Beruflich Dokumente
Kultur Dokumente
Product Preview i-CHROMA Boditech PDF
Hochgeladen von
UMARALEKSANA, CVOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Product Preview i-CHROMA Boditech PDF
Hochgeladen von
UMARALEKSANA, CVCopyright:
Verfügbare Formate
Overview
One of the noticeable trends in diagnostic market is the rapid shift of technology from the conventional instruments used in major hospitals or clinical laboratories to the point of care tests (POCT), where tests are performed in doctors offices, patients bedside and even at homes. We are on the forefront in this technology and have developed an integrated POCT system in line with this trend in diagnostics.
Our i-CHROMA is a POCT diagnostic system optimized for the screening of major "life- style related" diseases including, but not limited to, cardiac diseases, diabetes and cancers.
i-CHROMA system delivers the result in 3~15 minutes after taking the blood sample from the patient's fingertip right at the doctor's office. It is one-of-a-kind multi-parameter POCT system in the world. We have adopted ISO 13485 system to ensure continued quality assurance for our diagnostic systems and test devices. As we strive toward higher quality and reliability, we have been approved by USFDA and certified for CE. We are pioneering a new paradigm of POCT in the diagnostic world and your interests and encouragement would be healthy stimulus for us to grow upon.
Visit Us : www.umaraleksana.co.id
We are represented in over 40 countries worldwide.
We constantly expand out network through major tradeshows and strengthen ties with existing customers through long-term commitments.
I CHROMA Reader
TM
i-CHROMADUO
HEMOCRHOMATM
Mini FLOUROMETER
Visit Us : www.umaraleksana.co.id
Reader
General Information
The i-CHROMATM reader is a portable instrument dedicated to scan, detect and process the signal from the diagnostic testing devices provided by Boditech Med Inc. Once the testing device cartridge is loaded into this reader after the preprocessing procedure, the cartridge is sent to a designated location where it is irradiated with a beam of laser with 647nm wavelength. The fluorescence signal generated by fluorophores labeled onto capture molecules are optically collected and converted into clinically meaningful quantities by proprietary firmware stored in the onboard processor automatically. This result is displayed TM on the face plate of the reader. The i-CHROMA reader can forward this information to a receipt printer, to an external PC or even to the LIS via optional accessories. The i-CHROMATM reader is equipped with a set of self-diagnostic routines that continuously monitor the system integrity. The built-in interlocking errorprevention system consists of a coded ID Chip provided with the test device and the barcode on each test device. Any discrepancy among them would produce a warning message and prevents further operation until the problem is resolved.
TM The firmware on i-CHROMA reader can be upgraded easily by the user. The user can use the proprietary firmware upgrade kit from Boditech Med or download the binary file from Boditech homepage and execute the upgrade process.
Key Features of i-CHROMATM reader
Quantitative results Closed system One-dimensional scanner geometry High throughput: up to 45 samples per hour Simple user interface Supports up to 50 parameters (customizable) Automatically identifies sample types (Whole blood or Serum/Plasma): no user input necessary Simplified firmware upgrade Supports remote technical service Flexible communications via a RS232C port (printer, PC, LIS). Optional scanner and keypad connection interface available Enhanced mobility: can be retrofitted for battery operation in a travel package Comparable performance with high end reference analyzers Power requirement:100~240VAC 50~60Hz, 12V battery pack available. Weight 1.2 kg, Dimension 185x80x 250 mm (WHL)
Principle
Laser energy irradiating the scan line on the test device induces the emission of characteristic fluorescence off the fluorophores immobilized on the detection biomaterials, which were captured by capture molecules previously immobilized on the test device. The amount of this fluorescent energy is closely correlated with the relative amount of the TM analyte under measurement. i-CHROMA reader is equipped with the confocal optical arrangement widely adopted and proven in most confocal fluorescence microscopes. This guarantees the best possible background rejection, which is of crucial importance when one wants to increase the detection sensitivity. The firmware takes care of the whole operation after accepting the test device into the carriage. The user inputs via keys located on the faceplate or via external commands from externally connected control computer.
Visit Us : www.umaraleksana.co.id
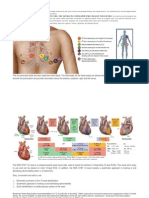
Cardiac Markers
Connectivity
The i-CHROMATM reader can be networked indirectly through its communication port (RS232c). One may use off-the-shelf serial receipt printers for immediate printout. An external computer will be handy in controlling and managing the accumulated data from the reader and in establishing and maintaining the connection to other networks, most likely a LIS server. An interface module is also available that enables the entry of the patient ID via a keyboard and/or a hand-held barcode scanner.
Visit Us : www.umaraleksana.co.id
Cardiac Markers
General Information
Cardiac troponins are currently the most sensitive and specific biochemical markers of myocardial necrosis. There are three troponin proteins in heart muscle fibers,Troponin C, I, and T. Together they cooperate to make muscle fibres contract. The clinical measurement of serum cardiac troponin I(Tn-I) has become an important tool in the diagnosis of acute myocardial infarction. Cardiac troponin I has been observed in people with renal failure, sepsis, stroke and other conditions. The average concentration of Tn- I in healthy adults is below 1ng/ml but it shows a great increase in patients with damaged heart muscle as in acute coronary syndrome, cardiomyopathy, and myocardial infarction for example. Serum troponin I is a more powerful prognostic marker in people with ischemic chest pain than CK-MB and shows significant increases in serum. National and international scientific organizations have suggested the use of these markers when implementing new diagnostic strategies in patients with acute coronary syndrome. Since Tn I is well known to be an important prognostic indicator of heart diseases, its most definitive role is in monitoring post-treatment clinical status and the post therapeutic evaluation of patients.
Key Features of i-CHROMATM Tn-I Test
Quantitative Test Result Sample Type : Serum, Plasma. Sample volume : 75 l Detection Limit : 0.01 ng/ml Working range : 0.01 ~ 50 ng/ml Cut Off : 0.6 ng/ml Precision : CV % 2~15 in working range Log Shelf Life : 20 months Fast Test Result : 12 minutes Comparable Test Result with Full Automatic AnalyzerNo Calibration Needed
Principle
The i-CHROMATM Tn-I Test is based on fluorescence immunoassay technology. The i-CHROMATM Tn-I Test uses a sandwich immunodetection method, such that the fluorescence-labeled detector antibody or antigen binds to the target protein in serum or plasma specimen. Signal intensity of fluorescence reflects amount of the Tn-I captured and is microprocessed from i-CHROMATM Reader to show the Tn-I concentration in the specimen.
Test Procedure
Visit Us : www.umaraleksana.co.id
Cardiac Markers
General Information
TM i-CHROMA hsCRP test kit is used to measure total CRP concentration in human serum, plasma or whole blood along with i-CHROMATM reader. The test kit consists of a test strip in a disposable plastic cartridge and detector buffer. The components of test strip are antibody-immobilized nitrocellulose membrane, a sample pad, an absorption pad, and a backing card. After the sample and detector are mixed well, the sample mixture is loaded onto a test TM device and waited 3 minutes for immune reaction. The device is then inserted into the holder of i-CHROMA reader for quantification of CRP TM concentration in blood. The i-CHROMA reader is a small fluorescence instrument for quantification of analyte concentration of immunoassays manufactured by Boditech Med Inc. The assays and instrument are for in vitro diagnostic use only. The i-CHROMATM reader can be used in point-of-care TM testing settings and central laboratories. The i-CHROMA reader uses a laser as the excitation light source. The emitted light from the fluorescence dye is detected and converted into an electrical signal. The signal is directly proportional to the amount of fluorescence present. The concentration of an analyte is calculated based on pre-programmed calibration. The specificity of each test is programmed into the test cartridges accepted by i-CHROMATM reader.
