Beruflich Dokumente
Kultur Dokumente
Egg Lab
Hochgeladen von
Melanie KileOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Egg Lab
Hochgeladen von
Melanie KileCopyright:
Verfügbare Formate
Melanie Kile
SCI 101 Mondays
Egg Lab
The purpose of our egg lab is to see what happens to an egg when it has sat in
vinegar, water and then syrup. Will the egg be permeable to these substances or not?
First take an egg and get it’s initial weight from a food scale or what have you.
Record the weight and date. Next fill a jar or plastic container with vinegar and place
your egg inside, the vinegar should mostly cover the egg. Wait a week then remove the
egg and weigh it, take note of its appearance and size as well. Repeat these steps with
water and syrup allowing a week in between for the egg to sit.
After a week in vinegar we saw that the egg had lost its shell and swelled a bit. To
begin with the egg weighed 59.9g and now it weighed 86.4g. This tells me that the
vinegar molecules were smaller than that of the eggs outer membrane. Next we were to
see if osmosis would occur. We placed the egg in water and waited a week. The egg then
weighed 91g bringing it up from 86.4g. Osmosis did occur, the egg swelled some more
and looked translucent. Next we placed the egg in syrup and waited a week. Our weigh in
for the syrup brought us down to 36g. This tells me that the egg was hypertonic which
means the water in the egg diffused outward into the hypertonic syrup, the egg weighed
in at 36g which means the syrup was most likely a bigger molecule that the eggs surface
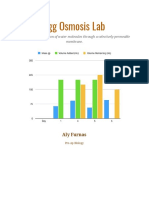
could not allow to pass through. Take a look at the following chart which shows the
results of four different eggs being tested.
1/31 2/3 2/7 2/10 2/14
BOB 60.8g 89.2g 85.3g 39g 41.5g
JEN 59.9g 86.4g 91g 36g 31.8g
HUMPTY 59.9g 89.5g 95g 43g 41.3g
SALLY FIELD 55.7g 80.8g 82.5g 32.2g 35.6g
(raw) (vinegar) (water) (syrup) (last reading)
When performing this lab I was unsure of many things. I knew the acids in the
vinegar would eat away the eggs shell but I didn’t expect the egg to receive the vinegar as
much as it did. Nor did I expect the egg to allow even more water to diffuse into. When
the egg was placed in syrup though, I predicted that the egg would shrivel through
osmosis, which it did.
This was a great lab to demonstrate osmosis as well as hypertonic and hypotonic
situations. Vinegar, water and syrup are great liquids to use to demonstrate this and I
think it would be interesting to see how other liquids effect the egg. This lab gave me a
clearer understanding of how osmosis and hypotonic and hypertonic situations work.
Das könnte Ihnen auch gefallen
- Lost Secrets of Baseball HittingDokument7 SeitenLost Secrets of Baseball HittingCoach JPNoch keine Bewertungen
- Lab Report (Egg and Osmosis)Dokument3 SeitenLab Report (Egg and Osmosis)almira garciaNoch keine Bewertungen
- 6lab207 Egg in Vinegar ExperimentDokument2 Seiten6lab207 Egg in Vinegar ExperimentHarry CaoNoch keine Bewertungen
- Egg Osmosis LabDokument3 SeitenEgg Osmosis Labapi-371081865Noch keine Bewertungen
- Egg Osmosis LabDokument4 SeitenEgg Osmosis Labnik farisNoch keine Bewertungen
