Beruflich Dokumente
Kultur Dokumente
Extraction Method
Hochgeladen von
soubi_yodiOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Extraction Method
Hochgeladen von
soubi_yodiCopyright:
Verfügbare Formate
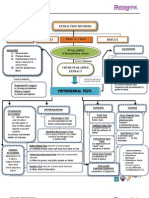
Extraction Method Reflux What is heating under a reflux?
? During extraction, heating under a reflux is heating of a mixture of compounds and the material for extraction in a flask with a condenser on top. This is done for its principle of heating without the loss of any solvents and compound. 1. Grind plant leaves to make 50g of powdered plant leaves 2. Fifty grams of the powdered plant leaves will be extracted by heating under reflux for 60 minutes with 750 mL 50% ethanol, accompanied by the addition of 250 mL 10% lead (II) acetate solution. Heating under reflux enables a mixture containing volatile liquids to be heated for a long time without the loss of the solvent. 3. The mixture is cooled and filtered; the filtrate is collected and is further extracted by shaking with three 375 mL quantities of dichloromethane/ isopropanol (3:2). The shaking should be gentle to avoid formation of emulsion. 4. The combined lower phases are filtered over anhydrous sodium sulfate and evaporated to remove the excess water. 5. The residue is collected and dissolved in 25 mL dichloromethane/ isopropanol (3:2). The solution obtained can then be ready for analysis for the presence of cardiac glycoside in saluyot leaves.
In heating under reflux, the water should enter from the bottom of the condenser. Also, there must not be a stopper at the top of the condenser, as the whole system cannot be sealed or else it may burst. The mixture of 50% ethanol, powdered saluyot leaf extract and 10% lead (II) acetate solution will be placed in the flask at the bottom.
General tests With FeCl3 A. Reagents 1. 4mL of glacial acetic acid 2. One drop of ferric chloride solution 3. 2mL of concentrated H2SO4 B. Materials and Apparatus 1. Serological pipette 2. Test tube 3. Dropping pipette 4. Beaker C. Procedure 1. Dissolve 1ml of extract in 4ml of glacial acetic acid containing one drop of ferric chloride solution. 2. Under lay the solution with 2ml of concentrated H2SO4. 3. A brown ring obtained at the interface indicates the presence of glycosides. D.Positive Result A brown ring obtained at the interface indicates the presence of glycosides. E. Principle The brown ring obtained is due to the presence of glycone sugar.
Foam test A. Reagents
1. 20mL distilled water. 2. 3gtts of olive oil B. Materials and Apparatus 1. Serological pipette 2. Graduated cylinder 3. Beaker 4. Filter paper 5. Hot paper C. Procedure 1. Boil about 2ml of sample in 20 ml of distilled water in a water bath and filter. 2. Mix 10ml of the filtrate with 5 ml of distilled water and shake vigorously for a stable persistent froth. 3. Mix the frothing with 3 drops of olive oil and shake vigorously, then observe for the formation of emulsion. D.Positive Result Formation of Emulsion E. Principle The formation of an emulsion is due to the presence of amphiphilic compound.
Specific tests Keller-Killiani A. Reagents 1.Glacial acetic acid 2.Ferric chloride solution 3.Concentrated sulphuric acid B. Materials and Apparatus
1.Dropping pipette 2.Test tubes C. Procedure 1.Treat five ml of each extracts with 2ml of glacial acetic acid containing one drop of ferric chloride solution. 2.Underlay the mixture with 1 ml of concentrated sulphuric acid. 3.A brown ring of the interface indicates a deoxysugar characteristic of cardenolides. 4.A violet ring may appear below the brown ring, while in the acetic acid layer, a greenish ring may form just gradually throughout thin layer D. Positive Result Presence of brown ring on the interface and greenish ring gradually on the upper phase. E. Principle Involved The brown ring indicates the presence of 2-deoxysugar in the glycone of the cardiac glycoside.
forms
portion
Salkowski A. Reagents 1. Chloroform 2. Sulfuric acid B. Materials and Apparatus 1. Dropper 2. Test tubes C. Procedure 1. Add 1ml of the extract to 2ml of chloroform. 2. Carefully add H2SO4. A reddish brown color at the interface indicates the presence of aglycone portion of cardiac glycoside. D. Positive Result Reddish-brown color at the interface E. Principle Involved
In the Salkowski reaction based method, cholesterol is oxidized in the presence of an excess amount of phosphoric acid and ferric ions to give a reddish brown derivative. The presence of the aglycone portion of the cardiac glycoside structure is detected. The aglycone part is the portion of the structure where there is the absence of sugar.
