Beruflich Dokumente
Kultur Dokumente
F334 Jan 06 - MS
Hochgeladen von
ExamStuffOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
F334 Jan 06 - MS
Hochgeladen von
ExamStuffCopyright:
Verfügbare Formate
Abbreviations, annotations and conventions used in the Mark Scheme
/ ; NOT () ecf AW ora
= = = = = = = =
alternative and acceptable answers for the same marking point separates marking points answers which are not worthy of credit words which are not essential to gain credit (underlining) key words which must be used to gain credit error carried forward alternative wording or reverse argument Expected answers Marks 1
Question 1 (a) 1 (b)
Improve properties / demand greater than nature can supply / reduce cost (1).
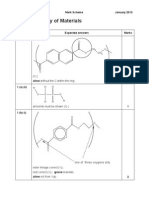
H H N (CH2)5
O C OH
1 (c) (i)
1 (c) (ii) 1 (d) (i) 1 (d) (ii) 1 (d) (iii) 1 (e) (i)
-COOH allow COCl (1); -NH2 (1) ONE of the groups must have the (CH2)5 for the second mark. 1,4-diaminobutane diaminobutane allow butyl/butan(e)diamine (1); 1,4 (1). ecf, 1,6-diaminohexane for 1 mark Any two from the following four points: lower Tg/ Tm/ strength/ rigidity ora (2). NOT b.p. nor density. 3 x 104 / 198 (1); 150152 (1) ecf for Mr. (Secondary) amide (1) NOT peptide. There will be greater number of hydrogen bonds (1); between chains (1); greater energy needed (to enable chains to move/flow) (1).
NH3+
+
2 2 1 3 2
H3N
may not be skeletal (1);
O HO
1 (e) (ii)
O (CH2)4 C OH (1).
ecf for C chain from (b).
H O H H N H O H O H O H N
Any one of the three atom arrangements above (1); Correct partial charges (1). Total 17
Question 2 (a) (i) 2 (a) (ii) +5 (1) accept 5+.
Expected answers
Marks 1 5
2 (b) (i) 2 (b) (ii) 2 (c) (i) 2 (c) (ii) 2 (d) (i)
hydrogen electrode (1); detailed drawing not required but should have H2 gas and H+(aq). a half-cell made from Pt (or C) dipping into a solution VO2+ and VO2+ ions (1); conditions given as 1 mol dm-3 /1M concentrations, 1 atmosphere pressure and 298 K (1); salt bridge dipping in solutions(1); voltmeter correctly connected (1). 0.74 V (1). B /V / V (may give more detail of half-cells) because it has the more negative/less positive electrode potential AW in terms of reducing agent/oxidizing agent or electron transfer (1). V3+ + e V2+ (1). V2+ + VO2+ + 2H+ V3+ + VO2+ + H2O Correct vanadium species in both reactants and products (1); equation given balanced correctly (1).
2+ 3+
1 1 1 2
3+
OH2 H2O V H2O OH2 OH2 OH2
Octahedral arrangement of ligands (1); O in H2O bonded to V for all ligands (1). Ignore charge on ion. 2 (d) (ii) 2
3d V V3+
Correct arrangement for V (1); correct arrangement for V3+ (1). 2 (d) (iii)
4s
Ligands cause/interact with d orbital/energy levels AW (1); to split into two groups / E = h or in words (1); visible light/frequencies absorbed to excite electrons (1); rest of visible light transmitted as colour AW (1). Total
19
Question 3 (a)
Expected answers 2-propylpentanoic acid pentanoic acid (1) 2-propyl (1) allow 2-propan(e) or propyl-2-/; Dipropylethanoic/dipropanethanoic acid gains 1 mark.