Key Features of i-CHROMATM HsCRP Test
Quantitative Test Result Sample Type: Whole blood, Serum, and Plasma Sample volume: 10 l Detection Limit: 0.1 mg/L Working range: 0.1~10 mg/L Cut Off: < 1 mg/L Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 3 minutes User Friendliness: No Pipette Used Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
TM The i-CHROMATM hsCRP Test is based on fluorescence immunoassay technology. The i-CHROMA hsCRP Test uses a sandwich immunodetection method, such that the fluorescence-labeled detector antibody or antigen binds to the target protein in blood specimen. Signal intensity of TM fluorescence reflects amount of the CRP captured and is microprocessed from i-CHROMA Reader to show the CRP concentration in blood specimen.
Test Procedure
Visit Us : www.umaraleksana.co.id
Cardiac Markers
General Information
D-dimer, a degradation product of cross-linked fibrin formed during activation of the coagulation system, is commonly used to exclude thromboembolic disease in outpatients suspected of having deep venous thrombosis (DVT) and pulmonary-embolism(PE). By providing a quick, noninvasive alternative to costly imaging, D-dimer has demonstrated to aid in the exclusion of venous thromboembolism(VTE) in outpatients. Deep vein thrombosis(DVT) and Pulmonary Embolism(PE) are relatively common and can cause sudden, fatal embolic events in the pulmonary arteries and other regions. Although prompt detection and treatment of DVT are necessary, DVT is usually subclinical and often underdiagnosed. Measurement of plasma D-dimer level has been used as a screening strategy for subclinical DVT. A systematic review reported that a normal range of a highly sensitive D-dimer level accurately ruled out DVT in patients classified as having a low or moderate clinical probability of DVT. Stroke is a highrisk factor for DVT because of advanced age, hemiplegia, and coagulation disorders; DVT can cause paradoxical embolic stroke via a right-to left shunt. Thus, it is important to understand the incidence and characteristics of DVT in acute stroke patients. Plasma D-dimer level has proven to be useful for DVT screening in chronic stroke patients undergoing rehabilitation. National and international scientific organizations have suggested the use of these markers when implementing new diagnostic strategies in patients with coronary syndrome. Since D-Dimer is well known to be an important prognostic indicator of heart diseases, its most definitive role is in monitoring post-treatment clinical status and the post therapeutic evaluation of patients.
Key Features of i-CHROMATM D-Dimer Test
Quantitative Test Result Sample Type: Plasma Sample volume: 75 l Detection Limit: 50 ng/ml Working range: 50 ~ 10,000 ng/ml Cut Off: 300 ng/ml (DDU:D-Dimner Unit) Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 5 minutes Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
TM The i-CHROMA D-Dimer Test is based on fluorescence immunoassay technology. The i-CHROMATM D-Dimer Test uses a sandwich immunodetection method, such that the fluorescence-labeled detector antibody or antigen binds to the target protein in serum or plasma specimen. Signal intensity of fluorescence reflects amount of the D-Dimer captured and is microprocessed from i-CHROMATM Reader to show the D-Dimer concentration in serum or plasma specimen.
Test Procedure
Visit Us : www.umaraleksana.co.id
Cancer Markers
General Information
Prostate specific antigen (PSA) is a neutral serine protease with chymotrypsin-like activity. It is composed of a single polypeptide chain of 237 amino acids. It is an intracellular glycoprotein containing 7-8% carbohydrate as a single N-linked oligosaccharide side chain, and has a molecular weight of approximately 33 KDa. PSA is synthesized by the glandular epithelium of the prostate and present in benign hyperplastic and malignant prostatic tissue, in metastatic prostatic carcinoma, in prostatic fluid, and seminal plasma. Low levels of PSA are found in the blood of normal male as a result of leakage of antigen from the prostate gland into circulation. The elevated levels of PSA in the blood are associated with prostatic pathology, including prostatitis, benign prostatic hyperplasia(BPH), and prostate cancer. The i-CHROMATM PSA Test measures quantitative PSA concentration in human serum, plasma, or whole blood.
Key Features of i-CHROMATM PSA Test
Quantitative Test Result Sample Type : Whole blood, Serum, and Plasma. Sample volume :Whole blood - 30 l, Serum, Plasma - 75 l Detection Limit : Whole blood - 0.5 ng/ml, Serum, Plasma - 0.1 ng/ml Working range : Whole blood - 0.5 ng/ml ~ 100 ng/ml Serum, Plasma - 0.1 ng/ml ~ 100 ng/ml Cut Off : 4ng/ml Precision : CV% 2-10 in working range. Long Shelf Life : 20 months Fast Test Result : 15 minutes User Friendliness : Pipette Used Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The i-CHROMATM PSA Test is based on fluorescence immunoassay technology. The i-CHROMATM PSA Test uses a sandwich immunodetection method, such that the fluorescence-labeled detector antibody or antigen binds to the target protein in blood specimen. Signal intensity of fluorescence TM reflects amount of the PSA captured and is microprocessed from i-CHROMA Reader to show the PSA concentration in blood specimen.
Test Procedure
Visit Us : www.umaraleksana.co.id
Cancer Markers
General Information
Alpha-fetoprotein (AFP) is a member of a1-globulin family of human plasma proteins and a glycoprotein with a molecular weight approximately 70 kDa. AFP is produced primarily in the liver of developing fetus; therefore can be found in maternal blood and in amniotic fluid. The concentration of AFP in healthy adult is below 20 ng/ml but it shows a great increase in several malignant diseases, mostly primary hepatocelluar carcinoma and nonseminomatous testicular cancer. Some 70-90% of patients with primary hepatocelluar carcinoma and non-seminomatous testicular cancer have been observed to have high levels of serum AFP. High levels of serum AFP also have been found in a limited number of patients diagnosed with various diseases such as gastrointestinal tract cancer, viral hepatitis, chronic active hepatitis, alcoholic cirrhosis, and adenocarcinomas of lung, pancreas, and gall bladder. Since AFP is well known to be an important prognostic indicator of non-seminomatous testicular cancer, its most definitive role is in monitoring post-treatment clinical status and the post therapeutic evaluation of patients.
Key Features of i-CHROMATM AFP Test
Quantitative Test Result Sample Type: Whole blood, Serum, and Plasma. Sample volume:Whole blood - 30 l, Serum, Plasma - 15 l Detection Limit: 5ng/ml Working range: 5 ng/ml ~ 350 ng/ml Cut Off: 20ng/ml Precision: CV% 2-10 in working range. Long Shelf Life: 20 months Fast Test Result: 15 minutes User Friendliness: Pipette Used Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
TM The i-CHROMATM AFP Test is based on fluorescence immunoassay technology. The i-CHROMA AFP Test uses a sandwich immunodetection method, such that the fluorescence-labeled detector antibody or antigen binds to the target protein in blood specimen. Signal intensity of fluorescence reflects amount of the AFP captured and is microprocessed from i-CHROMATM Reader to show the AFP concentration in blood specimen.