- The Peppers, Cracklings, and Knots of Wool Cookbook The Global Migration of African CuisineDokument486 SeitenThe Peppers, Cracklings, and Knots of Wool Cookbook The Global Migration of African Cuisinemikefcebu100% (1)
- Incredible Egg Lab: 1. TITLEDokument2 SeitenIncredible Egg Lab: 1. TITLEapi-296931788Noch keine Bewertungen
- Egg LabDokument6 SeitenEgg Labapi-540200599Noch keine Bewertungen
- Egg in Vinegar Research PaperDokument7 SeitenEgg in Vinegar Research Paperc9hpjcb3Noch keine Bewertungen
- Osmosis and The Egg Lab ReportDokument3 SeitenOsmosis and The Egg Lab ReportVale EspinelNoch keine Bewertungen
- Egg Lab: Charlotte NealDokument4 SeitenEgg Lab: Charlotte Nealapi-391552921Noch keine Bewertungen
- Laboratory Worksheet BIO 1 (NAKED EGG) 2Dokument7 SeitenLaboratory Worksheet BIO 1 (NAKED EGG) 2JC SantosNoch keine Bewertungen
- Egg Osmosis LabDokument4 SeitenEgg Osmosis Labapi-371081506Noch keine Bewertungen
- Bjective of The XperimentDokument5 SeitenBjective of The XperimentWhitneythegoddessNoch keine Bewertungen
- Magic Egg ExperimentDokument2 SeitenMagic Egg Experimentapi-505898069Noch keine Bewertungen
- Names: Course & Year: BSED-SCCIENCE 3: Osmosis EggsDokument8 SeitenNames: Course & Year: BSED-SCCIENCE 3: Osmosis EggsNichole AlbaracinNoch keine Bewertungen
- What Is The Effect of Vinegar and SyrupDokument6 SeitenWhat Is The Effect of Vinegar and Syrupmyesha_oNoch keine Bewertungen
- ChemistryDokument3 SeitenChemistryEduardo Sebastián Sosa HernándezNoch keine Bewertungen
- Egg Lab: Andrew WinkleDokument2 SeitenEgg Lab: Andrew Winkleapi-391704115Noch keine Bewertungen
- Egg CoagulationDokument1 SeiteEgg CoagulationisabelmirakeltooNoch keine Bewertungen
- Pre-Activities: Do The Egg ExperimentDokument50 SeitenPre-Activities: Do The Egg ExperimentMarry Jane Lustre CanabalNoch keine Bewertungen
- Mosenabre - ACTIVITY-2-Osmosis (BSN1-7)Dokument8 SeitenMosenabre - ACTIVITY-2-Osmosis (BSN1-7)Charade MosenabreNoch keine Bewertungen
- Bouncing Egg ExperimentDokument4 SeitenBouncing Egg ExperimentkristelNoch keine Bewertungen
- Osmosis Egg MassDokument2 SeitenOsmosis Egg Massapi-239937542Noch keine Bewertungen
- Egg-Speriment PresentationDokument11 SeitenEgg-Speriment Presentationapi-238168777Noch keine Bewertungen
- Week 1 Lab BIO 101Dokument2 SeitenWeek 1 Lab BIO 101Amber BlackmoreNoch keine Bewertungen
- Omosis LabDokument3 SeitenOmosis Labapi-194648770Noch keine Bewertungen
- JHS Modules 1ST QuarterDokument11 SeitenJHS Modules 1ST QuarterJohnson FernandezNoch keine Bewertungen
- Research Paper On Eggs and VinegarDokument6 SeitenResearch Paper On Eggs and Vinegarzeiqxsbnd100% (1)
- Osmosis LabDokument3 SeitenOsmosis LabAlisa LeNoch keine Bewertungen
- 11-HE Cookery Week 1Dokument25 Seiten11-HE Cookery Week 1LD 07Noch keine Bewertungen
- Naked Egg ExperimentDokument7 SeitenNaked Egg ExperimentAubrey PerezNoch keine Bewertungen
- Sarah Green: Scientific ExperimentDokument7 SeitenSarah Green: Scientific Experimentapi-350322099Noch keine Bewertungen
- RecipeDokument5 SeitenRecipeapi-612427779Noch keine Bewertungen
- Osmosissabrinasharmaandlaurengarcia StilleDokument11 SeitenOsmosissabrinasharmaandlaurengarcia Stilleapi-312048131Noch keine Bewertungen
- Egg Lab ReportDokument5 SeitenEgg Lab Reportapi-340581896Noch keine Bewertungen
