impairs the synthesis of vita m i n K-clepenclent
clotti ng factors II, VII, IX, and X (vita m i n K
antagon ist)
DURATION O F ACTI O N
Acute ( hours)
Chronic (clays)
I N H IBITS COAGULATION
Yes
No
Protamine sulfate
IV vita m i n K and fresh frozen plasma
MONITO R I N G
PTT (intrinsic pathway)
PT/INR (extrinsic pathway)
CROSSES PLACENTA
No
Yes (teratogenic)
MECHAN I SM OF ACTION
I N VITRO
TREATMENT O F ACUTE
OVERDOSE
Thrombolytics
Alteplase (tPA) , reteplase (rPA) , tenecteplase (TN K-tPA) .
MECHANISM
Directly or indirectly a iel conversion of plasm inogen to plasm i n , which cleaves thrombin and fibrin
clots. t PT, t PTT, no change in platelet count.
CLINICAL USE
Early Ml, early ischemic stroke, direct thrombolysis of severe pul monary embol ism.
TOXICITY
Bleeding. Contra ind icated in patients with active bleeding, h istory of i ntracranial bleeding, recent
s urgery, known bleeding diatheses, or severe hypertension . Treat toxicity with a minocaproic acid,
an inhibitor of fibrinolysis.
Aspirin (ASA)
MECHAN ISM
CLI N ICAL USE
TOXICITY
ADP receptor inhibitors
MECHANISM
Irreversibly inh ibits cyclooxygenase (both COX-I and C OX-2) enzyme by covalent acetylation.
Platelets cannot synthesize new enzyme, so effect lasts until new platelets are produced :
t bleeding time, TXA2 and prostaglandins. No effect on PT or PTT
Antipyretic, analgesic, anti-inflammatory, antiplatelet ( aggregation ) .
Gastric ulceration, tinnitus (CN V I I I ) . Chronic use can lead to acute renal failure, i nterstitial
nephritis, and upper GI bleeding. Reye's syndrome in children with viral infection . Overdose
causes respiratory alkalosis and metabol ic acidosis.
Clopiclogrel, ticlopicline, prasugrel , ticagrelor.
Inhibit platelet aggregation by irreversibly blocking ADP receptors. I n h ibit fibrinogen binding by
preventing glycoprotein l ib/lila from binding to fibrinogen .
CLIN ICAL USE
Acute coronary syndrome; coronary stenting. incidence or recurrence of thrombotic stroke.
TOXICITY
Neutropen ia (ticlopid ine) .
HEMATO LOGY AND ONC O LOGY
H E M ATO L O G Y A N D O N C O L O G Y-P H A RMA C O LO G Y
SECTION Ill
3 69
Cilostazol, dipyridamole
MECHANISM
Phosphodiesterase I I I inh ibitor; t cAM P in platelets, thus i n h ibiting platelet aggregation ;
vasodilators.
CliN ICAL USE
I nterm ittent claudication, coronary vasodilation, prevention of stroke or TIAs (combined with
aspirin), angina prophylaxis.
TOXICITY
Nausea, headache, facial flushing, hypotension, abdom inal pain.
GP l i b/ l i la inhibitors
Abciximab, eptifibatide, tirofiban.
MECHANISM
Bind to the glycoprotein receptor lib/Ilia on activated platelets, preventing aggregation . Abciximab
is made from monoclonal antibody Fab fragments .
CliN ICAL USE
Acute coronary syndromes, percutaneous transluminal coronary angioplasty.
TOXICITY
Bleeding, thrombocytopenia.
Cancer drugs-cell cycle
G2
Synthesis
of components
needed for
mitosis
G,
Synthesis
of components
needed for
DNA synthesis
Go
'
'\\
Resting ;
s
DNA
Anti metabolites
(Adapted, with permission, from Katzung BG, Trevor AJ. USMLE Road Map: Pharmacology, I st ed. New York:
McGraw-Hill, 200 3 : 1 33.)
3 70
SECTION Ill
HE MATOLOGY AND ONCOLOGY
H E M ATO L O G Y A N D O N C O LO G Y-P H A R M A C O L O G Y
Antineoplastics
Nucleotide synthesis
...- DNA ---- RNA ---- Protei n --- Ce llular d i v i s i o n
---
J
Methotrexate, 5-FU:
J.. t h y m i d i n e synthesis
_
Alkylating agents, cisplatin :
cross-link DNA
6 - M P:
J.. p u r i n e synthesis
Dactinomycin, doxoru bicin :
DNA i ntercalators
Etoposide:
i n h i b i ts topoisomerase I I
Vinca alkaloids:
i n h i b i t m i c rotu b u le formation
Paclitaxel:
i n h i bits m i c rotu b u le d i sassem bly
3 40
S E CTI O N I l l
G A ST R O I N T E ST I N A L
GASTROINTESTINAL-PHARMA COLOGY
Chronic pancreatitis
Chronic inflammation,atrophy,calcification of the pancreas. Major causes are alcohol abuse and
idiopathic.
