Beruflich Dokumente
Kultur Dokumente
Occupational Hazards in Orthodontics: A Review of Risks and Associated Pathology
Hochgeladen von
Dhiren SoniOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Occupational Hazards in Orthodontics: A Review of Risks and Associated Pathology
Hochgeladen von
Dhiren SoniCopyright:
Verfügbare Formate
REVIEW ARTICLE
Occupational hazards in orthodontics: A review of risks and associated pathology
Nikolaos Pandis,a Brandi D. Pandis,a Vasilios Pandis,a and Theodore Eliadesb Corfu and Thessaloniki, Greece The purpose of this article was to review the occupational hazards related to the practice of orthodontics. A systematic approach was used to include all risks involved in an orthodontic practice. The classication of hazards was based on major sources of risks by system or tissue and by orthodontic ofce area (dental chair, laboratory, sterilization area, x-ray developing area). Potentially hazardous factors relate to the general practice setting; to specic materials and tools that expose the operator to vision and hearing risks; to chemical substances with known allergenic, toxic, or irritating actions; to increased microbial counts and silica particles of the aerosols produced during debonding; to ergonomic considerations that might have an impact on the providers muscoleskeletal system; and to psychological stress with proven undesirable sequelae. The identication and elimination of these risk factors should be incorporated into a standard practice management program as an integral part of orthodontic education. Professional organizations can also assist in informing practitioners of potential hazards and methods to deal with them. (Am J Orthod Dentofacial Orthop 2007;132:280-92)
ccupational hazard is dened as a risk accepted as a consequence of a particular occupation.1 Professionals in dentistry are exposed to many occupational hazards; their effects appear as ailments that affect the dental practitioner and tend to intensify with age. These problems include musculoskeletal conditions due to improper body posture; physical hazards from light, noise, and trauma; biological risks from irradiation and microorganisms; and chemical detrimental sources. Risk factors for professionals have been studied mainly with survey questionnaires directed to clinicians. However, the results of surveys might apply only to the respondents and are valid at that specic time. Because some professionals do not respond to these surveys, the pool of respondents is biased because the respondents are usually the ones with problems. In general, the literature lists a limited number of investigations, which conrmed the responders medical problems by further clinical and laboratory testing. With the exception of Scandinavian countries and North America, where the level of awareness of potential health risks for operators is high, studies dealing with occupational hazards among orthodontists are
Private practice, Corfu, Greece. Associate professor, Department of Orthodontics, School of Dentistry, Aristotle University of Thessaloniki, Thessaloniki, Greece. Reprint requests to: Theodore Eliades, 57 Agnoston Hiroon St Nea Ionia GR-14231, Greece; e-mail, teliades@ath.forthnet.gr. Submitted, August 2006; revised and accepted, October 2006. 0889-5406/$32.00 Copyright 2007 by the American Association of Orthodontists. doi:10.1016/j.ajodo.2006.10.017
scarce. Jacobsen and Hensten-Pettersen2 investigated the occupation-related complaints of Norwegian orthodontists relative to data obtained in 1987. Most complaints were skin reactions on the hands and ngers from handling acrylics and bonding materials and from gloves. A few respiratory and systemic reactions were observed. The most recent study found signicantly fewer adverse reactions to detergents and soaps, but reactions to biomaterials persisted.3 Similarly, Munskgaard et al,4 in a survey of Danish dentists, found skin symptoms at a prevalence of 38%, with acrylic resin the key etiological factor. A comparable survey among Swedish dentists found a similar prevalence accompanied with eye and respiratory system reactions; the sources of the adverse effects were cold-curing acrylics and bonding materials.5 Recent studies found that methacrylates, natural rubber latex proteins, rubber glove allergens, and glutaraldehyde caused reactions ranging from cell-mediated contact allergy to urticaria and occupational asthma.6,7 Sinclair reported that over 40% of dentists had experienced symptoms of hand dermatoses and irritations to eyes, nose, and airway at some point in their practicing lives, and women had twice the odds of experiencing allergy symptoms.8 The prevalence of contact allergy to acrylates was below 1% of the responding dentists and, in most cases, did not have serious medical, social, or occupational consequences.9 Hand dermatitis experienced by the dental personnel has been attributed to hand washing, occupational exposure to many possible sensitizers, and frequent latex glove use. Although
280
American Journal of Orthodontics and Dentofacial Orthopedics Volume 132, Number 3
Pandis et al 281
Table I.