C3H7 H3C C O C C O CH2 CH3
Marks 2
3 (b)
C3H7 O
3 (c) (i) 3 (c) (ii) 3 (c) (iii) 3 (c) (iv) 3 (d)
Allow if only COO/both attached C atoms is/are circled. Prevent loss of product D/volatile reactants/gases (1). (Fractional) distillation (1). Add dilute hydrochloric/sulphuric/ acid (1). NOT conc. (Potassium) ethanoate/ CH3COO (K ) (1). C2H5OH (1); Any two from three: relative molar mass = 46 (1); since peak furthest right is due to molecular ion(may be shown on diagram) (1); any one use of fragmentation pattern e.g. peak at 29, due to ethyl group /difference between peaks at 29 and 45 = 16, suggests O present (1). chemical shift type of proton relative intensity from spectrum 1.0 1.4 2.2 3.7 CH3 CH2 O=CCH3 CH2OC=O/ CH3OC=O 9 8 3 2
+
1 1 1 1 3
3 (e)
3 (f)
two types of proton correctly identified (1); all four correct gains second mark (1); correct relative intensities (1). (Broad) peak around 25003200 cm-1 (1) indicates OH (in carboxylic acid) (1); (Strong) peak around 17001725 cm-1 (1) indicates C=O (in carboxylic acid) (1). Any two of the following points: Solids are easier to administer; taste/smell reduced; not acidic; not corrosive; easier to make sure correct dosage, more soluble in water (1 mark for each point). Total Expected answers Stereoisomerism/optical (isomerism) (1). 10
3 (g)
19 Marks 1
Question 4 (a) (i)
4 (a) (ii)
(Molecule has) an asymmetric carbon atom / chiral centre / carbon bonded to four different atoms/groups / mirror image is non-superimposable (1);
CH2COOH CH2COOH
H2N
COOH H
HOOC H
NH2
4 (b) (i)
4 (b) (ii) 4 (b) (iii) 4 (b) (iv) 4 (b) (v) 4 (c) 4 (d) (i)
Correct 3D structural formula for one enantiomer(1); mirror image (1). 850 25 (1) years for 1st reading; 850 25 years for 2nd reading and 3rd reading not greater than 925 (1) units need to be present for at least one of the readings to gain both marks; suitable construction on graph to show calculation of half-life (1). Half-life is constant (1). Rate = k x [L-aspartic acid]; [L-aspartic acid] (1); Rate = k (1). s-1 /yr-1/time-1 (1). k is the rate of reaction (1). Zwitterion (1).
1 2 1 1 1
[ion F] . [H ] Kc = [ion E] ion not necessary for mark (1).
[H ] = 1.38 x 10-4 x 0.50 (1); [H+] = 8.30 or 8.31 x 10-3 mol dm-3 (1); 2 or 3 sig. figs (1). Order/sequence of amino acids (in protein chain) (1); shape taken up by protein chain e.g. folding of chains AW (1); the (extra) COOH/COO in aspartic acid (1) ; forms/increases the hydrogen bonding/ ion- dipole forces/interactions with water (molecules) (1); charged groups on side/R groups of substrates (may give example NH3+ / COO groups) (1); can attract charged groups/(may give example -NH3+ / COO groups) in the active sites/AW of enzymes (1). Accept polar side chains for charged groups but 1 mark not 2. QWC See next page (1). Total
+ 2
4 (d) (ii)
4 (e)
24
11
Question 5 (a) 5 (b) (i) 5 (b) (ii) 5 (c) (i) 5 (c) (ii)
Expected answers Any answer relating to railway tracks, points, frogs etc.(1). To remove sulphur (1). Blowing oxygen through (1); turns the carbon to carbon dioxide accept carbon monoxide (1). Acidic (oxide) (1). 6CaO + P4O10 2Ca3(PO4)2
Marks 1 1 2 1 3
5 (c) (iii) 5 (d) 5 (e)
correct formula for P4O10 / P2O5 (1); correct formula for CaO rest correct (1). Correct amount of P added later AW (1). To remove (dissolved) oxygen (1). Analysing mixtures of steels/ sorting out different steels/ removing non steel materials/rust from the scrap/cleaning steel/contains unwanted elements (1). Total
1 1 1 11
Guidelines for the Award of S(P)AG QWC marks in Salters paper 2849 Jan 2006 1 2 The QWC mark is graded at E/U, and it is therefore expected that the majority of candidates will be awarded this mark. Award the mark if there is only one error in spelling, (punctuation) or grammar in any two relevant sentences. A repeated mis-spelling of the same word would count as one error; a repeated grammatical error (e.g. no verb) would count each time. Ignore all but the most blatant errors involving commas, because their use varies with individual preference. There should be at least two sentences in the answer. These should start with a capital letter but do not penalise lack of full stops at the end. Allow bullet points, provided each point is a sentence (or more), i.e. not note form. Bullet points need capitals at the start but not full stops at the end. Give the benefit of the doubt where unsure; especially avoid penalising obscure grammatical points.
