Beruflich Dokumente
Kultur Dokumente
Histological Features of Rats' Normal Lung Tissue: Dr. Koptyev M.M
Hochgeladen von
ManMan AR-llOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Histological Features of Rats' Normal Lung Tissue: Dr. Koptyev M.M
Hochgeladen von
ManMan AR-llCopyright:
Verfügbare Formate
European International Journal of Science and Technology
Vol. 3 No. 3
April, 2014
HISTOLOGICAL FEATURES OF RATS NORMAL LUNG TISSUE
Dr. Koptyev M.M. 1
E-mail: mn_koptev@ukr.net,
Dr. Pronina O.M. 1
E-mail: elenan_pronina@mail.ru,
Dr. Danylchenko S.I. 1
E-mail: svetlana_danilch@mail.ru,
Dr. Avetikov D.S. 1
E-mail: davidplast@rambler.ru,
Dr. Stavitskiy S.O. 1
stanislav_stavickii@mail.ru,
Department of Emergency Medicine with Operative Surgery and Topographic Anatomy, Higher State
Educational Establishment of Ukraine Ukrainian Medical Stomatological Academy,
23 Shevchenko Str., Poltava, 36011, Ukraine
Corresponding Author:
Dr. Danylchenko Svitlana Ivanivna,
PhD, Associate Professor
Department of Emergency Medicine with Operative Surgery and Topographic Anatomy, Higher State
Educational Establishment of Ukraine
Ukrainian Medical Stomatological Academy,
23Shevchenko Str., Poltava, 36011, Ukraine
E-mail: svetlana_danilch@mail.ru
33
European International Journal of Science and Technology
ISSN: 2304-9693
www.eijst.org.uk
ABSTRACT
Modeling on laboratory animals is widely used for experimental morphological studies. To interpret
the findings properly it is crucial to know the specific differences of their anatomical organization. The
purpose of the research was the study of histological features of normal lung texture of Wistar white rats.
Histological analysis showed that areas of destructive changes of alveolar walls with accumulation of
alveolocytes in the alveoli lumen on different stages of destruction, as well as fibrin and individual red blood
cells were observed in some of the test samples of rats normal lung tissue. In the hollows of some alveoli
few alveolar macrophages and the Type II alveolocytes were found. The special feature of
hemomicrocirculatory flow of rats normal lungs is accumulation of erythrocyte in the capillaries of
alveolar septums. In the areas of erythrocyte accumulation a local damage, destruction and demolition of
cytolemma of capillaries endothelium has been detected, resulted in endothelium basal membrane exposure
and accumulation of pinocytic vesicles in the lumen of capillaries.
Keywords: rats, morphology, lungs
INTRODUCTION
Nowadays respiratory apparatus diseases are the most widespread pathology in the structure of
morbidity of Ukrainian population. In this way, pulmonary pathology morbidity rate in 2011 constituted
17395, 6 per 100 thousand of adult population, and the spread rate constituted 24005, 4 per 100 thousand
[5]. One of the reasons that facilitate the increase of morbidity rate in respiratory system is stress effect [1].
Modeling on laboratory animals is widely used for carrying out the experiments aiming at study the
technique of bronchopulmonary pathology development under stresses [2]. At the same time it is extremely
important to know the features of normal anatomical organization of respiratory apparatus, as they have
significant specific differences [3].
PURPOSE
The purpose of the research was the study of histological features of normal lung texture of Wistar
white rats.
MATERIALS AND METHODS
Study has been carried out on 20 Wistar white male rats of 240-260 g body weight and 8-10 months
old, which were housed in standard conditions at vivarium of Ukrainian Medical Stomatologial Academy
and were not involved in any other experiments. Before euthanasia the animals were not fed during 24
hours. The rats were killed by decapitation under thiopental anesthesia. Fragments of lungs were preserved
in 10% solution of neutral formalin, and then, after appropriate alcoholization of increasing concentration,
were embedded into paraffin according to standard technique. Microtome specimens were colored by
hematoxylin-eosin according to Hart Van Gizon and Mallory.
The experimental part of the research has been carried out in compliance with the requirements of
international principals of the European Convention for the Protection of Vertebrate Animals Used for
Experimental and Other Scientific Purposes (Strasbourg, 18.03.1986) and corresponding Law of Ukraine
For the Protection of Pet Animals (No.3446-IV, 21.02.2006, Kyiv).