Hazards in orthodontic ofce by work area
Respiratory Muscoloskeletal Neck, shoulder, upper and lower back pain CTS Tendinopathies Repetitive strain injuries Neck, shoulder, upper and lower back pain Hearing Handpiece noise High volume Suction Ultrasonic scaler Vision Dry-eye syndrome Maculopathies Cataract Eye trauma Eye strain Infection Dry-eye syndrome Eye trauma Chemical burn Infection Dry-eye syndrome Eye trauma Chemical burn Infection Dry eye syndrome Eye trauma Chemical burn Infection Skin Allergy (chemicals) Trauma Infection
Dental chair area
Inhaling of chemicals (composites) Allergens Infection
Sterilization area
Inhaling of chemicals Allergens Infection Inhaling of chemicals Allergens Infection
Ultrasonic cleaner
Laboratory area
X-ray developer area
Inhaling of chemicals Allergens
Neck, shoulder, upper and lower back pain CTS Tendinopathies Repetitive strain injuries Neck, shoulder, upper and lower back pain
Model trimmer Vibrators Low-speed handpieces
Allergy (chemicals) Trauma Infection Burning Allergy (chemicals) Trauma Infection Burning Allergy (chemicals) Trauma
there is a dispute in the literature as to the true frequency of allergic contact dermatitis in dental personnel,10 extreme caution in handling these substances and close attention to the manufacturers directions are paramount for diminishing the adverse effects og substances that have not been thoroughly documented in clinical settings.11 The purpose of this article was to review and classify the health risks of practicing orthodontics and associated pathology. Additionally, a guide to the management of hazards in everyday practice is provided, with evidence to increase professionals awareness of this issue.
CATEGORIES AND SOURCES OF HAZARDS
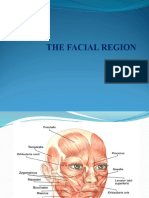
Table I categorizes the hazards of orthodontic practice by work area; the Figure gives a general classication of the hazards based on the source of risk.
HEALTH HAZARDS
A general classication of potential operator hazards in orthodontics includes the following. 1. Health hazards impose threats to a persons biological balance from exposure to physical factors (lights, noise, vibration, heat, trauma), chemical irritating or toxic factors (latex, monomers, sterilization and radiology uid, aerosols during debonding), and biological factors (infections from microorganisms). 2. Other hazards include risks to the professionals well-being, associated with physical or psychological factors such as ergonomic considerations (insufcient or inappropriate equipment, inappropriate work area design) and psychological stress (dealing with patients in general, difcult patients, employees, legal action, and work organization).
Health hazards for clinical orthodontists include physical factors such as lights, noise, vibration, heat, and trauma. Lights affect the eyes and vision. Ofce lighting and dental chair light are critical for optimal working conditions in an orthodontic setting. Additionally, other forms of light are used during daily procedures; the most important is the curing light for polymerization of bonding materials. The hazards associated with various forms of lighting in the orthodontic practice are summarized in Table II. Recently, lasers were introduced in orthodontics for ceramic bracket debonding12-14 and cosmetic gingival contouring.15-17 The hazards associated with laser light range from corneal/lens to retinal damage depending on the wavelength of the beam produced by each appliance. Eyestrain can also be a problem, due to concentration, insufcient lighting, and inappropriate position of working light in relation to the orthodontist.18 Maculopathies can be caused by poor lighting. Photoreceptor cells called rods are responsible for peripheral and dim light vision; they receive light and cones, which provide central, bright light, ne detail, and color vision. The photoreceptors convert light into nerve impulses, which are then processed by the retina
282 Pandis et al
American Journal of Orthodontics and Dentofacial Orthopedics September 2007
Fig. Taxonomy chart of sources of hazards in orthodontics.