3 4 5 6
12
Das könnte Ihnen auch gefallen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Minerals To Medicines Mark Scheme Jan 2003Dokument3 SeitenMinerals To Medicines Mark Scheme Jan 2003ExamStuffNoch keine Bewertungen
- F334 Jun 07 - MSDokument5 SeitenF334 Jun 07 - MSExamStuffNoch keine Bewertungen
- Minerals To Medicines Mark Scheme June 2003Dokument3 SeitenMinerals To Medicines Mark Scheme June 2003ExamStuffNoch keine Bewertungen
- Minerals To Medicines Mark Scheme June 2002Dokument3 SeitenMinerals To Medicines Mark Scheme June 2002ExamStuffNoch keine Bewertungen
- F334 Jun 06 - MSDokument7 SeitenF334 Jun 06 - MSExamStuffNoch keine Bewertungen
- F334 Jun 10 - MSDokument7 SeitenF334 Jun 10 - MSExamStuffNoch keine Bewertungen
- L A Level Chemistry Salters MS Jan 08Dokument24 SeitenL A Level Chemistry Salters MS Jan 08ExamStuffNoch keine Bewertungen
- Minerals To Medicines Mark Scheme Jan 2002Dokument4 SeitenMinerals To Medicines Mark Scheme Jan 2002ExamStuffNoch keine Bewertungen
- Minerals To Medicines Mark Scheme Jan 204Dokument3 SeitenMinerals To Medicines Mark Scheme Jan 204ExamStuffNoch keine Bewertungen
- F334 Jun 09 - MSDokument8 SeitenF334 Jun 09 - MSExamStuffNoch keine Bewertungen
- f334 Jun 10 - New SpecDokument20 Seitenf334 Jun 10 - New SpecExamStuffNoch keine Bewertungen
- F334 Jun 06Dokument16 SeitenF334 Jun 06ExamStuffNoch keine Bewertungen
- L A Level Chemistry Salters MS Jan 06Dokument52 SeitenL A Level Chemistry Salters MS Jan 06ExamStuffNoch keine Bewertungen
- F334 June 10 Mark SchemeDokument16 SeitenF334 June 10 Mark SchemeExamStuffNoch keine Bewertungen
- F334 Jun 09Dokument20 SeitenF334 Jun 09ExamStuffNoch keine Bewertungen
- F334 Jun 08 - MSDokument6 SeitenF334 Jun 08 - MSExamStuffNoch keine Bewertungen
- f334 Jan 10 - New SpecDokument20 Seitenf334 Jan 10 - New SpecIbrahimAhmed1994Noch keine Bewertungen
- F334 Jun 04Dokument8 SeitenF334 Jun 04ExamStuffNoch keine Bewertungen
- F334 Jun 02Dokument8 SeitenF334 Jun 02ExamStuffNoch keine Bewertungen
- F334 JAN 10 - MS (New Spec)Dokument13 SeitenF334 JAN 10 - MS (New Spec)ExamStuffNoch keine Bewertungen
- F334 - 07 Jun - QPDokument20 SeitenF334 - 07 Jun - QPExamStuffNoch keine Bewertungen
- F334 Jun 03Dokument8 SeitenF334 Jun 03ExamStuffNoch keine Bewertungen
- F334 Jan 10 - MSDokument8 SeitenF334 Jan 10 - MSExamStuffNoch keine Bewertungen
- F334 Jan 10Dokument20 SeitenF334 Jan 10ExamStuffNoch keine Bewertungen
- F334 Jan 09 - MSDokument9 SeitenF334 Jan 09 - MSExamStuffNoch keine Bewertungen
- F334 Jan 08 - MSDokument5 SeitenF334 Jan 08 - MSExamStuffNoch keine Bewertungen
- F334 - 06 Jan - QPDokument20 SeitenF334 - 06 Jan - QPExamStuffNoch keine Bewertungen
- F334 Jan 07Dokument16 SeitenF334 Jan 07ExamStuffNoch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- E.bs 3rd-Unit 22Dokument46 SeitenE.bs 3rd-Unit 22DUONG LE THI THUYNoch keine Bewertungen
- Administrations whose CoCs are accepted for CECDokument1 SeiteAdministrations whose CoCs are accepted for CECGonçalo CruzeiroNoch keine Bewertungen
- Silicone Bonding BrochureDokument4 SeitenSilicone Bonding BrochureAmir ShahzadNoch keine Bewertungen
- Graphic Organizers for Organizing IdeasDokument11 SeitenGraphic Organizers for Organizing IdeasMargie Tirado JavierNoch keine Bewertungen