Test Procedure
Visit Us : www.umaraleksana.co.id
Cancer Markers
General Information
CEA is an oncofetal glycoprotein, which is found at high levels in the fetal colon and at lower levels in the normal adult colonic epithelium. CEA occurs at abnormally high levels in several benign disorders and in some malignant tumors, including those of the stomach, small intestine, colon, rectum, pancreas, liver, breast, ovary, cervix and lung. CEA is a 180-kD glycoprotein that occurs at high levels in colon epithelial cells during embryonic development. Levels of CEA are significantly lower in colon tissue of adults, but can become elevated when inflammation or development of tumor occurs in any endodermal tissue, including in the gastrointestinal tract, respiratory tract, pancreas and breast. CEA is also expressed by epithelial cells in several non-malignant disorders, including diverticulitis, pancreatitis, inflammatory bowel disease, cirrhosis, hepatitis, bronchitis and renal failure and also in individuals who smoke. This fact has made it difficult to use serum CEA determination as a sensitive method for cancer screening. However, serum CEA levels have been useful in monitoring individuals for the recurrence of cancer.
Key Features of i-CHROMATM CEA Test
Quantitative Test Result Sample Type: Serum / Plasma. Sample volume:150 l Detection Limit: 3ng/ml Working range: 3 ng/ml ~ 500 ng/ml Cut off : 4 ng/ml Precision: CV(%) <10% in working range. Long Shelf Life: 20 months Fast Test Result: 15 minutes User Friendliness: Pipette Used Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
TM The i-CHROMA CEA is based on fluorescence immunoassay technology. i-CHROMA CEA uses a sandwich immunodetection method, such that by mixing detection buffer with serum or plasma sepecimen in a test vial, the fluorescence-labeled detector anti-CEA antibody in buffer binds to CEA antigen in surm or plasma specimen. As the sample mixture is loaded onto the sample well of the Test Device and migrates the nitrocellulose matrix of test strip by capillary action, the complexes of detector antybody and CEA ara captured to anti-CEA sandwich pair antibody that has been immobilized on test strip. Thus the presence of more CEA antigen in the specium, would lead to more complex accumulation on the test strip. Signal intensity of fluorescence of detector antibody reflects amount of CEA captured and is calcualted from i-CHROMATM Reader to show CEA concentration in serum or plasma specimen. The default result unit of i-CHROMATM CEA Test is displayed as a ng/ml on i-CHROMATM Reader. TM
Test Procedure
Visit Us : www.umaraleksana.co.id
Cancer Markers
General Information
Colorectal cancer is the third most common cancer in world, with about 1 million new cases and more than 500,000 deaths per year. Screening method for colorectal cancer include the fecal occult blood (FOB) test, barium enema, sigmoidoscopy and colonoscopy. Large randomized controlled trials have shown that FOB screening can result in decreased colorectal cancer mortality. The standard FOB test uses the chemical Guaiac, which is sensitive to Hb peroxidase activity. However, the standard Guaiac-FOB test has low sensitivity for clinically significant colorectal
Key Features of i-CHROMATM FOB Test
Quantitative Test Result Sample Type: feces Sample volume:25l Detection Limit: 3ng/ml Working range: 25ng/ml ~ 1000 ng/ml Cut Off: 50 ng/ml Precision: CV% 2-10 in working range. Long Shelf Life: 20 months Fast Test Result: 10 minutes Comparable Test Result with Full Automatic Analyzer
Principle
i-CHROMA FOB is based on fluorescence immunoassay technology. The i-CHROMA FOB uses a sandwich immuno-detection method, such that by mixing detection buffer with human Hb specimen in sample collection tube, the fluorescence-labeled detector anti-Hb antibody in buffer binds to Hb in fecal specimen. As the sample mixture is loaded onto the sample well of the test device and migrates the nitrocellulose matrix of test strip by capillary action, the complexes of detector antibody and FOB are captured by anti-human Hb sandwich pair antibody that has been immobilized on test strip. Thus the presence of more Hb in fecal specimen would lead to accumulation of more complexes on the test strip. Signal intensity of fluorescence of detector antibody reflects amount of human Hb captured. i-CHROMA Reader processes and shows Hb concentration in fecal specimen. The result unit of i-CHROMA FOB is displayed as an ng/ml from iCHROMA Reader. The working range of i-CHROMA FOB test system are 25-1,000 ng/ml.
TM TM TM TM TM TM
Test Procedure
Visit Us : www.umaraleksana.co.id
Diabetes Markers
General Information
Key Features of i-CHROMATM D-Dimer Test
Quantitative Test Result Sample Type: Whole blood Sample volume: 5l Detection Limit: 4 % Working range: 4 %~15 % Cut Off: < 6.5 % Precision: CV(%) =5% in working range. Long Shelf Life: 20 months User Friendliness: Pipette Used Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
TM The i-CHROMA HbA1c is based on fluorescence immunoassay technology. It uses competition immunodetection method. Whole blood from blood sepcimen is added to the mixture of hemolysis buffer and detection buffer which results in hemolysis of red blood cells. The mixture containing HbA1c from the hemolyzed red blood cells and fluorescence-labeled HbA1c peptides from detection buffer is loaded onto the sample well of Test Device and migrates the nitrocellulose matrix of test strip by capillary action. HbA1c of whole blood competes with fluorescencelabeled HbA1c peptides for binding sites on HbA1c antibodies of nitrocellulose maxtrix. Thus, the concentration of HbA1c antigen in blood specimen shows inversely proportional reationship with intensity of fluorescence of HbA1cpeptides. The result is displayed on i-CHROMATM Reader in units of percentage.
Test Procedure
Visit Us : www.umaraleksana.co.id
Glycated proteins are formed post-translationally from the slow, nonenzymatic reaction between glucose and amino groups on proteins. For hemoglobin, the rate of synthesis of Hemoglobin A1c (HbA1c) is principally a function of the concentration of glucose to which the erythrocytes are exposed. HbA1c is a clinically useful index of mean glycemia during the preceding 120 days, the average life span of erythrocytes. Carefully controlled studies have documented a close relationship between the concentrations of HbA1c and mean glycemia, while routine determinations of blood glucose by patients or by their healthcare providers are not considered as reliable as HbA1c to quantify mean glycemia. Concentrations of other blood-based glycated proteins (e.g., glycated serum or plasma proteins, "fructosamine") also reflect mean glycemia, but over a much shorter time than HbA1c: 15-30 days and 60-120 days, respectively.
Diabetes Markers
General Information
Urinary Albumin (UA) test evaluates urine for the presence of a protein called albumin. Albumin is normally found in the blood and filtered by the kidneys. When the kidneys are working properly, albumin is not present in the urine. When the kidneys are damaged, small amount of albumin leaks into the urine. This condition is called microalbuminuria (MAU). MAU is most frequently caused by kidney damage from diabetes. However, many other conditions can lead to kidney damage, such as high blood pressure, heart failure, cirrhosis, or systemic lupus erythmatosus(SLE). If early kidney damage is not treated, larger amounts of albumin and other proteins may leak into the urine. This condition is called macroalbuminuria or proteinuria. When the kidneys spill protein, it can mean serious kidney damage is present. This can lead to chronic kidney disease. A microalbumin urine test can be done on a sample of urine collected randomly (usually the first morning urine), a 24-hour collection sample, or a sample collected over a specified time period, such as 4 hours or overnight.