RESULTS AND DISCUSSION
Histological study showed that the respiratory part of rats lung consisted of alveolus system, located
on the walls of respiratory bronchioles, alveolar ducts and saccules. Alveoli were surrounded by the dense
34
European International Journal of Science and Technology
Vol. 3 No. 3
April, 2014
capillary nets, forming numerous plexuses. Alveoli were separated from each other by thin alveolar septums,
in which alveolar pores, i.e., junction between adjacent alveoli, became evident.
Internally, rats alveoli were covered by the layer of epithelial cells, interconnected by dense
contacts. Epithelium was located on the basal membrane, blended with endothelium basal membrane in
some areas and somewhere was separated from it by fissures. Such fissures contained reticular and elastic
fibers and cells. Among the epithelial cells, covering alveoli, the majority of them were represented by the
Type I respiratory alveolocytes and the minority were represented by the Type II secretory alveolocytes.
Apart from these cells, occurrence of alveolar macrophages and the Type III brush alveolocytes were also
evident.
The Type I alveolocytes are plane cells of irregular oblong shape with very thin cytoplasm and
nucleus with off-center positioning. The thickness of respiratory alveolocytes expands in the nucleate
portion of the cell. On the free surface of the cytoplasm, faced the alveolus cavity, wide cytoplasmic
processes, which extend the contact area of the epithelium surface with air, are detected. Pinocytotic
vesicles are detected in the cytoplasm. Anucleate areas of endothelial cells of capillaries are adjacent to the
anucleate areas of the Type I alveolocytes. Here the basal membrane of alveolocytes, approaching to the
basal membrane of endothelial of capillaries, blends with it. Due to this the aerohematic barrier is extremely
thin. It is formed by the layer of epithelial cells, covering the alveoli, basal membranes of alveoli epithelium
and endothelium of capillaries and cytoplasm of endothelial capillaries.
The Type II alveolocytes are bigger than respiratory ones. They are cells with cubic form, which are
projected into the alveoli lumen. Due to dense contacts the secretory alveolocytes are joined with respiratory
ones. On the surface, faced the alveolus cavity, secretory alveolocytes have microvilli, and their cytoplasm
contains secretory granules.
Apart from the Type II alveolocytes, another rather large orbicular cells, i.e., alveolar macrophages,
project into the alveoli lumen from the alveolar walls. Cytolemma of alveolar macrophages has numerous
folds, containing phagocytic particles. Cytoplasm of alveolar macrophages is of basophil origin and contains
numerous phagosomes, lysosomes and pinocytotic vesicles. Nuclei are small and of orbicular, bean-shaped
or of irregular form and contain large lumps of chromatin.
The Type III alveolocytes, similar to secretory ones, contain microvilli on the free surface of the
cytoplasm, and their cytoplasm contains numerous vesicles.
The areas of destruction of cytoplasmic processes of the Type I alveolocytes, as well as
micropinocytotic vesicles, accumulated in them, were detected in some of the test samples of rats normal
lung tissue. Rejection of destroyed respiratory alveolocytes leads to exposure of basal membrane and
alternation of damaged areas of alveoli epithelium with invariable ones. In the lumen of alveoli near
damaged areas numerous cells on different stages of destruction, fibrin and individual red blood cells have
been observed. In the hollows of some alveoli few destroyed and undamaged alveolar macrophages and the
Type II alveolocytes were also found.
According to our observations, histological structure of rats intrapulmonary bronchi varies,
depending on the bronchus size, but has common features. The wall of intrapulmonary bronchi consists of
four membranes: mucous, submucous, fibro- cartilaginous and adventitious. The less is the bronchus size,
the less sizes are cartilages in the fibro- cartilaginous membrane. Only smooth myocytes are detected in the
wall of bronchial tubes.
Epithelium of mucous membrane of bronchial tubes is presented by double-row ciliary epithelium
and in bronchial tubes of smaller size by the one-row ciliary epithelium. In the bronchial tubes among
epithelial cells, except ciliary, goblet, endocrine and basal ones, the Clara's secretory cells are detected, as
well as brush cells.
35
European International Journal of Science and Technology
ISSN: 2304-9693
www.eijst.org.uk
Proper lamina of mucous membrane of bronchial tubes is thin and contains collagenous and elastic
fibers, few cellular elements. Muscle plate of mucous membrane of bronchial tubes of small size is
significantly evident as compared with thickness of the entire wall. It is less developed in bronchial tubes as
compared with bronchi of bigger diameter.
In the mucous membrane of rats bronchial tubes elements of local immune barrier, i.e., lymphocytes
and lymphoid nodules have been detected.