- Primavera Inspire For Sap: Increased Profitability Through Superior TransparencyDokument4 SeitenPrimavera Inspire For Sap: Increased Profitability Through Superior TransparencyAnbu ManoNoch keine Bewertungen
- C++ NotesDokument129 SeitenC++ NotesNikhil Kant Saxena100% (4)
- Filler SlabDokument4 SeitenFiller Slabthusiyanthanp100% (1)
- Delhi Mumbai Award Status Mar 23Dokument11 SeitenDelhi Mumbai Award Status Mar 23Manoj DoshiNoch keine Bewertungen
- Chams 1Dokument78 SeitenChams 1Das RavindraNoch keine Bewertungen
- Current Developments in Testing Item Response Theory (IRT) : Prepared byDokument32 SeitenCurrent Developments in Testing Item Response Theory (IRT) : Prepared byMalar VengadesNoch keine Bewertungen
- Project Report VajDokument15 SeitenProject Report VajTamil SelvanNoch keine Bewertungen
- Ownership and Governance of State Owned Enterprises A Compendium of National Practices 2021Dokument104 SeitenOwnership and Governance of State Owned Enterprises A Compendium of National Practices 2021Ary Surya PurnamaNoch keine Bewertungen
- B2PLUS UNIT 6 Test Answer Key HighDokument2 SeitenB2PLUS UNIT 6 Test Answer Key HighАндрій НікітінNoch keine Bewertungen
- RoutineHub - R Download - iOS 13, 14, 15, 2Dokument1 SeiteRoutineHub - R Download - iOS 13, 14, 15, 2Gabriell AnjosNoch keine Bewertungen
- Biotox Gold 2.0-2021 Relaunch ReviewDokument6 SeitenBiotox Gold 2.0-2021 Relaunch ReviewChinthaka AbeygunawardanaNoch keine Bewertungen
- Expt 1 Yarn Formation (Sherley Trash Analyser)Dokument7 SeitenExpt 1 Yarn Formation (Sherley Trash Analyser)Yashdeep Sharma0% (1)
- Science SimulationsDokument4 SeitenScience Simulationsgk_gbuNoch keine Bewertungen
- Fictional Narrative: The Case of Alan and His FamilyDokument4 SeitenFictional Narrative: The Case of Alan and His Familydominique babisNoch keine Bewertungen
- ComputerDokument26 SeitenComputer29.Kritika SinghNoch keine Bewertungen
- Strategy 13 Presentation - Social Emotional LearningDokument29 SeitenStrategy 13 Presentation - Social Emotional Learningapi-588940234Noch keine Bewertungen
- Oreilly Design For Voice InterfacesDokument37 SeitenOreilly Design For Voice InterfacesHarmony JordenNoch keine Bewertungen
- Beyond VaR OfficialDokument76 SeitenBeyond VaR OfficialmaleckicoaNoch keine Bewertungen
- Writing A Formal Letter To The PresidentDokument1 SeiteWriting A Formal Letter To The PresidentPiaAnaisNoch keine Bewertungen
- English For Academic Purposes (EAP) : Lecture 5: Past SimpleDokument11 SeitenEnglish For Academic Purposes (EAP) : Lecture 5: Past Simplealmastar officeNoch keine Bewertungen
- History of English Prose PDFDokument21 SeitenHistory of English Prose PDFMeisyita QothrunnadaNoch keine Bewertungen
- Intraoperative Nursing Care GuideDokument12 SeitenIntraoperative Nursing Care GuideDarlyn AmplayoNoch keine Bewertungen
- Psalms Magick of The Old Testament PDFDokument129 SeitenPsalms Magick of The Old Testament PDFirrrs100% (1)
- Horizontal Machining Centers: No.40 Spindle TaperDokument8 SeitenHorizontal Machining Centers: No.40 Spindle TaperMax Litvin100% (1)
- APM200 Outdoor Power Supply System User Manual-20060628-B-1.0Dokument52 SeitenAPM200 Outdoor Power Supply System User Manual-20060628-B-1.0Andrés MarroquínNoch keine Bewertungen
- Canterburytales-No Fear PrologueDokument10 SeitenCanterburytales-No Fear Prologueapi-261452312Noch keine Bewertungen