Key Features of i-CHROMATM Urinary Albumin Test
Quantitative Test Result Sample Type: Urine Sample volume: 10 l Detection Limit: 2.0 mg/L Working range: 2.0~300 mg/L Cut Off: <20mg/L Precision: CV% =10 in working range Long Shelf Life: 20 months Fast Test Result: 12 minutes User Friendliness: Pipette Used Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
TM TM The i-CHROMA UA Test is based on fluorescence immunoassay technology. The i-CHROMA UA Test uses a sandwich immunodetection method, such that by mixing detection buffer with urine specimen in test vial, the fluorescence-labeled detector albumin antibody in buffer binds to albumin antigen in urine specimen. As the sample mixture is loaded onto the sample well of the test device and migrates the nitrocellulose matrix of test strip by capillary action, the complexes of detector antibody and albumin are captured to anti-albumin sandwich pair antibody that has been immobilized on test strip. Thus the presence of more albumin antigen in urine specimen would lead to accumulation of more complexes on the test strip. Signal intensity of fluorescence of detector antibody reflects amount of albumin captured and is micro processed from iCHROMATM Reader to show albumin concentration in blood specimen. The default result unit of i-CHROMATM UA is displayed as a mg/L from iCHROMATM Reader. The working range and the detection limit of i-CHROMATM UA system are 2~300mg/L.
Test Procedure
Visit Us : www.umaraleksana.co.id
Hormone Markers
General Information
Testosterone (17-hydroxyandrost-4-en-3-one) is an anabolic steroid synthesized primarily by Leydig cells in the testes of males, the ovaries of females, and adrenal glands of both sexes. It is synthesized from cholesterol, androstenediol, dehydroepiandrosterone (DHEA), progesterone, and pregnenolone acting as some of the intermediate substrates. Testosterone levels in males increase 10 to 20-fold during puberty, causing the physiological changes associated with male puberty. It also exerts a powerful, wide-ranging influence over emotional well-being, sexual function, muscle mass and strength, energy, cardiovascular health, bone integrity, and cognitive ability throughout a mans entire life. In the blood only 1 to 15% of testosterone is in its unbound or biologically active form. The remaining testosterone is bound to serum proteins. Unbound testosterone enters the saliva via intracellular mechanisms. The majority of testosterone in saliva is not protein-bound. Salivary testosterone levels are unaffected by salivary flow rate or salivary enzymes.
Key Features of i-CHROMATM Testosterone Test
Quantitative Test Result Sample Type: Serum, and Plasma Sample volume: 75 l Detection Limit: 0.01 ng/ml Working range: 0.01-10 ng/ml Cut Off(normal range): 2-8 ng/ml for male Precision: CV% 2-10 in working range. Long Shelf Life: 20 months Fast Test Result: 15 minutes User Friendliness: 75l Pipette Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The test uses a competitive immunodetection method, such that the detector antibody in buffer binds to testosterone in blood sample. The antigenantibody complexes compete with testosterone covalently coupled to BSA that has been immobilized on test strip as sample mixture migrates nitrocelluose matrix. Thus the presence of more testosterone antigen in blood would lead to less accumulation of antigen-antibody complexes on the test strip. Signal intensity of fluorescence on detector antibody reflects amount of antigen captured. It is processed from i-CHROMATM Reader to show testosterone concentration in the specimen. The working range of i-CHROMATM TESTOSTERONE test is 0.01 ~ 10 ng/mL.
Test Procedure
Visit Us : www.umaraleksana.co.id
Hormone Markers
General Information
Cortisol is a steroid hormone or glucocorticoid produced by the adrenal gland. Its primary functions are to increase blood sugar, suppress the immune system, and aid in fat, protein and carbohydrate metabolism. Cortisol test is performed on patients who may have malfunctioning adrenal glands. Cortisol levels normally rise and fall during the day in what is called a diurnal variation, so that the concentration of cortisol is at its highest level between 6 ~ 8 A.M. and gradually falls, reaching its lowest point around midnight. When testing for cortisol levels with the blood, a blood specimen is usually collected at 8 A.M. and again at 4 P.M. It should be noted that normal values may be transposed in individuals who have worked during the night and slept during the day for long periods of time.
Key Features of i-CHROMATM Cortisol Test
Quantitative Test Result Sample Type: whole blood, Serum, and Plasma Sample volume: whole blood-50 l , serum/plasma-30 l Detection Limit: 90 nmol/L Working range: 90-800 nmol/L Cut Off (normal range) : moring -140~700 nmol/L midnight-80-350 nmol/L Precision: CV% 2-10 in working range. Long Shelf Life: 20 months Fast Test Result: 10 minutes User Friendliness: 30l, 50l, 75l Pipette Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The i-CHROMATM Cortisol assay system uses a competitive immunodetection method, such that the antibody in Detection Buffer binds to cortisol in blood sample. As the mixture of blood and Detection Buffer migrates nitrocelluose matrix, the antigen-antibody complexes competes with covalently coupled cortisol-BSA that has been immobilized on test strip. Thus the presence of more cortisol antigen is in blood sample would lead to less accumulation of antigen-antibody complexes on the test strip. Signal intensity of fluorescence on detector antibody reflects amount of antigen captured on test line and is processed from i-CHROMATM Reader to show cortisol concentration in specimen. The working range of i-CHROMATM Cortisol test is 90 ~ 800 nmol/L.
Test Procedure
Visit Us : www.umaraleksana.co.id
Hormone Markers
General Information
Human chorionic gonadotropin (hCG) is a glycoprotein hormone secreted by the developing placenta shortly after implantation. hCG can be detected in the urine and serum of pregnant women as early as 6 to 15 days after conception. The concentration of hCG increases to 50 mIU/ml one week post implantation and reaches to about 100 mIU/ml at the time of the first missed menstrual period and the peak at 100,000-200,000 mIU/ml at the the first trimester.
Key Features of i-CHROMATM hCG Test
Quantitative Test Result Sample Type: Whole blood, Serum, and Plasma Sample volume: Whole blood-50l, Serum/Plasma-75 l Detection Limit: 2 mIU/ml Working range: 2-200,000 mIU/ml Cut Off: 10 mIU/ml Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 15 minutes User Friendliness: 75l Pipette Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The test uses a sandwich immunodetection method, such that the detector antibody in buffer binds to hCG in blood sample and antigen-antibody complexes are captured by antibody that has been immobilized on test strip as sample mixture migrates nitrocelluose matrix. Thus the presence of more hCG antigen in blood would lead to accumulation of more antigen-antibody complexes on the test strip. Signal intensity of fluorescence on TM detector antibody reflects the amount of antigen captured and is processed by i-CHROMA Reader to show hCG concentration in specimen. The working range of i-CHROMATM hCG test is 2-200,000 mIU/ml.
Test Procedure
Visit Us : www.umaraleksana.co.id
Hormone Markers
General Information
Luteinizing hormone(LH) is produced in both men and women from the anterior pituitary gland in response to luteinizing hormone-releasing hormone(LH-RH or Gn-RH), which is released by the hypothalamus. LH, also called interstitial cell-stimulating hormone(ICSH) in men, is a glycoprotein with a molecular weight of approximately 30,000. In women, LH helps regulate the menstrual cycle and egg production(ovulation). The level of LH in a womans body varies with the phase of the menstrual cycle. It increases rapidly just before ovulation occurs, about midway through the cycle(day 14 of a 28-day cycle). This is called an LH rise and fall together during the monthly menstrual cycle. In men, LH stimulates the production of testosterone, which plays a role in sperm production.