In contrast to histological structure of submucous connective tissue layer of other bronchi, the
absence of trophochrome glands and fibrocartilage membrane in the histological structure of submucous
connective tissue layer of bronchial tubes has been detected.
In the outer adventitious membrane of bronchial tubes a fibrous connective tissue has been detected.
Its fibers blend with interstitial connective tissue.
Histological study of rats medium bronchi stated that its inner membrane was mucous, lined with
monolayer plural-row ciliated epithelium, consisted of epithelial cells of different structure and functional
purpose. Epithelial cells of mucous membrane of medium bronchi are of various height, their nuclei are
located on the different levels, composing several rows. The less is bronchi size, the less is epithelium height
of its mucous membrane, due to change of cells shape from high prismatic to low cubical ones. Among
epithelial cells, except ciliated ones, goblet, endocrine and basal ones are detected. Ciliated cells have
ciliated cilia, which facilitate excretion of mucus and dust particles from the bronchi lumen. Goblet cells,
excreting mucin, are located between ciliated ones. Neuroendocrine cells are few and isolated; its cytoplasm
contains small dense granules. Basal or cambial cells are cubical with optically dense nucleus and small
amount of low-basophilic cytoplasm. They preserved the ability for mitotic division and are located in the
basal layer.
One of the peculiarities of epithelium structure of bronchi mucous membrane is the location of cells
nuclei on the various levels in relation to the basal membrane. Nuclei are of orbicular shape with evenly
colored chromatin and one nucleolus. One cells (ciliated, goblet ones) reach the surface of epithelial layer,
and the other (basal ones) do not reach it.
Proper lamina of mucous membrane of medium bronchi, where epithelial layer is located, is
indurated; the basal membrane is of sharp contour.
The middle part of the proper lamina contains numerous longitudinal elastic fibers, providing
bronchi with the ability to stretch.
On the boundary of mucous membrane and submucous layer, the muscle plate has been identified,
generated by obliquely circular fascicles of smooth myocytes.
Histological study of rats medium bronchi showed that the end parts of mixed trophochrome glands
were laid down here. Excretory ducts of these glands are opened on the surface of epithelium; their
secretion moistens the mucosa and facilitates the excretion of foreign particles from the bronchi lumen.
On the histological sections of medium bronchi the fibrocartilaginous membrane is presented by the
cartilaginous plates of irregular shape and insulae of elastic cartilaginous tissue. On the cross-sections of
medium bronchi, the cartilaginous plates are of crescent or oval shape. Spaces between cartilages are filled
with connective tissue. Cellular elements are not numerous, with prevailing majority of fiber component.
The outer adventitious membrane is formed by the fibrous connective tissue, which blends with
interparticle tissue of lung. Among cells of hematogenous origin, lymphocytes, macrophages and mastocytes
are detected, which participates in regulation of local homeostasis. Interstitial connective tissue mainly
consists of elastic and collagenous fibers and amorphous substance, and also contains resident cells
fibroblasts and migratory cells macrophages, lymphocytes, plasmacytes and mastocytes. Blood (arteries,
36
European International Journal of Science and Technology
Vol. 3 No. 3
April, 2014
veins) and lymphatic vessels, as well as nerve fibers, are located here. Collagenous fibers are randomly
located in different directions, are of multiple length and undulating coiled shape.
Elastic fibers are thinner than collagenous ones; on the cross-section the elastic fibers were of
orbicular or oblate shape.
Blood supply of lungs is performed on two vessel systems. Venous blood is supplied to lungs on
pulmonary arteries of lesser circulation. Branches of pulmonary artery accompany bronchial tree.
Stenosinuous capillary network is formed at the tela of alveoli. Each alveolar duct corresponds to arteriole,
from which precapillaryies are branched and bifurcated into capillaries. The latter blend with postcapillaries,
gathered into venule.
Arterioles are the smallest arterial vessels of muscle type, which walls are composed of low-grade
three tunics.
Inner tunic is formed by the endothelial cells, basal membrane, thin subendothelial layer and thin
inner elastic membrane.
Middle tunic consists of 1-2 circular layers of smooth myocytes. Bigger arterioles have the outer
elastic membrane.
Outer tunic of arterioles consists of collagenous and elastic fibers.