Can lead to pancreatic insufficiency-+ steatorrhea,fat-soluble vitamin deficiency,diabetes mellitus,
and t risk of pancreatic adenocarcinoma.
Amylase and lipase are less elevated (compared to levels in acute pancreatitis).
Pancreatic
adenocarcinoma
Prognosis averages 6 months or less; very aggressive tumor arising from pancreatic ducts; usually
already metastasized at presentation; tumors more common in pancreatic head (-+ obstructive
jaundice). Associated with CA-1 9-9 tumor marker (also CEA,less specific).
Risk factors:
Tobacco use (but not EtOH)
Chronic pancreatitis (especially > ZO years)
Age > 5 0 years
Jewish and African-American males
Often presents with:
Abdominal pain radiating to back
Weight loss (clue to malabsorption and anorexia)
Migratory thrombophlebitis-redness and tenderness on palpation of extremities (Trousseau's
syndrome)
Obstructive jaundice with palpable,nontender gallbladder (Courvoisier's sign)
Treatment: Whipple procedure,chemotherapy,radiation therapy.
GASTROINTESTINAL-PHARMA COLOGY
Cil
therapy
Somatostatin
(octreotide)
Enteric
ST2
Fundus
Antrum
G (CCK-B)
- ,......_
Antacids
Stomach lumen
(Adapted, with permission, from Katzung BG, Trevor AJ . USMLE Road Mop: Pharmacology, I st ed. New York: McGraw-Hill, 2003 : 1 59.)
G A S T R O I N T E ST I N A L
H2 blockers
GASTRO INTEST INAL-PHARMA COLOGY
Cimetidine,ranitidine,famotidine,nizatidine.
SECTION I l l
Take H z blockers before you dine. Think "table
for 2" to remember H2 .
MECHANISM
Reversible block of histamine Hrreceptors
CLINICAL USE
Peptic ulcer,gastritis,mild esophageal reflux.
TOXICITY
Cimetidine is a potent inhibitor of cytochrome P-45 0 (multiple drug interactions); it also has
antiandrogenic effects (prolactin release,gynecomastia,impotence, libido in males); can
cross blood-brain barrier (confusion,dizziness,headaches) and placenta. Both cimetidine and
ranitidine renal excretion of creatinine. Other H z blockers are relatively free of these effects.
Proton pump inhibitors
-+
34 1
H+ secretion by parietal cells.
Omeprazole,lansoprazole,esomeprazole,pantoprazole,dexlansoprazole.
MECHANISM
Irreversibly inhibit H+fK+ ATPase in stomach parietal cells.
CLINICAL USE
Peptic ulcer,gastritis, esophageal reflux,Zollinger-Ellison syndrome.
TOXICITY
Increased risk of C. difficile infection,pneumonia. Hip fractures, serum MgZ+ with long-term use.
Bismuth, sucralfate
MECHANISM
CliNICAl USE
Misoprostol
MECHANISM
Bind to ulcer base,providing physical protection and allowing HC0 3 - secretion to reestablish pH
gradient in the mucous layer.
t ulcer healing, traveler's diarrhea.
A PGE 1 analog. t production and secretion of gastric mucous barrier, acid production.
CLINICAL USE
Prevention of NSAID-induced peptic ulcers; maintenance of a patent ductus arteriosus. Also used
to induce labor (ripens cervix).
TOXICITY
Diarrhea. Contraindicated in women of childbearing potential (abortifacient).
Odreotide
MECHANISM
Long-acting somatostatin analog.
CliNICAl USE
Acute variceal bleeds,acromegaly,VIPoma,and carcinoid tumors.
TOXICITY
Nausea,cramps,steatorrhea.
Antacid use
Can affect absorption,bioavailability,or urinary excretion of other drugs by altering gastric and
urinary pH or by delaying gastric emptying.
All can cause hypokalemia.
Overuse can also cause the following problems.
Aluminum hydroxide
Constipation and hypophosphatemia; proximal
muscle weakness, osteodystrophy,seizures
Aluminimum amount of feces.