Table II. Eye strain
Hazards related to eyes and vision
Dry-eye syndrome Decreased tear production Increased tear evaporation Maculopathies Blue-light hazard Cataract Injuries UV lights Allergy Latex glove cornstarch Other Trauma Burs Wire Lab projectiles Infection Splatter Aerosol contamination Trauma Materials Chemical burns Radiology and sterilization uids Disinfectants
Concentration
and sent through nerve bers to the brain. Until recently, light sensitivity was believed to depend on the rod and cone photoreceptor cells of retinas. Recent research, however, showed that some of our ganglion cells might perform as a third type of photoreceptor called intrinsically photosensitive retinal ganglion cells.19,20 These sparsely situated cells are most sensitive to blue light. They seem to exist principally to assist in differentiating between day and night, thus modulating the sleep/wake cycles, known as circadian rhythms.21-23 When light hits a photoreceptor, the cell bleaches and becomes useless until it recovers through a metabolic process called the visual cycle.24 Absorption of blue light, however, has been shown to reverse the process in rodent models. The cell becomes unbleached and responsive again to light before it is ready. This greatly increases the potential for oxidative damage, which leads to a buildup of lipofuscin in the retinal pigment epithelium layer.25 Drusen are then formed from excessive amounts of lipofuscin, hindering the retinal pigment epithelium in its ability to provide nutrients to the photoreceptors, which then wither and die. Drusen are yellow or white deposits in the retina of the eye or on the optic nerve head. The presence of drusen is a common early sign of agerelated macular degeneration. Their presence alone does not indicate disease, but it can imply that the eye is at risk for developing more severe age-related macular degeneration. In addition, if the lipofuscin absorbs
blue light in high quantities, it becomes phototoxic; this can lead to oxidative damage to the retinal pigment epithelium and further cell death (apoptosis).26 Blue light is an important element in natural lighting, and it can also contribute to our psychological health.27 Research, however, shows that high illumination levels of blue light can be toxic to cellular structures, test animals, and human fetal retinas.28 The eyes of people operating curing lamps are at risk from acute and cumulative effects, mainly due to back reection of the blue light. Satrom et al29 evaluated 11 curing lights systems that produced visible blue light in the 400 to 500 nm range and found that no unit posed a health risk. A more recent and relevant study, comparing the effects of halogen, plasma, and lightemitting diode units on vision, reported that the exposure time required for plasma and light-emitting diode lamps to achieve curing depth similar to the tungstenhalogen light was longer than the irradiation times recommended by the manufacturers. This is important to know because blue light or ultraviolet (UV) hazard is related to exposure time, and thus requirements for prolonged irradiation can adversely affect vision. Additionally, some plasma units were been found to emit light in the ultraviolet-A region.30 A cataract is a condition with clouding or loss of transparency of the lens. Light transmission through the lens is hindered, and this results in dim, distorted, or blurred images on the retina and decreased vision.