Capillaries are contiguous with one alveole by one of their surface, and with adjacent alveole by
another one. In the alveolar blood capillaries the red blood cells are arranged in one row, creating optimal
conditions for gas exchange. Blood capillaries are covered with continuous layer of endotheliocytes, located
on the basal membrane. Pre-nucleate part of endothelial cell (perikaryon) is thickened, and peripheral part is
thinned. Plasmalemma of endothelial cells are covered with thin glycocalyx, has cytoplasmic processes of
multiple length and protrusions inside cytoplasm, forming micropinocytic vesicles to transfer substances
through endothelium. On the periphery, cytoplasm of endotheliocytes becomes thinner, stretching along the
basal membrane to considerable distances, forming the thinnest portions of aerohaematic barrier.
Basal membrane of endothelium, similar to alveolar epithelium, is formed of thin intertwined fibrils,
deepened into basal substance of connective tissue. In thin portions of aerohaematic barrier, the basal
membranes of endothelium and epithelium are approaching each other in ways that look like generic basal
membrane.
In some areas the cells with fibrils in cytoplasm, i.e., pericytes, are identified in breakdowns of basal
membrane, which are probable precursors of fibroblasts and smooth myocytes and sources of synthesis of
components of basal substance of connective tissue.
Capillaries on their venous end transit into postcapillary venules, and the greater diameter is, the
greater number of pericytes is. Such venules flow into gathering venules, which, apart from the pericytes,
have also outer tunic, which is formed from the fibroblasts and collagenous fibers. Internally, venules are
covered with endotheliocytes, which in some parts are dense and in other parts fissures are formed between
their membranes. Just after the endothelium the basal membrane is detected. Along or around venous
endothelial tube the processes of pericytes are stretching. Pericytes and their processes are also covered by
the basal membrane, on the outer surface of which the collagenous fibers are located.
The special feature of hemomicrocirculatory flow of rats normal lungs is the occurrence of
erythrocyte aggregation in the capillaries of alveolar septums. Some of the capillaries are fully filled with
agglutinate erythrocytes. In the areas of erythrocyte aggregation, especially if it is accompanied by
erythrocyte adhesion to cytoplasmic processes of endotheliocytes, a local damage, destruction and
demolition of cytolemma of capillaries endothelium is detected. In some cases in the cytoplasm of damaged
endotheliocytes the dense inclusions are identified. Rejection of destroyed endotheliocytes leads to
endothelium basal membrane exposure and accumulation of pinocytic vesicles in the lumen of capillaries.
37
European International Journal of Science and Technology
ISSN: 2304-9693
www.eijst.org.uk
CONCLUSION
The study of structure of rats lungs in control group concluded that rats normal lungs had specific
generic histological features. The areas of destruction of cytoplasmic processes of the Type I alveolocytes
and accumulation of micropinocytotic vesicles in them were observed in some of the test samples of rats
normal lung tissue. Rejection of destroyed respiratory alveolocytes leads to exposure of basal membrane
and alternation of damaged areas of alveoli epithelium with invariable ones. In the lumen of alveoli near
damaged areas numerous cells on different stages of destruction, fibrin and individual red blood cells have
been detected. In the hollows of some alveoli few destroyed and undamaged alveolar macrophages and the
Type II alveolocytes have been found. The special feature of hemomicrocirculatory flow of rats normal
lungs is the occurrence of erythrocyte aggregation in the capillaries of alveolar septums. Some of the
capillaries are fully filled with agglutinate erythrocytes. In the areas of erythrocyte aggregation, especially if
it is accompanied by erythrocyte adhesion to cytoplasmic processes of endotheliocytes, a local damage,
destruction and demolition of cytolemma of capillaries endothelium have been detected. In some cases in the
cytoplasm of damaged endotheliocytes the dense inclusions have been identified. Rejection of destroyed
endotheliocytes leads to endothelium basal membrane exposure and accumulation of pinocytic vesicles in
the lumen of capillaries.
Thus, rats normal lungs have certain specific differences of anatomical organization, which should
be taken into consideration while carrying out experimental studies.
REFERENCES
1. Bryndina I.G., Danilov G.Ye. (2002). Substantsia kak factor, povyshayuschiy ustoichivost
surfaktnoi sistemy lyegkikh k khronicheskomy immobilizatsionnomy stressu, Rossiyskiy fisiologicheskiy
zhurnal, 1, 84-89.
2. Danilov L.N., Lyebedeva Ye.S., Kirilov Yu.A. (2005). Modelirovaniye zabolyevaniy lyegkikh,
Posobiye dlya nauchnykh rabotnikov. S-Pb, p.31.
3. Zaitseva K.K., Simonenkova V.A., Komar Yu.A. (1985). Ultrastrukturnaya organizatsiya
aerogematicheskogo baryera legkikh laboratornykh zhyvotnykh. Arkh. Anat. Gist. i Embriol, 9, 59-66.