Magnesium hydroxide
Diarrhea,hyporeflexia,hypotension,cardiac
arrest
Mg = Must go to the bathroom.
Calcium carbonate
Hypercalcemia,rebound acid t
Can chelate and effectiveness of other drugs
(e.g.,tetracycline).
3 42
SECTION I l l
Osmotic laxatives
G A ST R O I N T E S T I N A L
GASTROINTESTINAL-PHARMACOLOGY
Magnesium hydroxide,magnesium citrate,polyethylene glycol,lactulose.
MECHANISM
Provide osmotic load to draw water out.
Lactulose also treats hepatic encephalopathy since gut flora degrade it into metabolites (lactic acid
and acetic acid) that promote nitrogen excretion as NH4 +.
CLINICAL USE
Constipation.
TOXICITY
Diarrhea,dehydration; may be abused by bulimics.
lnfliximab
MECHANISM
Monoclonal antibody to TNF-a.
CLINICAL USE
Crohn's disease,ulcerative col itis,rheumatoid arthritis.
TOXICITY
Infection (including reactivation of latent TB),fever,hypotension.
Sulfasalazine
MECHANISM
A combination of sulfapyridine (antibacterial) and 5 -aminosalicylic acid (anti-inflammatory).
Activated by colonic bacteria.
CLINICAL USE
Ulcerative colitis,Crohn's disease.
TOXICITY
Malaise,nausea,sulfonamide toxicity,reversible oligospermia.
Ondansetron
MECHANISM
5 - H T 3 antagonist. Powerful central-acting
antiemetic.
CLINICAL USE
Control vomiting postoperatively and in patients
undergoing cancer chemotherapy.
TOXICITY
Headache,constipation.
Metoclopramide
MECHANISM
CLINICAL USE
TOXICITY
At a party but feeling queasy? Keep on dancing
with ondansetron !
D2 receptor antagonist. t resting tone,contractility, LES tone, motility. Does not influence colon
transport time.
Diabetic and post-surgery gastroparesis,antiemetic.
t parkinsonian effects. Restlessness,drowsiness,fatigue,depression,nausea,diarrhea. Drug
interaction with digoxin and diabetic agents. Contraindicated in patients with small bowel
obstruction or Parkinson's disease.
EN DOCRINE
ENDOCRINE-PHA R M ACOLOGY
SECTION Ill
305
ENDOCRINE-PHA R M ACOLOGY
Diabetes drugs
Treatment strategy for type l DM -low-sugar diet, insulin replacement.
Treatment strategy for type 2 DM-dietary modification and exercise for weight loss; oral
hypoglycemics and insulin replacement.
DRUG CLASSES
ACTION
Insulin :
Bind insulin receptor (tyrosine
kinase activity).
Liver: t glucose stored as glycogen.
Muscle : t glycogen and protein
synthesis, K+ uptake.
Fat: aids TG storage.
Lispro (rapid-acting)
Aspart (rapid-acting)
Glulisine (rapid-acting)
Regular (short-acting)
NPH (intermediate)
Glargine (long-acting)
Detemir (long-acting)
Biguanides :
M etformin
Sulfonylurea s :
First generation :
Tol butamide
Chlorpropamide
Second generation:
G lyburide
Glimepiride
G l ipizide
G litazones/
thiazolidinediones :
Piogl itazone
Rosiglitazone
a-glucosidase
inhibitors :
Acarbose
M igl itol
CLINICAL USE
Type l DM, type 2 DM,
gestational diabetes, life
threatening hyperkalemia,
and stress-induced
hyperglycemia.
TOXICITIES
Hypoglycemia, very rarely
hypersensitivity reactions.
Exact mechanism is unknown.
gluconeogenesis, t glycolysis,
t peripheral glucose uptake
(insulin sensitivity).
Oral. First-line therapy in
type 2 DM.
Can be used in patients
without islet function.
GI upset; most serious
adverse effect is
lactic acidosis (thus
contraindicated in renal
failure).
Close K+ channel in -cell
membrane, so cell depolarizes
triggering of insulin release via
t Ca 2+ influx.
Stimulate release of
endogenous insulin in type
2 DM. Require some islet
function, so useless in type
l DM.
First generation : disulfiram
like effects.
Second generation :
hypoglycemia.
Used as monotherapy in type
2 DM or combined with
above agents.
Weight gain, edema.
Hepatotoxicity, heart failure.
Used as monotherapy in type
2 DM or in combination
with above agents.
GI disturbances.