Key Features of i-CHROMATM LH Test
Quantitative Test Result Sample Type: Serum, and Plasma Sample volume: 75 l Detection Limit: 5 mIU/ml Working range: 5-100 mIU/ml Cut Off: 20 mIU/ml Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 15 minutes User Friendliness: 75l Pipette Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The test uses a sandwich immunodetection method, such that the detector antibody in buffer binds to LH in blood sample and antigen-antibody complexes are captured to antibody that has been immobilized on test strip as sample mixture migrates nitrocellulose matrix. Thus the presence of more LH antigen in blood would lead to accumulation of more antigen-antibody complexes on the test strip. Signal intensity of fluorescence on detector antibody reflects amount of antigen captured. It is processed from i-CHROMA Reader to show LH concentration in specimen. The working range of i-CHROMA LH test is 5-100 mIU/ml.
Test Procedure
Visit Us : www.umaraleksana.co.id
Hormone Markers
General Information
The determination of serum or plasma levels of thyroid stimulating hormone (TSH or thyrotropin) is recognized as an important measurement in the assessment of thyroid function. Thyroid stimulating hormone is secreted by the anterior lobe of the pituitary gland, and induces the production and release of thyroxine(T4) and triiodothyronine (T3) from the thyroid gland. It is a glycoprotein with a molecular weight of approximately 28,000 daltons, consisting of two chemically different subunits, alpha and beta. Although the concentration of TSH in the blood is extremely low, it is essential in the maintenance of normal thyroid function. The release of TSH is regulated by a TSH-releasing hormone (TRH) produced by the hypothalamus. The levels of TSH and TRH are inversely related to the level of thyroid hormone. When there is a high level of thyroid hormone in the blood, less TRH is released by the hypothalamus, so less TSH is secreted by the pituitary. The opposite action will occur when there are decreased levels of thyroid hormones in the blood. This process, known as a negative feedback mechanism, is responsible for maintaining the proper blood levels of these hormones. The i-CHROMA TSH Test measures quantitatively TSH concentration in human Serum/plasma.
Key Features of i-CHROMATM TSH Test
Quantitative Test Result Sample Type: Serum, and Plasma Sample volume: 150 l Detection Limit: 0.10 IU/mL Working range: 0.10 - 100 IU/mL Normal Range : 0.40 - 5.00 IU/mL Precision: CV% 0.25 - 0.50 IU/mL <20%, 0.50 100 IU/mL <10% in working range Long Shelf Life: 20 months Fast Test Result: 12 minutes User Friendliness: 75l pipette Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The test uses a sandwich immunodetection method, such that the detector antibody in buffer binds to TSH in blood sample and antigen-antibody complexes are captured to antibody that has been immobilized on test strip as sample mixture migrates nitrocelluose matrix. Thus the presence of more TSH antigen in blood would lead to accumulation of more antigen-antibody complexes on the test strip. Signal intensity of fluorescence on detector antibody reflects the amount of antigen captured and is processed by i-CHROMA Reader to show TSH concentration in specimen. The working range of i-CHROMA TSH test is 0.1-100 IU/mL.
Test Procedure
Visit Us : www.umaraleksana.co.id
Hormone Markers
General Information
Thyroxine (T4) is one of two major hormones produced by the thyroid gland (the other is called triiodothyronin, or T3). T4 and T3 are regulated by a sensitive feedback system involving the hypothalamus and pituitary gland. The hypothalamus releases the thyrotropin releasing hormone (TRH), which stimulates the pituitary to release the thyroid stimulating hormone (TSH). This causes the thyroid to release T3 and T4 and these in turn regulate the release of TRH and TSH via a feedback control mechanism. Normally, increased blood levels of T4 and T3 act to decrease the amount of TSH secreted, thereby reducing the production and release of T4 and T3. Greater than 99% of T4 is reversibly bound to three plasma proteins in blood; thyroxine binding globulin (TBG) binds 70%, thyroxine binding pre-albumin (TBPA) binds 20%, and albumin binds 10%. Approximately 0.03% of T4 is in the free, unbound state in blood at any one time. T4 is a useful marker for the diagnosis of hypothyroidism and hyperthyroidism. The level of T4 is decreased in hypothyroidism, myxedema, chronic thyroiditis (Hashimotos disease). Increased levels of T4 have been found in hyperthyroidism due to Graves disease and Plummers disease.
Key Features of i-CHROMATM T4 Test
Quantitative Test Result Sample Type: Serum, and Plasma Sample volume: 10 l Detection Limit: 20 nmol/L Working range: 20-300 nmol/L Cut Off(normal range): 60-160 nmol/L Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 10 minutes User Friendliness: 10 l, 75 l Pipette Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The test uses a competitive immunodetection method, such that the detector antibody in buffer binds to T4 in blood sample and antigen-antibody complexes competes with T4 covalently coupled to BSA that has been immobilized on test strip as sample mixture migrates nitrocelluose matrix. Thus the presence of more T4 antigen in blood would lead to accumulation of less antigen-antibody complexes on the test strip. Signal intensity of fluorescence on detector antibody reflects amount of antigen captured and is processed from i-CHROMA Reader to show T4 concentration in specimen. The working range of i-CHROMA T4 test is 20~300 nmol/L.
Test Procedure
Visit Us : www.umaraleksana.co.id
Hormone Markers
General Information
Human Prolactin (PRL: lactogenic hormone) is secreted from the anterior pituitary gland in both men and women. PRL is a single chain polypeptide hormone with a molecular weight of approximately 23,000 daltons. Women normally have slightly higher basal PRL levels than men; apparently, there is an estrogen-related rise at puberty and a corresponding decrease at menopause. During pregnancy, PRL levels increase progressively to between 10 and 20 times normal values, declining to non-pregnant levels by 3-4 weeks post-partum. The determination of PRL concentration is helpful in diagnosing hypothalamic-pituitary disorders. Microadenomas (small pituitary tumors) may cause hyperprolactinemia, which is sometimes associated with male impotence. High PRL levels are commonly associated with galactorrhea and amenorrhea. PRL concentrations have been shown to be increased by estrogens, thyrotropin-releasing hormone (TRH), and several drugs affecting dopaminergic mechanism. PRL levels are elevated in renal disease and hypothyroidism, and in some situations of stress, exercise, and hypoglycemia. Additionally, the release of PRL is episodic and demonstrates diurnal variation.
Key Features of i-CHROMATM PRL Test
Quantitative Test Result Sample Type: Serum, and Plasma Sample volume: 75 l Detection Limit: 1 ng/ml Working range: 1-100 ng/ml Cut Off(normal range): non-pregnant female : 5~20 ng/ml pregnant female : 10~209 ng/ml Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 10 minutes User Friendliness: 75l Pipette Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The test uses a sandwich immunodetection method, such that the detector antibody in buffer binds to PRL in blood sample. The antigen-antibody complexes are captured by antibody that has been immobilized on test strip as sample mixture migrates nitrocellulose matrix. Thus the presence of more PRL antigen in blood would lead to more antigen-antibody complexes on the test strip. Signal intensity of fluorescence on detector antibody reflects amount of antigen captured and is processed from i-CHROMA Reader to show PRL concentration in specimen. The working range of iCHROMA PRL test is 1-100 ng/ml.
Test Procedure
Visit Us : www.umaraleksana.co.id
Infection Markers
General Information
The hepatitis B virus (HBV) is responsible for acute and chronic hepatitis infections, possibly evolving to cirrhosis or primary liver cancer. Chronicicity occurs in 5 to 10% of cases in adults, but up to 90% of cases in infants following perinatal transmission. Currently, more than 350 million people worldwide are chronic carriers of the virus. The discovery of the Australia antigen in 1970 - later known as HBs antigen- combined with viral hepatitis was a major breakthrough in the diagnosis of hepatitis B. HBs antigen appears several days to several weeks after contact with the virus and can persist for several months. Persistence of HBs antigen for more than 6 months serologically defines chronic HBV infection. Disappearance of the HBs antigen is normally followed by the appearance of anti-HBs antibodies, which is a sign of recovery.