4. Zakon Ukrayiny Pro zakhyst tvaryn vid zhorstokogo povodzhennya (2006), No. 3447 IV
issued 21.02.2006, p.18.
5. Porivnyalni dani pro rozpovsyudzhennist khvorob organiv dykhannya i medychnu dopomogu
khvorym na khvoroby pulmonologichnogo ta alergologichnogo profilyu v Ukrayini za 2006-2012 rr.
[Online] Available: http://www.ifp.kiev.ua/doc/staff/pulmukr2012.pdf (2013).
6. European Convention for the Protection of Vertebrate Animals Used for Experimental and Other
Scientific Purposes (1986). Council of Europe, Strasbourg, p. 53.
38
Das könnte Ihnen auch gefallen
- Pulmonary Endocrine Pathology: Endocrine Cells and Endocrine Tumours of the LungVon EverandPulmonary Endocrine Pathology: Endocrine Cells and Endocrine Tumours of the LungNoch keine Bewertungen
- Milestones in Immunology: Based on Collected PapersVon EverandMilestones in Immunology: Based on Collected PapersNoch keine Bewertungen
- Anatomy and Histology of The Normal Lung and AirwaysDokument20 SeitenAnatomy and Histology of The Normal Lung and AirwaysFahd Abdullah Al-refaiNoch keine Bewertungen
- NHLBI Workshop Summary: The Mysterious Pulmonary Brush CellDokument4 SeitenNHLBI Workshop Summary: The Mysterious Pulmonary Brush CellcholoconvertiNoch keine Bewertungen
- Respiratory DisordersDokument20 SeitenRespiratory DisordersjeromeNoch keine Bewertungen
- Cyto Lungtumorbook1Dokument104 SeitenCyto Lungtumorbook1ixNoch keine Bewertungen
- Final Written Report 4)Dokument13 SeitenFinal Written Report 4)chocoholic potchiNoch keine Bewertungen
- Histology RespiDokument4 SeitenHistology RespiKris TejereroNoch keine Bewertungen
- 2nd Lec of Respiratory Histology by DR RoomiDokument39 Seiten2nd Lec of Respiratory Histology by DR RoomiMudassar Roomi100% (1)
- Diagnostic Examination of Pulmonary TuberculosisDokument5 SeitenDiagnostic Examination of Pulmonary TuberculosisMark BerbanoNoch keine Bewertungen
- Ijms-21-03075 IniDokument14 SeitenIjms-21-03075 IniMila KarmilaNoch keine Bewertungen
- Assignment NO 1: Name:Adeel Afzal Section: C Sap I'd: 70126263 Assignment: Physiology Submitted To: DR Urfah ZaighamDokument6 SeitenAssignment NO 1: Name:Adeel Afzal Section: C Sap I'd: 70126263 Assignment: Physiology Submitted To: DR Urfah ZaighamAdeel AfzalNoch keine Bewertungen
- Ultrastructural Changes in Alveolar Epithelium in Response To Freund's Adjuvant (Faulkner & Esterly 1971)Dokument16 SeitenUltrastructural Changes in Alveolar Epithelium in Response To Freund's Adjuvant (Faulkner & Esterly 1971)gd_hbarNoch keine Bewertungen
- INTRODUCTIONDokument43 SeitenINTRODUCTIONNikita NishanNoch keine Bewertungen
- Upper Respiratory TractDokument6 SeitenUpper Respiratory TractGeraldine Gallaron - CasipongNoch keine Bewertungen
- Respiratory Histology by DR R.MDokument44 SeitenRespiratory Histology by DR R.Mdr_RMNoch keine Bewertungen
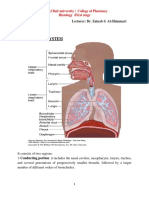
- Respiratory System: A) The Conducting Portion, Which Consists of The Nasal CavitiesDokument7 SeitenRespiratory System: A) The Conducting Portion, Which Consists of The Nasal CavitiesSalmah Alimah FitriNoch keine Bewertungen
- Human Lung DevelopmentDokument20 SeitenHuman Lung DevelopmentMila KarmilaNoch keine Bewertungen
- The Oropharynx Is Located Posterior To The Oral Cavity and Extends From The Soft Palate To The Level of The Hyoid BoneDokument6 SeitenThe Oropharynx Is Located Posterior To The Oral Cavity and Extends From The Soft Palate To The Level of The Hyoid BoneCecilia PerezNoch keine Bewertungen