-+
t insulin sensitivity in peripheral
tissue. Binds to PPAR-y nuclear
transcription regulator."
Inhibit intestinal brush-border
a-glucosidases.
Delayed sugar hydrolysis
and glucose absorption
postprandial hyperglycemia.
-+
Amylin analogs:
glucagon.
Type l and type 2 DM.
t insulin, glucagon release.
Type 2 DM.
Nausea, vomiting;
pancreatitis.
t insulin, glucagon release.
Type 2 DM.
Mild urinary or respiratory
infections.
Pra m lintide
GLP-1 analogs :
Exenatide
Liraglutide
DPP-4 inhibitors :
Linagliptin
Saxagliptin
Sitagliptin
Hypoglycemia,
nausea, diarrhea.
"Genes activated by PPAR-y regulate fatty acid storage and glucose metabolism. Activation of PPAR-y t insulin sensitivity and
levels of adiponectin.
30 6
SECTION Il l
ENDOCRINE
ENDOC R I NE- P H A R M ACO LOG Y
Propylthiouracil. methimazole
MECHANISM
Block peroxidase, thereby inhibiting organification of iodide and coupling of thyroid hormone
synthesis. Propylthiouracil also blocks 5 '-deiodinase, which peripheral conversion of T4 to T 3 .
CLINICAL USE
Hyperthyroidism.
TOXICITY
Skin rash, agranulocytosis (rare), aplastic anemia, hepatotoxicity (propylthiouracil). Methimazole is
a possible teratogen.
Levothyroxine. triiodothyronine
MECHANISM
Thyroxine replacement.
CLINICAL USE
Hypothyroidism, myxedema.
TOXICITY
Tachycardia, heat intolerance, tremors, arrhythmias.
Hypothalamic/pituitary d rugs
DRUG
CLINICAL USE
GH
GH deficiency, Turner syndrome.
Somatostatin
(octreotide)
Acromegaly, carcinoid, gastrinoma, glucagonoma, esophageal varices.
Oxytocin
Stimulates labor, uterine contractions, milk let-down; controls uterine hemorrhage.
ADH (desmopressin)
Pituitary (central, not nephrogenic) DI.
Demeclocycline
MECHANISM
ADH antagonist (member of the tetracycline family).
CLINICAL USE
SIADH.
TOXICITY
Nephrogenic DI, photosensitivity, abnormalities of bone and teeth.
Glucocorticoids
MECHANISM
Hydrocortisone, prednisone, triamcinolone, dexamethasone, beclomethasone.
the production of leukotrienes and prostaglandins by inhibiting phospholipase A 2 and expression
of COX-2.
CliNICAl USE
Addison's disease, inflammation, immune suppression, asthma.
TOXICITY
Iatrogenic Cushing's syndrome -buffalo hump, moon facies, truncal obesity, muscle wasting, thin
skin, easy bruisability, osteoporosis, adrenocortical atrophy, peptic ulcers, diabetes (if chronic).
Adrenal insufficiency when drug stopped abruptly after chronic use.
56 2
SECTION Ill
RESPIRATORY
RESPIRATORY-PHARMACOLOGY
RESPIRATORY-PHARMACOLOGY
H1 blockers
Reversible inhibitors of H1 histamine receptors.
1st generation
Diphenhydramine, dimenhydrinate,
chlorpheniramine.
CLINICAL USES
Allergy, motion sickness, sleep aid.
TOXICITY
Sedation, antimuscarinic, anti-a-adrenergic.
2nd generation
Loratadine, fexofenadine, desloratadine,
cetirizine.
CLINICAL USES
Allergy.
TOXICITY
Far less sedating than lst generation because of
entry into CNS.
Names contain "-en/-ine" or "-en/-ate."
Names usually end in "-adine."
RESPIRATORY
Asthma drugs
RESPIRATORY-PHAR M A CO lOGY
SECTION Ill
5 63
Bronchoconstriction is mediated by ( l) inflammatory processes and (2) parasympathetic tone;
therapy is directed at these 2 pathways.
-agonists
Albuterol -relaxes bronchial smooth muscle W2 ). Use during acute exacerbation.
Salmeterol, formoterol -long-acting agents for prophylaxis. Adverse effects are tremor and
arrhythmia.
Methylxanthines
Theophylline -likely causes bronchodilation by inhibiting phosphodiesterase, t hereby ! cAM P
hydrolysis. Usage is limited because of narrow t herapeutic index (cardiotoxicity, neurotoxicity);
metabolized by P-450. Blocks actions of adenosine.