- Lesson 1: Composition: Parts of An EggDokument22 SeitenLesson 1: Composition: Parts of An Eggjohn michael pagalaNoch keine Bewertungen
- Group 2: Laboratory Sheet # 2.1 "Naked Egg Experiment"Dokument2 SeitenGroup 2: Laboratory Sheet # 2.1 "Naked Egg Experiment"Mikel NinalgaNoch keine Bewertungen
- Egg Osmosis LabDokument5 SeitenEgg Osmosis Labapi-391626668Noch keine Bewertungen
- Amazing Spinning Egg!Dokument2 SeitenAmazing Spinning Egg!Powerhouse MuseumNoch keine Bewertungen
- Práctica de Laboratorio.Dokument5 SeitenPráctica de Laboratorio.Juan PabloNoch keine Bewertungen
- Egg Lab: HypothesisDokument4 SeitenEgg Lab: Hypothesisapi-391720200Noch keine Bewertungen
- Eggs 101 PDFDokument27 SeitenEggs 101 PDFtryabcdefNoch keine Bewertungen
- Egg Osmosis LabDokument11 SeitenEgg Osmosis Labchizzy gNoch keine Bewertungen
- Egg Osmosis LabDokument2 SeitenEgg Osmosis Labapi-391540671Noch keine Bewertungen
- Egg Osmosis Lab 1Dokument6 SeitenEgg Osmosis Lab 1api-603235637Noch keine Bewertungen
- Osmosis Egg LabDokument3 SeitenOsmosis Egg Labapi-391711797Noch keine Bewertungen
- Science OsmosisDokument6 SeitenScience Osmosisapi-197380918Noch keine Bewertungen
- Osmosis Eggsperiment: NAME: Dianne M. Baniel Strand: 11 Stem 2 DATE: OCTOBER 10, 2019Dokument2 SeitenOsmosis Eggsperiment: NAME: Dianne M. Baniel Strand: 11 Stem 2 DATE: OCTOBER 10, 2019Cindy AgustinNoch keine Bewertungen
- Jumping EggDokument7 SeitenJumping Eggapi-302127208Noch keine Bewertungen
- Reviewer 2Dokument2 SeitenReviewer 2Yasmien MajulNoch keine Bewertungen
- Modeling OsmosisDokument3 SeitenModeling Osmosisapi-208339580Noch keine Bewertungen
- U.S. Egg Equivalents: Shell Eggs To Frozen/Liquid or Dried EggsDokument1 SeiteU.S. Egg Equivalents: Shell Eggs To Frozen/Liquid or Dried EggsJeremy ShepherdNoch keine Bewertungen
- Egg Osmosis PosterDokument2 SeitenEgg Osmosis Posterapi-495153723100% (1)
- Egg LabDokument3 SeitenEgg Labapi-283204633Noch keine Bewertungen
- Geitheim Modeling OsmosisDokument3 SeitenGeitheim Modeling Osmosisapi-208377940Noch keine Bewertungen
- ExperimentDokument2 SeitenExperimentSarah Jean TraballoNoch keine Bewertungen
- Food Science Lab Report 2Dokument9 SeitenFood Science Lab Report 2api-340272103100% (1)
- How To Preserve Eggs: Freezing, Pickling, Dehydrating, Larding, Water Glassing, & MoreVon EverandHow To Preserve Eggs: Freezing, Pickling, Dehydrating, Larding, Water Glassing, & MoreBewertung: 5 von 5 Sternen5/5 (1)
- Heavenly Deviled Eggs: Tips and Tricks for Fun, Flavorful FillingsVon EverandHeavenly Deviled Eggs: Tips and Tricks for Fun, Flavorful FillingsNoch keine Bewertungen
- Amazing (Mostly) Edible Science: A Family Guide to Fun Experiments in the KitchenVon EverandAmazing (Mostly) Edible Science: A Family Guide to Fun Experiments in the KitchenNoch keine Bewertungen
- Occupant Response To Vehicular VibrationDokument16 SeitenOccupant Response To Vehicular VibrationAishhwarya Priya100% (1)
- Medical Surgical Nursing Nclex Questions 5Dokument18 SeitenMedical Surgical Nursing Nclex Questions 5dee_day_8Noch keine Bewertungen
- Faust Part Two - Johann Wolfgang Von GoetheDokument401 SeitenFaust Part Two - Johann Wolfgang Von GoetherharsianiNoch keine Bewertungen