Key Features of i-CHROMATM HBsAg Test
Quantitative Test Result Sample Type: Serum, and Plasma Sample volume: 100 l Detection Limit: 1 IU/ml Working range: 1- 250 IU/ml Cut Off: 1 IU/ml Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 12 minutes Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The i-CHROMA HBsAg Test is based on fluorescence immunoassay technology. Thei-CHROMA HBsAg Test uses a sandwich immunodetection method, such that the fluorescence-labeled detector antibody or antigen binds to the target protein in blood specimen. Signal intensity of fluorescence reflects amount of the HBsAg captured and is microprocessed from iCHROMA Reader to show the HBsAg concentration in blood specimen.
Test Procedure
Visit Us : www.umaraleksana.co.id
Infection Markers
General Information
Key Features of i-CHROMATM Anti - HBs Test
Quantitative Test Result Sample Type: Serum, and Plasma Sample volume: 75 l Detection Limit: 0 mIU/ml Working range:0.0 ~ 750 mIU/ml Cut Off: 101mIU/ml Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 12 minutes Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The i-CHROMA HBsAg Test is based on fluorescence immunoassay technology. The i-CHROMA HBsAg Test uses a sandwich immunodetection method, such that the fluorescence-labeled detector antibody or antigen binds to the target protein in blood specimen. Signal intensity of fluorescence reflects amount of the HBsAg captured and is microprocessed from iCHROMA Reader to show the HBsAg concentration in blood specimen.
Test Procedure
Visit Us : www.umaraleksana.co.id
The hepatitis B virus (HBV) is responsible for acute and chronic hepatitis infections, possibly evolving to cirrhosis or primary liver cancer. Currently, more than 350 million people worldwide are chronic carriers of the virus. HBs antigen (HBsAg) appears several days to several weeks after contact with the virus and can persist for several months: in this case, the infection is considered to be chronic. Disappearance of the HBs antigen is normally followed by the appearance of anti-HBs, which is a sign of recovery. In this case, the presence of anti-HBs is associated with that of antiHBc. The detection of anti-HBs is thus performed to monitor infected patients, but also to check the efficacy of immunization against the hepatitis B virus (vaccination by HBsAg). The persistence of anti-HBs is related to the initial titer obtained after the complete vaccination series has been administered. The European Consensus Group recommends performing anti-HBs determination 1-3 months following vaccination. It is only possible to affirm that the immunological memory will confer long-term protection to immunocompetent adults if titers are greater than 100 mIU/ml. Nevertheless, numerous countries have adopted 10 mIU/ml as the lowest titer indicating protective immunity against HBV infection.
Infection Markers
General Information
The i-CHROMA CRP test kit is used for measurement of total CRP concentration in human serum , plasma or whole blood along with i-CHROMA reader. The test kit consists of a test strip in a disposable plastic cartridge and detector buffer. The test strip is composed of an antibody- immobilized nitrocellulose membrane, a sample pad, an absorption pad, and a backing card. After sample and detector are mixed well, the sample mixture is loaded onto a test device and waited 3 minutes for immune reaction. The device is then inserted into a holder of i-CHROMA reader for quantification of CRP concentration in blood. The i-CHROMA reader is a small fluorescence instrument for quantification of analyte concentration of immunoassays manufactured by BodiTechMed Incorporated. The assays and instrument are for in vitro diagnostic use only. The i-CHROMA reader can be used in point-of-care testing settings and the central laboratories. The i-CHROMA reader uses a laser as the excitation light source. The emitted light from the fluorescence dye is detected and converted into an electrical signal. The signal is directly proportional to the amount of fluorescence present. The concentration of analyte is calculated based on pre-programmed calibration. The i-CHROMA reader accepts test cartridges that are designed specifically for use with this instrument. - serological parameters - including the C-reactive protein - acute phase reactants: elevated CRP value (c-reactive protein) - duration of arthritis: symptoms lasting six weeks or longer Thus, the most commonly used CRP blood tests include C-reactive protein. Our CRP test can measure CRP simultaneously with one drop of finger tip blood within 3 minutes.
Key Features of i-CHROMATM D-Dimer Test
Quantitative Test Result Sample Type: Whole blood, Serum, and Plasma Sample volume: 10 l Detection Limit: 2.5 mg/L Working range: 2.5-300 mg/L Cut Off: < 10 mg/L Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 3 minutes User Friendliness: No Pipette Used Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The i-CHROMA CRP Test is based on fluorescence immunoassay technology. The i-CHROMA CRP Test uses a sandwich immunodetection method, such that the fluorescence-labeled detector antibody or antigen binds to the target protein in blood specimen. Signal intensity of fluorescence reflects amount of the CRP captured and is microprocessed from i-CHROMA Reader to show the CRP concentration in blood specimen.
Test Procedure
Visit Us : www.umaraleksana.co.id
Rheumatoid Arthritis Markers
General Information
Rheumatoid arthritis (RA) is the most common chronic autoimmune arthritis worldwide leading to disability and substantial economic costs. It is a chronic, systemic inflammatory disorder that may affect many tissues and organs, but principally attacks synovial joints. About 1% of the world's population is afflicted by rheumatoid arthritis, women three times more often than men. Onset is most frequent between the ages of 40 and 50, but people of any age can be affected. It can be a disabling and painful condition, which can lead to substantial loss of functioning and mobility if not adequately treated. Four areas are covered in the diagnosis of RA: - joint involvement - depending on the type and number of joints - serological parameters - including the rheumatoid factors - acute phase reactants: elevated ESR or elevated CRP value (c-reactive protein) - duration of arthritis: symptoms lasting six weeks or longer Thus, the most commonly used RA blood tests include rheumatoid factor and C-reactive protein. Our RA double test can measure RF and CRP simultaneously with one drop of finger tip blood within 5 minutes.
Key Features of i-CHROMATM RA Double Test
Quantitative Test Result Sample Type: Whole blood, Serum, and Plasma Sample volume: Whole blood-10 l, Serum/Plasma-5l Detection Limit: RF(IgM)-15 IU/ml, CRP- 5 mg/L Working range: RF(IgM): 15-200 IU/ml, CRP 5-200 mg/L Cut Off: RF- 20 IU/ml Precision: CV% 2-10 in working range Long Shelf Life: 20 months Fast Test Result: 5 minutes User Friendliness: No Pipette Used Comparable Test Result with Full Automatic Analyzer No Calibration Needed
Principle
The i-CHROMA RF(IgM)/CRP is based on fluorescence immunoassay technology. The i-CHROMA RF(IgM)/CRP Test uses a sandwich immunodetection method, such that the fluorescence-labed detector antibody or antigen binds to the target protein in blood specimen. Signal intensity of fluorescence reflects amount of the RF(IgM) and CRP captured and is microprocessed from i-CHROMA Reader to show the RF(IgM) and CRP concentration in blood specimen.