American Journal of Orthodontics and Dentofacial Orthopedics Volume 132, Number 3
Pandis et al 283
Penetrating injuries (eg, from wire segments or adhesive chips during debonding) and UV light (from photopolymerizing units) are risk factors for cataracts.31 Infections can be caused by splashing material, aerosols, and trauma from wires, burs, and other projectiles. Trauma associated with microorganisms could cause various eye infections.31 Chemical burns come from acids or alkaline substances. Acids are usually less dangerous than alkalis because they tend to precipitate tissue proteins, which form barriers and inhibit deeper penetration; therefore, lesions are limited to lids, conjunctiva, and cornea. Alkalis saponify lipids in the corneal epithelium and bind to mucoproteins and collagen in the corneal stroma. In this way, they disrupt the normal barriers of the cornea, gain rapid access to the more posterior parts of the eye, and can cause severe eye complications including cataract and secondary glaucoma.31 The risk of eye hazards from acids mostly relates to the patient during bonding; additional eye protection such as glasses might be necessary. The presence of alkalis and their potentially hazardous effects can arise from the careless use of disinfectants or other liquid materials sprayed on surfaces or appliances. The operator must use protective eyewear. Dry-eye syndrome is related to reduced blinking (prolonged concentration), decreased tear production, or increased tear evaporation caused by excessive lighting, heat, or air-conditioning. Symptoms of dryeye syndrome include irritation, foreign body sensations, stringy mucous, and transient blurred vision. Burning sensation, itching, photophobia, and tired or heavy feeling of the eyelids are less frequently reported.31-38
Noise
Table III.
Permissible noise exposure levels (OSHA)
Sound level dB (A) slow response 85 86 88 89 91 92 94 97 100
Duration per day (h) 8 6 4 3 2 1 1 or less Data from OSHA40
The effects of occupational noise in the orthodontic ofce can lead to noise-induced hearing loss (NIHL); symptoms can include difculty with speech communication and other auditory signals, fatigue, and tinnitus. The symptoms of NIHL increase gradually with continual exposure. The process of hearing begins when sound waves enter the outer ear and reach the middle ear, where the tympanic membrane vibrates and sets the 3 ossicles malleus, incus, and stapesinto motion. The eardrum and the ossicles amplify the vibrations and send them to the oval window via the stapes. From the oval window, the force moves through the uid-lled cochlea and stimulates tiny hair cells in the cochlea. Individual hair cells respond to specic sound frequencies, so, depending on the frequency of the sound, only certain hair cells are stimulated. Signals from these hair cells are
translated into nerve impulses, which are carried to the brain, via the acoustic nerve, where they are interpreted as a particular sound. NIHL, currently not treatable, occurs when exposure to harmful sounds causes damage to the tiny hair cells in the cochlea and to the acoustic nerve. The greatest damage is usually caused at 3000 to 6000 Hz. NIHL can be caused by repeated exposure to sounds at various loudness levels, measured in decibels (dB), over an extended time or by a 1-time exposure to an intense sound. Exposure to noise over a long time can cause a temporary threshold shift, which is a temporary elevation in the hearing threshold that gradually recovers. It might range from a change of a few decibels to a change that temporarily renders the ear severely impaired. A noise-induced permanent threshold shift occurs after a long period of continual exposure to hazardous noise combined with the effects of aging.39 According to the National Institutes of Health, sounds above 85 dB are potentially hazardous. To determine which sounds are hazardous, the frequency and the duration of the sound must be specied. Generally, a noise level of 85 dB (A) in the normal range of hearing, for an 8-hour per day exposure, over a period of years, might be damaging. Sound levels less than 75 dB (A) are considered unlikely to cause permanent hearing loss. DB (A) refers to the decibel scale usually used to measure sound to which people are exposed. Table III shows permissible noise exposures according to the Occupational Safety and Health Administration (OSHA).40 Several studies on used and new dental equipment recorded the sound levels of common sources of noise in dentistry; Table IV shows their ndings.41-44 A possible cause-and-effect relationship between the use of dental equipment and dentists hearing loss was the aim of several studies.45-48 Results of this research, however, do not provide a denitive answer
284 Pandis et al
American Journal of Orthodontics and Dentofacial Orthopedics September 2007
Table IV. Device
Noise levels from dental equipment
dB (A) 65.5-93 61.9-77 73-88 76 75 73 72-81 93.5 98.5 81.7-86.5
should be used during laboratory procedures, when injuries from projectiles are possible.