Baljet A. Reagents 1. Picric Acid 2. Ethanol 3. Sodium Hydroxide B. Materials and Apparatus 1.Dropper 2.Test tubes C. Procedure 1. To prepare Solution I for Baljet Test, place 1g of picric acid in of EtOH. 2. To prepare Solution II for Baljet Test, add 10g NaOH in 100mL 3. Combine the two solutions. 4. Add 2-3 drops of the combined solution to 2-3mg of sample; a reaction is indicated by orange to deep red color. D. Positive Result Orange to deep red coloration E. Principle Involved Reactions due to (-CH2-) group of the lactone ring. cardenolide + Baljet's reagent (picric acid +NaOH) orange or red
100mL water. positive
Barfoed A. Reagent Barfoeds reagent (mixture of acetic acid and copper (II) acetate B. Materials 1. Serological pipette 2. Aspirator 3. Parafilm 4. Test tube 5. Hot plate 6. Beaker C. Procedure 1. In a test tube, add 1mL of Barfoeds reagent to 5gtts of the extract. 2. Seal the test tube with parafilm so as to prevent volatile substances from evaporating. 3. Place the solution in a boiling water bath. 4. When the solution yielded a reaction, remove from the water bath. 5. Observe a brick red precipitate indicating the presence of monosaccharide. D. Positive result Brick red precipitate E. Principle Involved Cardiac glycosides contain a sugar portion called glycone and a non sugar portion called the aglycone. If a reducing sugar is present, a brick red precipitate of Copper (II) oxide is form. The reaction will be negative in the presence of disaccharide sugar as they are weaker reducing agents. One can distinguish monosaccharide from disaccharide based on how fast the brick red precipitate is form. Monosaccharide reacts within 2-3 minutes, whereas disaccharide takes longer. The reaction involved is oxidation in acidic medium.
Seliwanoff A. Reagents Seliwanoffs reagent (dissolving 50 mg of resorcinol in 100 ml of dilute HCl or resorcinol in 6M HCl) B. Materials 1. Dropper 2. Breaker 3. Hot plate 4. Test tube C. Procedure 1. Place 0.5ml of a sample solution is in a test tube. 2. Add two ml of Seliwanoff's reagent (a solution of resorcinol and 3. Heat the solution in a boiling water bath for two minutes. D. Positive result Cherry red solution E. Principle Involved Cherry red solution indicates the presence of ketohexoses. The test reagent dehydrates ketohexoses to form 5-hydroxymethylfurfural. 5hydroxymethylfurfural further reacts with resorcinol present in the test reagent to produce a red product within two minutes. Aldohexoses react to form the same product, but do so more slowly.
HCl.)
Das könnte Ihnen auch gefallen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Phytochemical Tests: Theoretical FrameworkDokument4 SeitenPhytochemical Tests: Theoretical Frameworksoubi_yodiNoch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Prep 22-27Dokument2 SeitenPrep 22-27soubi_yodi100% (1)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Internship 5 - 7Dokument62 SeitenInternship 5 - 7Ysmael MagnoNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- CPOEDokument1 SeiteCPOEsoubi_yodiNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Distillation From GavhaneDokument48 SeitenDistillation From GavhaneSolanum tuberosum100% (3)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- HYSYS LAB 1 SolutionDokument7 SeitenHYSYS LAB 1 SolutionDhanesh KumarNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- CHE CAL Module 3Dokument13 SeitenCHE CAL Module 3Beatrice AlejeNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Aspen Capital Cost EstimatorDokument28 SeitenAspen Capital Cost EstimatordimolinaNoch keine Bewertungen