Test Procedure
Visit Us : www.umaraleksana.co.id
.U CV
R MA
LE
A AN S
U . V C
A M
A R
E L
S K
A N A
Das könnte Ihnen auch gefallen
- Service Convergys X5Dokument142 SeitenService Convergys X5thanhtu987Noch keine Bewertungen
- BC-2800 Operation Maunal (1.8)Dokument283 SeitenBC-2800 Operation Maunal (1.8)Tekniah GlobalNoch keine Bewertungen
- Chem 7Dokument2 SeitenChem 7Rosa Maria Mosqueda Vazquez100% (1)
- Analytica 705 PresentationDokument12 SeitenAnalytica 705 PresentationBhupinder0% (1)
- Operation ManualDokument50 SeitenOperation ManualAhmed TawfikNoch keine Bewertungen
- Test Loader ExplainationDokument2 SeitenTest Loader ExplainationKP ServiceNoch keine Bewertungen
- PDFDokument526 SeitenPDFjairodiaz88Noch keine Bewertungen
- Service Manual For XL-100 With ISEDokument317 SeitenService Manual For XL-100 With ISESIELAB C.A.Noch keine Bewertungen
- DC-30 Auto Hematology Anazlyer Operational Manual-ILONGCAREDokument133 SeitenDC-30 Auto Hematology Anazlyer Operational Manual-ILONGCAREJason WangNoch keine Bewertungen
- Roche AVL9120,9130,9140,9180,9181 - Service Manual PDFDokument114 SeitenRoche AVL9120,9130,9140,9180,9181 - Service Manual PDFJose Rolando Orellana Rodriguez0% (1)
- Technical Specifications: Toll Free No: 1800 425 7151Dokument2 SeitenTechnical Specifications: Toll Free No: 1800 425 7151India DiscoverNoch keine Bewertungen
- Sedy12 ESR Manual U - E12 - NNDokument20 SeitenSedy12 ESR Manual U - E12 - NNSmart BiomedicalNoch keine Bewertungen
- Mek8222 TRM BDokument50 SeitenMek8222 TRM BSergioUrichNoch keine Bewertungen
- H30 Installation Guidance v1.1Dokument34 SeitenH30 Installation Guidance v1.1Romuald Eric TefongNoch keine Bewertungen
- Automated Hematology Analyzer: MEK-7300KDokument53 SeitenAutomated Hematology Analyzer: MEK-7300KСергей ПразднечновNoch keine Bewertungen
- AbacusDokument97 SeitenAbacusأنور مازوز أبو يوسفNoch keine Bewertungen
- XL 200 Service Manual v1.2Dokument322 SeitenXL 200 Service Manual v1.2Aahsan Iqbal احسن اقبالNoch keine Bewertungen
- Technical Specifications: System FunctionDokument2 SeitenTechnical Specifications: System FunctionAniket dubey0% (1)
- 9.ifam AU 400 MechanicalDokument17 Seiten9.ifam AU 400 Mechanicalmaran.suguNoch keine Bewertungen
- GH-900Plus Installation Guide 220329Dokument87 SeitenGH-900Plus Installation Guide 220329gerente soportecNoch keine Bewertungen
- Chem-7 Domestic v2012.01.02 Operator ManualDokument101 SeitenChem-7 Domestic v2012.01.02 Operator ManualSajanan S S ChathannurNoch keine Bewertungen
- Discover New Ways to Alleviate SufferingDokument6 SeitenDiscover New Ways to Alleviate SufferingFrancisco Arias0% (1)
- URIT-2900 Training (2020 - 08 - 20 02 - 50 - 44 UTC)Dokument53 SeitenURIT-2900 Training (2020 - 08 - 20 02 - 50 - 44 UTC)Arnoldo FelixNoch keine Bewertungen
- Dragon Lab CentrifDokument20 SeitenDragon Lab CentrifJaviAbadinNoch keine Bewertungen
- Vdocuments - MX - Chemray 240 Service Manual V10eDokument44 SeitenVdocuments - MX - Chemray 240 Service Manual V10eJoshua OkuraNoch keine Bewertungen
- RX DaytonaDokument16 SeitenRX Daytonajedi_exNoch keine Bewertungen
- BC-20s Auto Hematology Analyzer: Minimize Size, Maximize PossibilitiesDokument3 SeitenBC-20s Auto Hematology Analyzer: Minimize Size, Maximize PossibilitiesAniket DubeyNoch keine Bewertungen
- EasyLyte's ManualDokument113 SeitenEasyLyte's ManualAlex Sarmiento67% (3)
- Pentra 120 DX PrintDokument6 SeitenPentra 120 DX Printnbiolab6659Noch keine Bewertungen
- SOP05-5002F Service Manual ProlyteDokument41 SeitenSOP05-5002F Service Manual ProlyteRogger Ruffini100% (1)
- ABX Pentra 80 - Service Manual PDFDokument333 SeitenABX Pentra 80 - Service Manual PDFFrepa_ALNoch keine Bewertungen
- Abl90 Manual OperaçãoDokument59 SeitenAbl90 Manual OperaçãoMarlos CarapetoNoch keine Bewertungen
- BC-2800 Service Training: Hematology Global Technical Support Department Version V2.0 NO.:MXQ-12028-BC-2800Dokument157 SeitenBC-2800 Service Training: Hematology Global Technical Support Department Version V2.0 NO.:MXQ-12028-BC-2800Edgar Mendoza GarcíaNoch keine Bewertungen
- SUITPlus Protocol Description v4.0Dokument58 SeitenSUITPlus Protocol Description v4.0amr sabry100% (1)
- XL Series Parts Concept for DistributorsDokument12 SeitenXL Series Parts Concept for DistributorsIllusive ManNoch keine Bewertungen
- Crea 275 - 564 XL-1000 - Xsys0024 - 76 - FDokument4 SeitenCrea 275 - 564 XL-1000 - Xsys0024 - 76 - FNonameNoch keine Bewertungen
- 3.8 HDSD NeoChem 100Dokument117 Seiten3.8 HDSD NeoChem 100huy Lê xuan Thanh100% (1)
- Insight v5 Haematology Analyser Service ManualDokument127 SeitenInsight v5 Haematology Analyser Service ManualMicle RechaNoch keine Bewertungen
- Icon 5 User ManualDokument97 SeitenIcon 5 User ManualMon John GascoNoch keine Bewertungen
- Abbott I-Stat 1 Analyzer - System ManualDokument454 SeitenAbbott I-Stat 1 Analyzer - System ManualRastateNoch keine Bewertungen
- Mnchip Routine MaintenanceDokument8 SeitenMnchip Routine Maintenancedmantsio100% (1)
- Roche Midtron Junior II - Service ManualDokument137 SeitenRoche Midtron Junior II - Service Manualleopa78Noch keine Bewertungen
- Flyer Hemolyzer 3 ProDokument4 SeitenFlyer Hemolyzer 3 Prolantip rujitoNoch keine Bewertungen
- RT-2100C+ User's Manual V2.6eDokument44 SeitenRT-2100C+ User's Manual V2.6eJosian Robles AbreuNoch keine Bewertungen
- FT4NDokument8 SeitenFT4NTrisnoNoch keine Bewertungen
- H50&H50P Service Manual V7.0 enDokument262 SeitenH50&H50P Service Manual V7.0 enSunand Nambiar100% (1)
- DSG AU2700 AU5400 LISspecsDokument65 SeitenDSG AU2700 AU5400 LISspecsquangbo1310Noch keine Bewertungen
- Sfri Brochure-Hemix330 Eng BDDokument4 SeitenSfri Brochure-Hemix330 Eng BDYair Tigre100% (1)
- Bs 800 Chemistry Analyzer PDFDokument1 SeiteBs 800 Chemistry Analyzer PDFДимонNoch keine Bewertungen