- Pathophysiology of Respiratory System Feghiu I., Tacu L. Lutan VDokument55 SeitenPathophysiology of Respiratory System Feghiu I., Tacu L. Lutan VLunguVictoriaNoch keine Bewertungen
- 4 RESPIRATORY Lec4Dokument11 Seiten4 RESPIRATORY Lec4Tee bagNoch keine Bewertungen
- Pulmonary TuberculosisDokument6 SeitenPulmonary Tuberculosisjenilay0% (1)
- PTB Case StudyDokument6 SeitenPTB Case StudyTrisha Carolina SottoNoch keine Bewertungen
- Bilateral Nasolabial Cyst MarcoviceanuDokument4 SeitenBilateral Nasolabial Cyst MarcoviceanubamsusiloNoch keine Bewertungen
- Department of Histology, Cytology and EmbryologyDokument8 SeitenDepartment of Histology, Cytology and Embryologysubmen81Noch keine Bewertungen
- lr4 - ILHAASHINI (012021090323) - LAB 4Dokument6 Seitenlr4 - ILHAASHINI (012021090323) - LAB 4Ilhaashini krishnanNoch keine Bewertungen
- Respiratory System 2Dokument8 SeitenRespiratory System 2Shawn Gaurav JhaNoch keine Bewertungen
- TonsilasDokument15 SeitenTonsilasLuz InfanteNoch keine Bewertungen
- Adeno Tonsilitis SlideDokument17 SeitenAdeno Tonsilitis SlideChowdhury Mohammed Tawhid TasneefNoch keine Bewertungen
- Respiratory System HistologyDokument70 SeitenRespiratory System HistologytazeNoch keine Bewertungen
- Practical No 28 - Respiratory SystemDokument8 SeitenPractical No 28 - Respiratory Systemkartik.patil151106Noch keine Bewertungen
- Normal Histology of The Lung and Pathophysiology of COPD (T Year)Dokument36 SeitenNormal Histology of The Lung and Pathophysiology of COPD (T Year)khaledalameddineNoch keine Bewertungen
- Broncho PneumoniaDokument23 SeitenBroncho Pneumoniaanon-84769398% (43)
- Respritory System Part2Dokument30 SeitenRespritory System Part2Deniz asmazNoch keine Bewertungen
- Neonatal Respiration PhysiologyDokument6 SeitenNeonatal Respiration PhysiologyRaja Ahmad Rusdan MusyawirNoch keine Bewertungen
- Asthama - Pathology and PathophysiologyDokument5 SeitenAsthama - Pathology and PathophysiologyTejal ParulkarNoch keine Bewertungen
- Tonsillitis, Peritonsillarand Lateralpharyngeal Abscesses: Jonathan M. Tagliareni,, Earl I. ClarksonDokument8 SeitenTonsillitis, Peritonsillarand Lateralpharyngeal Abscesses: Jonathan M. Tagliareni,, Earl I. ClarksonAlfianRismawanNoch keine Bewertungen
- Respiratory System Development - EmbryologyDokument1 SeiteRespiratory System Development - EmbryologyRicardo david Reyes alvarezNoch keine Bewertungen
- RespiratoryDokument1 SeiteRespiratoryJames Gabriel SalardaNoch keine Bewertungen
- 1st Lecture of Respiratory Histology by DR RoomiDokument24 Seiten1st Lecture of Respiratory Histology by DR RoomiMudassar Roomi100% (1)
- Matthew E. Levison: PneumoniaDokument30 SeitenMatthew E. Levison: PneumoniaWira AisyaNoch keine Bewertungen
- Understanding The Nasal AirwayDokument21 SeitenUnderstanding The Nasal AirwayGera AguilarNoch keine Bewertungen
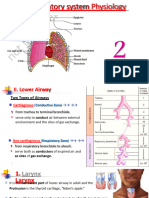
- 2- الجاهزة20.02.2022 lower Respiratory system physiologyDokument32 Seiten2- الجاهزة20.02.2022 lower Respiratory system physiologybessan alfqeatNoch keine Bewertungen
- Binant Et Al 2012Dokument5 SeitenBinant Et Al 2012Rachel AutranNoch keine Bewertungen
- Tight Junctions in Pulmonary Epithelia During Lung InflammationDokument13 SeitenTight Junctions in Pulmonary Epithelia During Lung InflammationbiancacampitelliNoch keine Bewertungen
- Nasopharynx, Larynx, Trachea, Bronchi, Bronchioles and Terminal BronchiolesDokument3 SeitenNasopharynx, Larynx, Trachea, Bronchi, Bronchioles and Terminal BronchiolesMichael UrrutiaNoch keine Bewertungen