Muscarinic
antagonists
lpratropium -competitive block of muscarinic receptors, preventing bronchoconstriction. Also
used for COPD, as is tiotropium, a long-acting muscarinic antagonist.
Corticosteroids
Beclomethasone, fluticasone -inhibit the
synthesis of virtually all cytokines. Inactivate
N F-KB, t he transcription factor that induces
the production of T F-a, among other
inflammatory agents. lst-line therapy for
chronic asthma.
Antileukotrienes
Montelukast, zafirlukast-block leukotriene
receptors. Especially good for aspirin-induced
asthma.
Zileuton -a 5 -lipoxygenase pathway inhibitor.
Blocks conversion of arachidonic acid to
leukotrienes.
Omalizumab
Monoclonal anti-IgE antibody. Binds mostly
unbound serum IgE. Used in allergic asthma
resistant to inhaled steroids and long-acting
z-agonists.
Bcoochodllatloo
+-0--Bro n c h i a l tone
+---0-- Theophy l l i ne
AMP
ACh
M usca ri n c
---0-7 Adenosine
ff
antagom sts
Theophyl l i ne
Moldoo
Antigen and lgE f--Omalizumab
on mast cells
Mediators
(leukotrienes, histamine, etc.)
-agonists
Theophylline
Muscarinic
antagonists
Steroids
Antileukotrienes
!l-ago o l "
cAM P
POE
c:p
Exposure to antigen
(dust, pollen, etc.)
Late response:
inflammation
Early response:
bronchoconstriction
Bronchial
hyperreactivity
Symptoms
T reatment strategies in asthma
Bronchoconstriction
(Adapted, with perm ission, from Katzung BG, Trevor AJ. Pharmacology: Examination 8 Board Review, 5th ed. Stamford, 0: Appleton & Lange, 1 9 9 8 : 1 59 and 1 6 1 .)
Expedorants
Guaifenesin
Expectorant-thins respiratory secretions; does not suppress cough reflex.
N-acetylcysteine
Mucolytic-can loosen mucous plugs in CF patients. Also used as an antidote for acetaminophen
overdose.
56 4
SECTION Ill
RESPIRATORY
RESPIRATORY-PHARMACOLOGY
Bosentan
Used to treat pulmonary arterial hypertension. Competitively antagonizes endothel in-l receptors,
decreasing pulmonary vascular resistance.
Dextromethorphan
Antitussive (antagonizes N M DA glutamate receptors). Synthetic codeine analog. Has mild opioid
effect when used in excess. aloxone can be given for overdose. Mild abuse potential.
Pseudoephedrine, phenylephrine
MECHANISM
Sympathomimetic a-agonistic nonprescription nasal decongestants.
CLINICAL USE
Reduce hyperemia, edema, and nasal congestion; open obstructed eustachian tubes.
Pseudoephedrine also used as a stimulant.
TOXICITY
Hypertension. Can also cause CNS stimulation/anxiety (pseudoephedrine).
Methacholine
Muscarinic receptor agonist. Used in asthma challenge testing.
Das könnte Ihnen auch gefallen
- QuizletDokument104 SeitenQuizletS.Noch keine Bewertungen
- Diseases - BiochemDokument4 SeitenDiseases - BiochemJay FeldmanNoch keine Bewertungen
- Retinoblastoma Cognitive Concept Map - DR Kumar Ponnusamy and DR Jegathambigai RN - Problem Based Learning (PBL) For Large Groups Medical StudentsDokument1 SeiteRetinoblastoma Cognitive Concept Map - DR Kumar Ponnusamy and DR Jegathambigai RN - Problem Based Learning (PBL) For Large Groups Medical StudentsPonnusamy Kumar100% (1)
- 7sgdfgf PDFDokument438 Seiten7sgdfgf PDFPratik JadhavNoch keine Bewertungen
- Anti FungalsDokument5 SeitenAnti FungalskakuNoch keine Bewertungen
- 2 Renal Buzzword ChartDokument6 Seiten2 Renal Buzzword ChartTyler KingNoch keine Bewertungen
- First Aid Step 1 2017 Sample RevisionsDokument9 SeitenFirst Aid Step 1 2017 Sample RevisionsFirst Aid/USMLE-Rx100% (2)
- Pharm TableDokument35 SeitenPharm TableHannah BaldwinNoch keine Bewertungen