- Organic Syntheses, Coll. Vol. 2, p.219 (1943) Vol. 16, p.25Dokument3 SeitenOrganic Syntheses, Coll. Vol. 2, p.219 (1943) Vol. 16, p.25ariesterNoch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- International Reactor Corporation: Standard and Custom ReactorsDokument8 SeitenInternational Reactor Corporation: Standard and Custom Reactorskumar_chemicalNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Chemsheets As 062 (Practical Guide) (1) - See NowDokument6 SeitenChemsheets As 062 (Practical Guide) (1) - See NowPrincess KimNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- 11 7845 JS Aspen HYSYS Dynamics Columns FINALDokument20 Seiten11 7845 JS Aspen HYSYS Dynamics Columns FINALkarthick100% (1)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Factors Affecting Distillation OperationDokument1 SeiteFactors Affecting Distillation OperationrahulNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- 10Dokument25 Seiten10aytajNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- P2CaseBook PDFDokument265 SeitenP2CaseBook PDFmfruge7Noch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Properly Employ Overhead Condensers For Vacuum ColumnsDokument8 SeitenProperly Employ Overhead Condensers For Vacuum ColumnsmrtiemannNoch keine Bewertungen
- Chapyer 01 Modern Distillation TechniquesDokument20 SeitenChapyer 01 Modern Distillation TechniquesYezdi Solaina100% (3)
- Lab Manual Analytical Organic Chemistry CLB 10803Dokument30 SeitenLab Manual Analytical Organic Chemistry CLB 10803Arif HilmiNoch keine Bewertungen
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Tutorial-Chapter 2 (June - Oct 2013)Dokument5 SeitenTutorial-Chapter 2 (June - Oct 2013)paulineanakmawatNoch keine Bewertungen
- Design of A Separation ProcessDokument8 SeitenDesign of A Separation Processdario delmoralNoch keine Bewertungen
- Research Article: Design of Batch Distillation Columns Using Short-Cut Method at Constant RefluxDokument15 SeitenResearch Article: Design of Batch Distillation Columns Using Short-Cut Method at Constant RefluxSanthosh RockNoch keine Bewertungen
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Amphetamine Sulphate SynthesisDokument12 SeitenAmphetamine Sulphate SynthesisLorenzo Tosi67% (9)
- Name: Safoora Shabbir Registration No: MCH172070 Class: M.Sc. Chemistry 2 Semester Section: B Submitted To: Dr. Riffat JawariaDokument7 SeitenName: Safoora Shabbir Registration No: MCH172070 Class: M.Sc. Chemistry 2 Semester Section: B Submitted To: Dr. Riffat JawariaSafooraShabbirNoch keine Bewertungen
- Wa0015 PDFDokument43 SeitenWa0015 PDFEstherNoch keine Bewertungen
- Presentación - Batch Processes Introduction - ETH ZurichDokument61 SeitenPresentación - Batch Processes Introduction - ETH ZurichizolatNoch keine Bewertungen
- Distillation ColumnDokument32 SeitenDistillation ColumnTatiana RosarioNoch keine Bewertungen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Making Methamphetamine at HomeDokument5 SeitenMaking Methamphetamine at Homenicholasworkingclass100% (1)
- Practicaldistillationcontrol 140307141500 Phpapp01Dokument549 SeitenPracticaldistillationcontrol 140307141500 Phpapp01syedmuhammadtariqueNoch keine Bewertungen
- Project Simualtion DR ShawalliahDokument17 SeitenProject Simualtion DR ShawalliahharrisNoch keine Bewertungen
- Distillation Experiment ProcedureDokument5 SeitenDistillation Experiment ProcedureZach Sayari PadagdagNoch keine Bewertungen
- N-Vinylpyrrolidin-2-One As A 3-Aminopropyl Carbanion Equivalent in The Synthesis of Substituted 1-Pyrrolines: 2-Phenyl-1-PyrrolineDokument5 SeitenN-Vinylpyrrolidin-2-One As A 3-Aminopropyl Carbanion Equivalent in The Synthesis of Substituted 1-Pyrrolines: 2-Phenyl-1-Pyrrolinezodd01Noch keine Bewertungen
- Distillation 1Dokument19 SeitenDistillation 1Salman HaniffaNoch keine Bewertungen
- Distillation Exp.Dokument5 SeitenDistillation Exp.Ibrahim DewaliNoch keine Bewertungen
- Aspen Plus Tutorial ÿËÈøË ÖÝÁ Óñ Ì° Texsas - University - Aspen - Plus - TutorialDokument55 SeitenAspen Plus Tutorial ÿËÈøË ÖÝÁ Óñ Ì° Texsas - University - Aspen - Plus - TutorialrajindoNoch keine Bewertungen
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)