Chemical Factors
Air turbine handpiece Micromotor handpiece Scaler Irrigator Power suction tube Saliva suction tube Ultrasonic scaler Gypsum cutting equipment Vibrator Aspirator and engine
as to whether work-related noise impacts dentists hearing.49-54 The degree of risk might depend on several factors including age, personal susceptibility, total daily exposure, exposure measured over many years, smoking, medication, and noise exposure outside the dental ofce.55
Injuries
Occupational injuries of health professionals are another area of interest, due to the increased awareness of patient-doctor cross contamination. In 1995, a survey sponsored by the American Dental Association56 found injuries at a yearly rate of 3.4% among dentists; this agreed with the 3.6% reported by a similar study.57,58 Among specialists,59 orthodontists had the second lowest prevalence (1.9%) after endodontists (1.3%); pedodontists had 5.5%, prosthodontists 4.5%, and oral surgeons 2.6%.56 Bagramian and McNamara59 conducted a survey sponsored by the American Association of Orthodontists targeting specically orthodontists and their staffs. The ndings for orthodontists showed a low yearly injury rate of less than 1%, with most incidents occurring extraorally; this is an important nding, since doctor-patient contact during injury is minimal. Most injuries occurred to the index nger and thumb and during wire changes. Other injuries were caused by burs, explorers, rotary disks, scalers, and other sharp instruments. No needle-stick injuries were reported.59 Under the same program, McNamara and Bagramian60 investigated injuries to orthodontic staff. Their ndings showed similar injury rates (1.4% per year) and patterns compared with the orthodontists. In a study of ocular trauma among orthodontists, it was found that 43% of the respondents reported ocular injuries, mainly during debonding and trimming acrylic.61 Other procedures included ligating materials, pumicing, and acid etching. Most of the injuries (83.5%) were treated on site without long-term effects. Extra caution
Chemical factors include latex and associated allergies, monomers, and sterilization and radiology uids. Although gloves enhance the barrier abilities of the skin and help decrease cross contamination, adverse reactions to latex are side effects. The general population has a low sensitivity to latex,62,63 but in the health care eld, due to the continuous exposure to the allergens, sensitivity has been reported to be much higher.64-69 According to OSHA, Allergy to latex was rst recognized in the late 1970s. Since then, it has become a major health concern as an increasing number of people in the workplace are affected. Health care workers exposed to latex gloves or medical products containing latex are especially at risk. It is estimated that 8-12% of health care workers are latex sensitive. Between 1988-1992, the Federal Drug Administration (FDA) received more than 1,000 reports of adverse health effects from exposure to latex, including 15 deaths due to such exposure.70 Natural rubber latex, the main ingredient of protective gloves, occurs naturally and is produced from liquid extracts of the Hevea brasiliensis tree. From the extract stage until the nal product, latex gloves go through a series of processes that introduce into them agents such as benzothiazol, thiuram, and carbamate and other groups with strong allergenic potential. Immediate allergic reactions to latex can appear in those who have been repeatedly exposed to latex proteins through glove wearing and have developed high levels of IgE antibodies.71 Clinical signs include rash, rhinitis, edema, bronchospasmus, and allergic shock. Alternatively, allergens absorbed by the skin combine with proteins and form antigens as T cells, and become activated custom T cells that circulate in the blood and lymphatic systems. Thisthe sensitization stage creates the environment for a more immediate response to these allergens on a future contact with the host. The binding of the custom T cells and the reentering antigen induce immune responses that result in usually localized tissue damage (contact dermatitis). The reaction is usually not life threatening and has a delayed onset. Signs and symptoms include rash, itching, and skin exfoliation. Cornstarch powder on the natural rubber latex gloves, in addition to its role in type I allergy, might also contribute to the development of irritation and type IV allergy.72 The prevalence of latex allergy is probably declining because of increased awareness, and a recent study showed that type I
American Journal of Orthodontics and Dentofacial Orthopedics Volume 132, Number 3
Pandis et al 285