- BIO-GEN INNO-VISION 2012 - DR Kumar Ponnusamy 1 Min Papers On Inborn Errors of AA Metab & DNA RNADokument12 SeitenBIO-GEN INNO-VISION 2012 - DR Kumar Ponnusamy 1 Min Papers On Inborn Errors of AA Metab & DNA RNADr Kumar PonnusamyNoch keine Bewertungen
- LeukemiaDokument2 SeitenLeukemiaAyeshaArifNoch keine Bewertungen
- Abnormal LFTsDokument2 SeitenAbnormal LFTsRenu RosyNoch keine Bewertungen
- Exam 1 DiseasesDokument1 SeiteExam 1 DiseasesSolomon Seth SallforsNoch keine Bewertungen
- Incorrect UWORLD Questions TemplateDokument29 SeitenIncorrect UWORLD Questions TemplateFeroz RaZa SoomrOoNoch keine Bewertungen
- Cocci Rod 4 Main Classifications: Gram Staph, Strep Bacillus Clostridium Neisseria Pleiomorphic Enterobact-EriceaeDokument2 SeitenCocci Rod 4 Main Classifications: Gram Staph, Strep Bacillus Clostridium Neisseria Pleiomorphic Enterobact-EriceaeKimberly KanemitsuNoch keine Bewertungen
- Antivirals, Rubella, Peecorna VIRUSDokument3 SeitenAntivirals, Rubella, Peecorna VIRUSErnie G. Bautista II, RN, MDNoch keine Bewertungen
- Lippin NotesDokument8 SeitenLippin Noteswalt65100% (1)
- 4.1d - Pathology of The Pituitary - Nov.10 - Dr. GalangDokument4 Seiten4.1d - Pathology of The Pituitary - Nov.10 - Dr. GalangMiel Raphael AranillaNoch keine Bewertungen
- Emergency Radiology - Dr. YantoDokument93 SeitenEmergency Radiology - Dr. YantoLeonardus William KuswaraNoch keine Bewertungen
- Anemia Type Pathogenesis Clinical Manifestations Diagnosis Peripheral Blood Lab FindingsDokument15 SeitenAnemia Type Pathogenesis Clinical Manifestations Diagnosis Peripheral Blood Lab FindingsDanielle FosterNoch keine Bewertungen
- Brenner and Stevens, Pharmacology 3 © 2010Dokument5 SeitenBrenner and Stevens, Pharmacology 3 © 2010PharAwayNoch keine Bewertungen
- Pathogens of The Vagina-Annie Espinosa - This Is The Revised VersionDokument1 SeitePathogens of The Vagina-Annie Espinosa - This Is The Revised VersionMicroposterNoch keine Bewertungen
- Small Intestine 01 PDFDokument9 SeitenSmall Intestine 01 PDFfadoNoch keine Bewertungen
- Nephrotic Nephritic SyndromsDokument4 SeitenNephrotic Nephritic SyndromsKimiwari100% (2)
- Block 13 Patho SlidesDokument62 SeitenBlock 13 Patho SlidesLennon Ponta-oyNoch keine Bewertungen
- Actinic KeratosisDokument19 SeitenActinic Keratosisattydoc1234Noch keine Bewertungen
- Ebr Hy CluesDokument16 SeitenEbr Hy CluesStaporn KasemsripitakNoch keine Bewertungen
- PnemoniaDokument4 SeitenPnemoniadhavalNoch keine Bewertungen
- Uworld EndocrineDokument211 SeitenUworld Endocrineهنادي رازمNoch keine Bewertungen
- Pathology - 2Dokument99 SeitenPathology - 2lusia playzNoch keine Bewertungen
- Poliomyelitis Haemophilus Influenzae Type B VariecellaDokument4 SeitenPoliomyelitis Haemophilus Influenzae Type B VariecellaJeanna Chong100% (1)
- DNA Viruses: P P P A H H PDokument2 SeitenDNA Viruses: P P P A H H PKimberly KanemitsuNoch keine Bewertungen
- AtelectasisDokument3 SeitenAtelectasisLouis FortunatoNoch keine Bewertungen
- Notes Apr 19, 2014 Obstetrics and Gynecology Part 2Dokument29 SeitenNotes Apr 19, 2014 Obstetrics and Gynecology Part 2Diwakesh C BNoch keine Bewertungen
- DR Kumar Ponnusamy Biochemistry-Genetics USMLE Preparatory Course BIOGEN Reusable On-Line Resources For Large Group Teaching-Learning in Relatively Short TimeDokument1 SeiteDR Kumar Ponnusamy Biochemistry-Genetics USMLE Preparatory Course BIOGEN Reusable On-Line Resources For Large Group Teaching-Learning in Relatively Short TimeDr Kumar Ponnusamy100% (1)