- Catalog Vacuum Circuit Breakers 3ah47 enDokument36 SeitenCatalog Vacuum Circuit Breakers 3ah47 enbajricaNoch keine Bewertungen
- Bibliography of Loyalist Source MaterialDokument205 SeitenBibliography of Loyalist Source MaterialNancyNoch keine Bewertungen
- Mohd Ali 17: By:-Roll NoDokument12 SeitenMohd Ali 17: By:-Roll NoMd AliNoch keine Bewertungen
- Project Dayan PrathaDokument29 SeitenProject Dayan PrathaSHREYA KUMARINoch keine Bewertungen
- Breast Cancer ChemotherapyDokument7 SeitenBreast Cancer Chemotherapydini kusmaharaniNoch keine Bewertungen
- Afghanistan Law Bibliography 3rd EdDokument28 SeitenAfghanistan Law Bibliography 3rd EdTim MathewsNoch keine Bewertungen
- MInor To ContractsDokument28 SeitenMInor To ContractsDakshita DubeyNoch keine Bewertungen
- Skin DseDokument9 SeitenSkin DsePapitas FritasNoch keine Bewertungen
- Measurement (Ques - Ch2 - Electromechanical Instruments) PDFDokument56 SeitenMeasurement (Ques - Ch2 - Electromechanical Instruments) PDFmadivala nagarajaNoch keine Bewertungen
- 4.dynamic Analysis of Earth Quake Resistante Steel FrameDokument28 Seiten4.dynamic Analysis of Earth Quake Resistante Steel FrameRusdiwal JundullahNoch keine Bewertungen
- Does Moore Succeed in Refuting IdealismDokument5 SeitenDoes Moore Succeed in Refuting IdealismharryNoch keine Bewertungen
- Introductory EconomicsDokument22 SeitenIntroductory Economicswedjefdbenmcve100% (1)
- The Old Man and The SeaDokument6 SeitenThe Old Man and The Seahomeless_heartNoch keine Bewertungen
- A Management and Leadership TheoriesDokument43 SeitenA Management and Leadership TheoriesKrezielDulosEscobarNoch keine Bewertungen
- MGMT 400-Strategic Business Management-Adnan ZahidDokument5 SeitenMGMT 400-Strategic Business Management-Adnan ZahidWaleed AhmadNoch keine Bewertungen
- Case Study On TQMDokument20 SeitenCase Study On TQMshinyshani850% (1)
- Gianna Pomata (Editor), Nancy G. Siraisi (Editor) - Historia - Empiricism and Erudition in Early Modern Europe (Transformations - Studies in The History of Science and Technology) (2006)Dokument493 SeitenGianna Pomata (Editor), Nancy G. Siraisi (Editor) - Historia - Empiricism and Erudition in Early Modern Europe (Transformations - Studies in The History of Science and Technology) (2006)Marcelo Rizzo100% (1)
- Sosa Ernest - Causation PDFDokument259 SeitenSosa Ernest - Causation PDFtri korne penal100% (1)
- Vocabulary Ladders - Grade 3 - Degree of FamiliarityDokument6 SeitenVocabulary Ladders - Grade 3 - Degree of FamiliarityfairfurNoch keine Bewertungen
- Supply Chain Analytics For DummiesDokument69 SeitenSupply Chain Analytics For DummiesUday Kiran100% (7)
- Acc 106 Ebook Answer Topic 4Dokument13 SeitenAcc 106 Ebook Answer Topic 4syifa azhari 3BaNoch keine Bewertungen
- Performance Task in Mathematics 10 First Quarter: GuidelinesDokument2 SeitenPerformance Task in Mathematics 10 First Quarter: Guidelinesbelle cutiee100% (3)
- PDF Document 2Dokument12 SeitenPDF Document 2Nhey VergaraNoch keine Bewertungen
- SKI Report2008 - 50 2Dokument46 SeitenSKI Report2008 - 50 2nada safitriNoch keine Bewertungen
- Chapter 2-EER and Relational Database SchemaDokument146 SeitenChapter 2-EER and Relational Database Schemagirmay tadeseNoch keine Bewertungen