- Invasive Epithelial Mesothelioma in A Dog: F. R, B. B, K. R, E. S, D. BDokument5 SeitenInvasive Epithelial Mesothelioma in A Dog: F. R, B. B, K. R, E. S, D. BjamesyuNoch keine Bewertungen
- Laporan Tutorial Blok 3.1: Disusun Oleh: Kelompok 2B Anggota KelompokDokument14 SeitenLaporan Tutorial Blok 3.1: Disusun Oleh: Kelompok 2B Anggota KelompokrezaNoch keine Bewertungen
- Sistema Respiratório InferiorDokument7 SeitenSistema Respiratório InferiorGero CabralNoch keine Bewertungen
- Respiratory EpitheliumDokument33 SeitenRespiratory EpitheliumMaham BaqaiNoch keine Bewertungen
- Respiratory System and AnemiaDokument112 SeitenRespiratory System and AnemiaJorie RocoNoch keine Bewertungen
- 1-Respiratory MechanicsDokument56 Seiten1-Respiratory MechanicsDoha JKNoch keine Bewertungen
- Avian Respiratory DistressDokument15 SeitenAvian Respiratory DistressJessica RuizNoch keine Bewertungen
- Bronchial Asthma ThesisDokument33 SeitenBronchial Asthma ThesisAbdul Hafiz40% (5)
- 234 FarmerDokument6 Seiten234 FarmerRaghavendra NalatawadNoch keine Bewertungen
- Histology of Lower Respiratory TractDokument42 SeitenHistology of Lower Respiratory TractRahma ShofiNoch keine Bewertungen
- Options For Histological Study of The Structure and Ultrastructure of Human Urinary Bladder EpitheliumDokument8 SeitenOptions For Histological Study of The Structure and Ultrastructure of Human Urinary Bladder EpitheliumSergio CerveraNoch keine Bewertungen
- Cleft Lip and Palate Management: A Comprehensive AtlasVon EverandCleft Lip and Palate Management: A Comprehensive AtlasRicardo D. BennunNoch keine Bewertungen
- Research in ProtozoologyVon EverandResearch in ProtozoologyTze-Tuan ChenNoch keine Bewertungen
- Obesity Prevention and Management: Patient Population: Objectives: Key PointsDokument14 SeitenObesity Prevention and Management: Patient Population: Objectives: Key PointsManMan AR-llNoch keine Bewertungen
- 2016 Maret: Kamis Rabu Selasa Jum at SeninDokument2 Seiten2016 Maret: Kamis Rabu Selasa Jum at SeninManMan AR-llNoch keine Bewertungen
- Cover Responsi - Kelompok 2 TiminDokument1 SeiteCover Responsi - Kelompok 2 TiminManMan AR-llNoch keine Bewertungen
- PICAPS Susunan Acara Gala Dinner PartyDokument1 SeitePICAPS Susunan Acara Gala Dinner PartyManMan AR-ll50% (2)
- Daftar Calon Tutor Pendikar Muslim 2015Dokument22 SeitenDaftar Calon Tutor Pendikar Muslim 2015ManMan AR-llNoch keine Bewertungen
- Standardization of Pharmacokinetic/pharmacodynamic (PK/PD) Terminology For Anti-Infective DrugsDokument4 SeitenStandardization of Pharmacokinetic/pharmacodynamic (PK/PD) Terminology For Anti-Infective DrugsManMan AR-llNoch keine Bewertungen
- Krstic Et Al. 2002 - ForensicDokument6 SeitenKrstic Et Al. 2002 - ForensicManMan AR-llNoch keine Bewertungen
- Commentary Biowaiver Monographs For Immediate Release Solid Oral Dosage Forms: CimetidineDokument11 SeitenCommentary Biowaiver Monographs For Immediate Release Solid Oral Dosage Forms: CimetidineManMan AR-llNoch keine Bewertungen
- Computer Vision Syndrome (CVS) : Recognition and Control in Software ProfessionalsDokument3 SeitenComputer Vision Syndrome (CVS) : Recognition and Control in Software ProfessionalsManMan AR-ll100% (1)
- DPNA Pendikar Muslim Untan 2013-2014Dokument88 SeitenDPNA Pendikar Muslim Untan 2013-2014ManMan AR-llNoch keine Bewertungen