- Bio Chem 1Dokument5 SeitenBio Chem 1Reynaldo RiveraNoch keine Bewertungen
- Bipolar Disorder Background: Hypomania Has The Same Symptoms of Mania Without Psychotic SymptomsDokument2 SeitenBipolar Disorder Background: Hypomania Has The Same Symptoms of Mania Without Psychotic SymptomshumdingerNoch keine Bewertungen
- Antifungal Agents: EchinocandinsDokument2 SeitenAntifungal Agents: EchinocandinsCourtney TownsendNoch keine Bewertungen
- ENDOCRINE PATHOLOGY WebpathDokument35 SeitenENDOCRINE PATHOLOGY Webpathapi-3766657Noch keine Bewertungen
- Goljan Blue NotesDokument1 SeiteGoljan Blue NotesCarl NieweldNoch keine Bewertungen
- Pharmacology Main DrugsDokument14 SeitenPharmacology Main DrugsSabir KhanNoch keine Bewertungen
- Clinical Medicine - Lecture: - Topic: - DateDokument3 SeitenClinical Medicine - Lecture: - Topic: - DateqselmmNoch keine Bewertungen
- Cognitive Concept Map - Case of The Week - USMLE Genetics - All About Cystic Fibrosis (CF) - DR Kumar Ponnusamy & DR Jegathambigai RNDokument2 SeitenCognitive Concept Map - Case of The Week - USMLE Genetics - All About Cystic Fibrosis (CF) - DR Kumar Ponnusamy & DR Jegathambigai RNPonnusamy KumarNoch keine Bewertungen
- Week 7. Renal Pathology Continued.Dokument9 SeitenWeek 7. Renal Pathology Continued.Amber LeJeuneNoch keine Bewertungen
- Pediatric Pathology: Disease Cause/Risk Factors SymptomsDokument12 SeitenPediatric Pathology: Disease Cause/Risk Factors SymptomsherethemindNoch keine Bewertungen
- Genetic Disorders-Www - Qworld.co - inDokument13 SeitenGenetic Disorders-Www - Qworld.co - inQworld100% (1)
- Valvular Heart DseDokument8 SeitenValvular Heart DseJane Pineda CuraNoch keine Bewertungen
- Hematology & Oncology. Anatomy 56Dokument60 SeitenHematology & Oncology. Anatomy 56Heran TeferiNoch keine Bewertungen
- Renal - PathDokument23 SeitenRenal - PathKimberly KanemitsuNoch keine Bewertungen
- Acute Myeloproliferative Acute Lymphoproliferative Chronic Myeloproliferative Chronic Lymphoproliferative Plasma Cell NeoplasmDokument1 SeiteAcute Myeloproliferative Acute Lymphoproliferative Chronic Myeloproliferative Chronic Lymphoproliferative Plasma Cell NeoplasmAudreySlitNoch keine Bewertungen
- (MED II) 1.05 Emergencies in Cancer PatientsDokument17 Seiten(MED II) 1.05 Emergencies in Cancer PatientsJearwin AngelesNoch keine Bewertungen
- UW Infectious Diseases + Microbiology Educational Objectives PDFDokument75 SeitenUW Infectious Diseases + Microbiology Educational Objectives PDFDrbee10Noch keine Bewertungen
- Firecracker Pathoma Companion 2017Dokument9 SeitenFirecracker Pathoma Companion 2017Zia HaywoodNoch keine Bewertungen
- Table of Genetic Disorders: Download A Copy of This Study GuideDokument11 SeitenTable of Genetic Disorders: Download A Copy of This Study Guideerica perezNoch keine Bewertungen
- Internal Medicine #1Dokument167 SeitenInternal Medicine #1Nikhil RayarakulaNoch keine Bewertungen
- Diseases Study Guide 2007Dokument6 SeitenDiseases Study Guide 2007Bahaa Ibrahim HelmiNoch keine Bewertungen
- Pathology Week 2 p1-18Dokument18 SeitenPathology Week 2 p1-18zeroun24100% (1)
- Arteriolar Dilator Decreases After Load Ejection FractionDokument1 SeiteArteriolar Dilator Decreases After Load Ejection FractionJack GuccioneNoch keine Bewertungen
- Ged 102 Mathematics in The Modern WorldDokument84 SeitenGed 102 Mathematics in The Modern WorldKier FormelozaNoch keine Bewertungen