allergy among US dentists decreased from 1996 to 2001 to less than 4%.73 The addition of powders such as cornstarch to simplify glove usage has also been implicated in allergies via dust transportation of allergenic substances. The cornstarch particles, which absorb great quantities of proteins, are widely dispersed in the air, particularly when the gloves are changed. It has been demonstrated that the number of cornstarch particles or natural latex proteins collected in the air of a health care setting can sometimes be up to a hundred times higher than that in a room where only powder-free gloves are used.74,75 Two interesting studies evaluated the tendency of cornstarch to bind natural latex proteins; 3 preparations of cornstarch were evaluated for total protein levels76 and allergenic protein levels77: clean, unused dusting powder; cornstarch exposed to natural latex protein extracts; and cornstarch extracted from powdered gloves. Unexposed cornstarch contained no allergenic proteins, but both natural latex exposed cornstarch preparations had signicant amounts of allergenic proteins bound to the particles. The results of both studies clearly demonstrated that cornstarch binds to allergenic proteins, which cannot be detached by simply washing the powder. Adverse reactions to latex occur in signicant numbers of dental students and dental practitioners, with those who reported personal and familial atopy more likely to be affected.8,78 Studies in the dermatologic literature suggest that glove powders can exacerbate irritant dermatitis and enhance the potential for adverse reactions to other components of natural rubber latex gloves.79 Several articles provide direct evidence that natural latex protein allergens, bound to cornstarch particles, are a cause of respiratory allergic reactions and asthma-like attacks.80,81 Synthetic gloves (vinyl, nitrile) were introduced as an alternative for latex-sensitive people. However, they have proven inferior to latex in respect to their capacity to withstand mechanical stress and tactile sensation.82 Evaluation of natural latex, synthetic rubber, and synthetic polymeric glove materials showed various degrees of cytotoxicity in vitro83; this prompted the introduction of silicone, powder-free gloves. Allergic contact dermatitis caused by methacrylates is common among dental professionals. Geukens and Goossens84 found that 13 of the 31 people diagnosed with contact allergy worked in the dentistry, and Ortengren85 found high rates of hand eczema among dentists. Researchers demonstrated severe cytotoxicity for some monomers used in dentistry,86-89 and glove material permeation by monomers and substantial swell-
ing with structural changes were observed after contact with monomers in relatively short time periods.90,91 Two types of impression dental materials, polyethers and vinyl polysiloxanes, were tested for cytotoxicity, and the polyether materials were found to be more toxic than vinyl polysiloxanes.92 Sterilization and radiology uids are used to decontaminate or sterilize instruments, surfaces, and impressions contaminated with blood and saliva. Sterilant chemicals include aldehydes, phenols, and quaternary ammonium compounds. These chemicals can cause lung problems and dermatitis.93-95 Radiology uids contain chemicals such as ammonium thiosulfate, potassium sulphite, potassium carbonate, hydroquinone, diethylene glycol, acetic acid, and glutaraldehyde. These substances can cause symptoms ranging from skin irritation to allergy and pulmonary edema if mishandled.96 Careful handling of uids, according to the manufacturers directions, and sufcient ventilation are recommended.
Biologic Factors
Biologic factors include microorganisms and particles. In the dental ofce, the main source of infection is through interaction of the patient with the health caregiver. This can occur from direct contact with blood, body uids, secretions, and excretions (except sweat), regardless of blood presence, nonintact skin and mucous membranes regardless of blood presence. A thorough analysis of the infection hazards in dental and orthodontic practices is beyond the scope of this article; this information is provided in standard sterilizationdisinfection courses in pre- and postgraduate dental curricula. Infection can occur indirectly by contact with contaminated instruments, surfaces, equipment, and materials. Contact of sensitive body areas with infected droplets expelled from infected persons at short range or inhalation of suspended microorganisms that can survive for long periods can occur in the ofce environment.97 Other possible sources of infectious contamination are dental unit waterlines,97-102 handpieces,103-108 saliva ejectors and suctions, other devices attached to air and waterlines,109-111 and radiology equipment (especially digital sensors). Impression materials and orthodontic appliances transported between the clinical area and the laboratory could be a source of infection. Microorganisms are transferred in dental impressions and on dental casts, and certain microorganisms can survive in the cast material for several days. Commissioning laboratory work to an outside facility can transmit microorganisms even farther.112117,118 116 Toroglou et al, in specialty-specic studies, evaluated the contents of aerosols produced during
286 Pandis et al
American Journal of Orthodontics and Dentofacial Orthopedics September 2007
Table V.