- Hypertensive CrisisDokument13 SeitenHypertensive Crisis.Katherine CalderonNoch keine Bewertungen
- Pregnancy ComplicationsDokument14 SeitenPregnancy Complicationsvienny kayeNoch keine Bewertungen
- Histological Comparison of Pulpal Inflamation in Primary Teeth With Occlusal or Proximal CariesDokument8 SeitenHistological Comparison of Pulpal Inflamation in Primary Teeth With Occlusal or Proximal CariesGilmer Solis SánchezNoch keine Bewertungen
- بنك الأسئلةDokument775 Seitenبنك الأسئلةسماح صلاح100% (1)
- ICU One Pager NIPPVDokument1 SeiteICU One Pager NIPPVNicholas HelmstetterNoch keine Bewertungen
- Funda DemoDokument10 SeitenFunda DemoariadnebabasaNoch keine Bewertungen
- Clear Sky Recovery's Ibogaine Treatment Program To Be Featured On National GeographicDokument3 SeitenClear Sky Recovery's Ibogaine Treatment Program To Be Featured On National GeographicPR.comNoch keine Bewertungen
- Buku Panduan Mahasiswa Blok 11 2021-2022 (Kurikulum 2012) - 25-34Dokument10 SeitenBuku Panduan Mahasiswa Blok 11 2021-2022 (Kurikulum 2012) - 25-34Qonita AztNoch keine Bewertungen
- Breast Cancer Mind MapDokument1 SeiteBreast Cancer Mind Maprjbar arian 69Noch keine Bewertungen
- Liver BiopsyDokument3 SeitenLiver BiopsyBiway RegalaNoch keine Bewertungen
- ISSN: 0975-833X: Case StudyDokument4 SeitenISSN: 0975-833X: Case StudyantonNoch keine Bewertungen
- Cells in The PBSDokument31 SeitenCells in The PBSDelzell Dame CasaneNoch keine Bewertungen
- 2012 OiteDokument256 Seiten2012 Oiteaddison wood100% (4)
- Nursing Care of CHF (Congestive Heart Failure)Dokument25 SeitenNursing Care of CHF (Congestive Heart Failure)Irwan100% (2)
- DR - Jetty - Executive Summary of Perioperative ManagementDokument28 SeitenDR - Jetty - Executive Summary of Perioperative ManagementDilaNoch keine Bewertungen
- Gastroenterology and HepatologyDokument42 SeitenGastroenterology and HepatologyCarlos HernándezNoch keine Bewertungen
- Vaccination Certificate 20435699572410Dokument1 SeiteVaccination Certificate 20435699572410MANOJ BHADANENoch keine Bewertungen
- Chapter XNXXXDokument3 SeitenChapter XNXXXclaudette100% (2)
- Diarrhoea Patient InformationDokument3 SeitenDiarrhoea Patient InformationIgor DemićNoch keine Bewertungen
- Ho CocDokument48 SeitenHo Cocmehadi100% (1)
- Exchange Blood Transfusion 2Dokument15 SeitenExchange Blood Transfusion 2Sarah100% (1)
- Health Grade10 4th QuarterDokument40 SeitenHealth Grade10 4th QuarterYnjel HilarioNoch keine Bewertungen
- 1000 Mcqs - Periodontics Plus September 2014 McqsDokument16 Seiten1000 Mcqs - Periodontics Plus September 2014 McqsSelvaArockiam86% (7)
- Failure of Weaning:: According To The European Respiratory Society (ERS) Task ForceDokument12 SeitenFailure of Weaning:: According To The European Respiratory Society (ERS) Task ForceAmr El Taher0% (1)
- Acute Kidney InjuryDokument49 SeitenAcute Kidney InjuryfikasywNoch keine Bewertungen
- Contact Urticaria Syndrome: Occupational Aspects: Becky S. Li, Iris S. Ale, and Howard I. MaibachDokument34 SeitenContact Urticaria Syndrome: Occupational Aspects: Becky S. Li, Iris S. Ale, and Howard I. MaibachsaskiakonitaNoch keine Bewertungen
- Diabetes MellitusDokument75 SeitenDiabetes MellitusIndika KarunamuniNoch keine Bewertungen
- BSS 2018Dokument24 SeitenBSS 2018gumoha01Noch keine Bewertungen
- Fetal DistressDokument111 SeitenFetal DistressSakariye SuleimanNoch keine Bewertungen
- Chapter 11 Obesity and NutritionDokument14 SeitenChapter 11 Obesity and NutritionAlan ShimNoch keine Bewertungen