- Controlled DemolitionDokument3 SeitenControlled DemolitionJim FrancoNoch keine Bewertungen
- Anviz T5 RFID ManualDokument52 SeitenAnviz T5 RFID ManualLuis Felipe Olaya SandovalNoch keine Bewertungen
- Icici PrudentialDokument52 SeitenIcici PrudentialDeepak DevaniNoch keine Bewertungen
- 1 Prof Chauvins Instructions For Bingham CH 4Dokument35 Seiten1 Prof Chauvins Instructions For Bingham CH 4Danielle Baldwin100% (2)
- FKTDokument32 SeitenFKTNeeraj SharmaNoch keine Bewertungen
- TAC42055 - HO01 Edition I2.0: Section 1 Module 1 Page 1Dokument69 SeitenTAC42055 - HO01 Edition I2.0: Section 1 Module 1 Page 1matheus santosNoch keine Bewertungen
- Rin Case StudyDokument4 SeitenRin Case StudyReha Nayyar100% (1)
- Dance Terms Common To Philippine Folk DancesDokument7 SeitenDance Terms Common To Philippine Folk DancesSaeym SegoviaNoch keine Bewertungen
- Heirs of Vinluan Estate in Pangasinan Charged With Tax Evasion For Unsettled Inheritance Tax CaseDokument2 SeitenHeirs of Vinluan Estate in Pangasinan Charged With Tax Evasion For Unsettled Inheritance Tax CaseAlvin Dela CruzNoch keine Bewertungen
- GA Power Capsule For SBI Clerk Mains 2024 (Part-2)Dokument82 SeitenGA Power Capsule For SBI Clerk Mains 2024 (Part-2)aa1904bbNoch keine Bewertungen
- Pontevedra 1 Ok Action PlanDokument5 SeitenPontevedra 1 Ok Action PlanGemma Carnecer Mongcal50% (2)
- Physico-Chemical Properties of Nutmeg (Myristica Fragrans Houtt) of North Sulawesi NutmegDokument9 SeitenPhysico-Chemical Properties of Nutmeg (Myristica Fragrans Houtt) of North Sulawesi NutmegZyuha AiniiNoch keine Bewertungen
- APA Citation Method For ERLACS: Reference Citations in TextDokument8 SeitenAPA Citation Method For ERLACS: Reference Citations in Textdanny_alfaro_8Noch keine Bewertungen
- Islamiyat ProjectDokument21 SeitenIslamiyat ProjectSubhan Khan NiaziNoch keine Bewertungen
- Book Speos 2023 R2 Users GuideDokument843 SeitenBook Speos 2023 R2 Users GuideCarlos RodriguesNoch keine Bewertungen
- National Employment Policy, 2008Dokument58 SeitenNational Employment Policy, 2008Jeremia Mtobesya0% (1)
- Ward 7Dokument14 SeitenWard 7Financial NeedsNoch keine Bewertungen
- Participatory EvaluationDokument4 SeitenParticipatory EvaluationEvaluación Participativa100% (1)
- Channel & Lomolino 2000 Ranges and ExtinctionDokument3 SeitenChannel & Lomolino 2000 Ranges and ExtinctionKellyta RodriguezNoch keine Bewertungen
- Route Clearence TeamDokument41 SeitenRoute Clearence Teamctenar2Noch keine Bewertungen
- Durability of Prestressed Concrete StructuresDokument12 SeitenDurability of Prestressed Concrete StructuresMadura JobsNoch keine Bewertungen
- 7 ElevenDokument80 Seiten7 ElevenakashNoch keine Bewertungen
- Paper II - Guidelines On The Use of DuctlessDokument51 SeitenPaper II - Guidelines On The Use of DuctlessMohd Khairul Md DinNoch keine Bewertungen
- Jurnal 1 Ieevee LPF PDFDokument4 SeitenJurnal 1 Ieevee LPF PDFNanda SalsabilaNoch keine Bewertungen
- A Brief Tutorial On Interval Type-2 Fuzzy Sets and SystemsDokument10 SeitenA Brief Tutorial On Interval Type-2 Fuzzy Sets and SystemstarekeeeNoch keine Bewertungen
- Zambia National FormularlyDokument188 SeitenZambia National FormularlyAngetile Kasanga100% (1)
- Introduction To AmplifierDokument8 SeitenIntroduction To AmplifierElaine BicolNoch keine Bewertungen
- Smart Plug Installation GuideDokument9 SeitenSmart Plug Installation GuideFrancisco GuerreroNoch keine Bewertungen
- Taxation Law 1Dokument7 SeitenTaxation Law 1jalefaye abapoNoch keine Bewertungen