Measures to reduce exposure to hazardous materials and procedures
Respiratory Muscoloskeletal Hearing Check noise level of operatory Vision Skin
Dental chair Fresh air access Adopt proper body posarea Ventilation ture during dental Use masks, aspiration chair work during debonding Use ample lighting and Follow guidelines for in direction that does infection control not produce awkward body posture Arrange intermittent work load Handle instrument properly Use stretching before work Sterilization Use ventilation and Ergonomically designed area masks area and appropriate Follow guidelines for bench height infection control Easy access to instruments/equipment Laboratory Masks, ventilation Ergonomically designed area (preferably fresh area and appropriate air access) bench height Follow guidelines for Adopt proper body posinfection control ture Easy access to frequently used instruments and equipment Take frequent breaks
Avoid prolonged concenUse powder-free, silicone tration and if necessary gloves if irritated by use assisting appliances conventional powdered Always use protective latex shield for photopolymer- Exercise measures sugization gested by Centers for Use protective eyewear for Disease Control for bonding and debonding infection control (patient and staff also) Cover cuts in exposed Avoid splashes during body areas (face) to rinsing and spraying avoid contamination by splashed liquids Use protective eyewear Cover all skin areas (wear long sleeves, gloves, mask)
Use insulation for ultrasonic baths
Use insulation when Disinfect impressions possible Exercise measures as in Use ear plugs durother areas for eye proing model prepatection ration and trimming
Avoid contact with methacrylates Use ventilation
debonding procedures in an orthodontic ofce. They concluded that orthodontists are exposed to high levels of aerosols and contaminants, and that debonding procedures are potentially hazardous to their health. Postexposure management is important to control and avoid further transmission of the infection. Detailed guidelines and information are given in the publication of the Centers for Disease Control and Prevention, Guidelines for Infection Control in Dental Care Settings2003.119 The foregoing concerns provoked the investigation of means to minimize bacterial counts in aerosols. Rinsing with antiseptic solutions before treatment was found to signicantly reduce the bacterial counts in aerosols during ultrasonic scaling.120 Apart from microorganism contamination, a concern was recently expressed on the composition of aerosol produced during the use of rotary instruments. Research indicated that these aerosols contain silica particles from the adhesive resin llers and various bur material byproducts. The sizes of these particles have been estimated between 2 and 30 , thus falling within the hazardous-product particle range of 2.5 .121 The concern about the small size of these particles relates to the fact that they are implicated in many diseases
because they can reach the alveoli. Thus, ventilation, use of masks and aspirators, and mechanical removal of as much resin as possible before using rotary instruments are suggested. In Table V, precautions and measures to reduce the exposure to hazardous materials and procedures are listed.