- Vital Scientific Vitalab - User ManualDokument142 SeitenVital Scientific Vitalab - User ManualSulehri EntertainmentNoch keine Bewertungen
- Ic-2600 Service ManualDokument30 SeitenIc-2600 Service ManualAlexeyNoch keine Bewertungen
- Market Review Blood Gas Analyser S 2010Dokument43 SeitenMarket Review Blood Gas Analyser S 2010Fercalo AndreiNoch keine Bewertungen
- EHT5 Product Manual PRDokument93 SeitenEHT5 Product Manual PRAlexeyNoch keine Bewertungen
- DXH 520 Implementation Workbook 2.2Dokument94 SeitenDXH 520 Implementation Workbook 2.2Eureca Chara firmanNoch keine Bewertungen
- INS00060 ECL 412 - EN - User Manual - A-l25RZTUQYHDokument86 SeitenINS00060 ECL 412 - EN - User Manual - A-l25RZTUQYHBereket TeketelNoch keine Bewertungen
- Prietest Touch User ManualDokument52 SeitenPrietest Touch User ManualRoger SilvaNoch keine Bewertungen
- Abbott I-Stat 1 Analyzer - System ManualDokument454 SeitenAbbott I-Stat 1 Analyzer - System ManualАлександрНовоселов100% (2)
- Medical Equipment Management A Complete Guide - 2020 EditionVon EverandMedical Equipment Management A Complete Guide - 2020 EditionNoch keine Bewertungen
- Antigen-Antibody Reactions In Vivo: Methods in Immunology and Immunochemistry, Vol. 5Von EverandAntigen-Antibody Reactions In Vivo: Methods in Immunology and Immunochemistry, Vol. 5Noch keine Bewertungen
- 68th AACC Annual Scientific Meeting Abstract eBookVon Everand68th AACC Annual Scientific Meeting Abstract eBookNoch keine Bewertungen
- Autoclave CatalogueDokument17 SeitenAutoclave CatalogueUMARALEKSANA, CVNoch keine Bewertungen
- AED and Defibrillator CatalogueDokument17 SeitenAED and Defibrillator CatalogueUMARALEKSANA, CVNoch keine Bewertungen
- Physiotherapy Catalogue PDFDokument49 SeitenPhysiotherapy Catalogue PDFUMARALEKSANA, CV100% (1)
- How To Install EKG / ECG Lead PDFDokument2 SeitenHow To Install EKG / ECG Lead PDFUMARALEKSANA, CV100% (1)
- Service Claim FormDokument1 SeiteService Claim FormUMARALEKSANA, CVNoch keine Bewertungen
- ECG EKG Cable and Leadwires ElectrodesDokument42 SeitenECG EKG Cable and Leadwires ElectrodesUMARALEKSANA, CVNoch keine Bewertungen
- ECG InterpretationDokument52 SeitenECG InterpretationMarcus, RN98% (44)
- Itc 400 D PDFDokument2 SeitenItc 400 D PDFUMARALEKSANA, CVNoch keine Bewertungen
- Alamat Laboratories SurabayaDokument1 SeiteAlamat Laboratories SurabayaUMARALEKSANA, CVNoch keine Bewertungen
- 60 91 EndocrineDokument34 Seiten60 91 EndocrineYaj CruzadaNoch keine Bewertungen
- Worksheet Ap04Dokument15 SeitenWorksheet Ap04mark rothNoch keine Bewertungen
- University of Saint Louis Biochemistry ModuleDokument19 SeitenUniversity of Saint Louis Biochemistry ModuleLy xyNoch keine Bewertungen
- Patologia Do Sistema Endócrino em CãesDokument45 SeitenPatologia Do Sistema Endócrino em CãesSaulo CardosoNoch keine Bewertungen
- Endocrine NotesDokument157 SeitenEndocrine NotesLaila Cristine CarvalhoNoch keine Bewertungen
- Thyroid & Antithyroid Drugs: Thyroglobulin, Hormone SynthesisDokument13 SeitenThyroid & Antithyroid Drugs: Thyroglobulin, Hormone SynthesisLatiNoch keine Bewertungen
- DR Ananta Thyroid SlideDokument73 SeitenDR Ananta Thyroid SlideRoshan Kumar PanditNoch keine Bewertungen
- Thyroid Function Ordering AlgorithmDokument1 SeiteThyroid Function Ordering AlgorithmMVKDSP RaoNoch keine Bewertungen
- HyperthyroidismDokument6 SeitenHyperthyroidismNader Smadi100% (2)
- Types of Thyroid MedicationsDokument3 SeitenTypes of Thyroid MedicationsfennarioNoch keine Bewertungen
- Hypo Thy Rod IsmDokument17 SeitenHypo Thy Rod IsmanusinghNoch keine Bewertungen
- Thyroid DisordersDokument45 SeitenThyroid Disorders9rbf7pkgppNoch keine Bewertungen
- Anatomy and Physiology,: Lecture OutlineDokument79 SeitenAnatomy and Physiology,: Lecture OutlineJorelyn Giron NaniongNoch keine Bewertungen
- Hyper Guidelines 2011Dokument65 SeitenHyper Guidelines 2011wrocha2000Noch keine Bewertungen
- BBLRDokument9 SeitenBBLREni RahmawatiNoch keine Bewertungen
- 70 Years of LevothyroxineDokument141 Seiten70 Years of LevothyroxinecavrisNoch keine Bewertungen
- Thyroid Disorder PDFDokument324 SeitenThyroid Disorder PDFdhilsss nabNoch keine Bewertungen
- Thy.2016.0229 1Dokument117 SeitenThy.2016.0229 1Peter InocandoNoch keine Bewertungen
- Thyroid Storm - UpToDateDokument21 SeitenThyroid Storm - UpToDateJENNYLA HAZEL SICLOTNoch keine Bewertungen
- Penyakit Jantung Tiroid2Dokument30 SeitenPenyakit Jantung Tiroid2Yunan ElfaNoch keine Bewertungen
- Anatomy. Case Study. It's Just StressDokument4 SeitenAnatomy. Case Study. It's Just Stressjonar parinasNoch keine Bewertungen
- Thyroid Gland: Dr. May GalalDokument71 SeitenThyroid Gland: Dr. May GalalKAMALNoch keine Bewertungen
- 1-Complete Blood Count - PO1106326185-399Dokument8 Seiten1-Complete Blood Count - PO1106326185-399Arup KumarNoch keine Bewertungen
- Nursing Care of Adults: Metabolic and Endocrine DisordersDokument134 SeitenNursing Care of Adults: Metabolic and Endocrine DisordersMary Ann Pardilla Alcober100% (4)
- TT3 15Dokument6 SeitenTT3 15drubaldoNoch keine Bewertungen
- 2022 - JER - Levothyroxine Conventional and Novel Drug DeliveryDokument24 Seiten2022 - JER - Levothyroxine Conventional and Novel Drug DeliveryLuiz Fernando Fonseca VieiraNoch keine Bewertungen
- 10 Things that Stopped My Thyroid Hair LossDokument115 Seiten10 Things that Stopped My Thyroid Hair LossAnonymous vI4dpAhcNoch keine Bewertungen
- Hypothalamic Pitutary Axis and Thyroid Hormone SynthesisDokument5 SeitenHypothalamic Pitutary Axis and Thyroid Hormone Synthesis78 shivangi mauryaNoch keine Bewertungen
- Thyroid EssayDokument2 SeitenThyroid EssayAine Graham100% (1)
- Thyroid Disorders Part I Hyperthyroidism Little 2006Dokument9 SeitenThyroid Disorders Part I Hyperthyroidism Little 2006Jing XueNoch keine Bewertungen