OTHER HAZARDS Musculoskeletal Problems
Dental professionals often develop musculoskeletal problems, which are related to suboptimal work-environment ergonomics that might be responsible for improper sitting postures and movements causing unnecessary musculoskeletal loading, discomfort, and fatigue. Insufcient or inappropriate equipment, inappropriate work-area design, direct injuries, repetitive movements from working with dental instruments, or sitting for extended times with a exed and twisted back are contributing factors to neck and low-back ailments.94,122-124 The limited research in the orthodontic literature showed increased risks for developing these types of pathology.124-127 Musculoskeletal problems happening outside the work environment can either worsen with work or
American Journal of Orthodontics and Dentofacial Orthopedics Volume 132, Number 3
Pandis et al 287
make work difcult.128 Various structures can be affectedmuscles, ligaments, tendons, nerves, joints, and supporting structures (intervertebral discs). A number of disorders are included under this category: upper and lower back pain, herniated disc, neck pain with or without cervical root problems, carpal tunnel syndrome, tendinopathies, shoulder pain, rotator cuff tendinopathies, and repetitive strain injuries. Upper and lower back pain and intervertebral disc disease (acute or chronic) are responsible for work absence in the general population. However, in evaluating absence from work, a differentiation is appropriate between employed and self-employed people, since the latter, often running a single clinician-operated practice, would be reluctant to miss from work for prolonged periods of time. The dental chair position and the dentists stool position and orientation relative to that of the patient, combined with the doctors effort to maintain visibility of the oral environment, result in awkward positions over long periods of time; these in turn result in back problems. The symptoms include low back pain, stiffness, and sciatica with neurological features such as tingling, paresthesia, and muscle weakness. Intervertebral disc herniation can be just simple bulging, causing pressure to the dura mater and resulting in backache, or it can be true herniation with direct pressure on a nerve root, causing pain, paresthesia, and muscle weakness to the corresponding neurotome. According to the natural history of disc herniation, the affected disc cannot be regenerated to its previous state, resulting in various degrees of loss of disc space and height, stiffness, spondyloarthosis (spondylosis), and spinal stenosis. This can result in chronic pain (mechanical low back pain) and difculty in performing various tasks such as bending, lifting, and driving long distances.128 Neck problems are associated with a similar etiology, especially awkward body and head posture, which are often required for direct vision into the mouth. The introduction of magnifying loupes is probably the only development over the years that helps dentists keep a more neutral or balanced posture.129 The symptoms include intermittent neck pain, often radiating to the shoulders (with stiffness); headaches; tingling, or pins and needles down the arms and ngers, resulting in weakness; and clumsiness. In more severe situations, disc prolapse can occur and, later, degeneration (cervical spondylosis). Because the shoulder muscles are innervated by the brachial plexus, there is also strain on the shoulder muscles (pain, weakness) that will complicate the situation further if there is coexisting rotator cuff pathology.130
The rotator cuff of the shoulder consists of the supraspinatus, infraspinatus, subscapularis, and teres minor muscles, which are responsible for abduction, rotation of the shoulder, and stabilization of the humerus head on the glenoid during movement. The most common tendon to be affected is the supraspinatus (tendonitis, partial tear, complete tear, and degeneration). Tendonitis usually causes pain and discomfort that worsens with movements. Tears also cause weakness in abduction; old and degenerative tears cause impingement in the subacromial region (arc pain in abduction, eased beyond 90-100). Although direct injuries are rare in dentistry, eccentric loading of the tendon or the muscle and working with the arm in an abducted position for a long time is common. Cervical spondylosis can cause further muscle weakness, which will give rise to more pain exacerbated by radiating pain from nerve root irritation.131,132 Carpal tunnel syndrome (CTS) is the most common nerve entrapment syndrome. It involves entrapment of the median nerve at the level of the wrist. In the work environment, CTS results from rapid, repetitive, and daily use of the hand and ngers for many hours at a time. The problem is compounded when working with a bent wrist, exerting force, working with vibratory tools, and in cold environments. Rapid movement of tendons in the synovial tube causes inammation and uid buildup. This can result in atrophy of the thenar muscles; tingling in the thumb, index, middle, and half of the ring nger; night pain; and pain when handling tools.133 Tendinopathies are inammations of the tendons or the tendon sheaths. The hand performs many complex tasks, and the tendons move inside tendon sheaths with synovial uid. Repetitive and forceful movements and the use of vibrating tools increase uid accumulation and inammation. The affected area is swollen and sensitive to touch, whereas pain is elicited with certain movements such as grasping and pinching. A typical example is the DeQuervain tenosynovitis. This affects the rst dorsal compartment of the extensor tendons (extensor pollicis brevis and abductor pollicis longus).134 Practically, this translates to difculty in handling instruments because it affects grasping and rotational and lateral bending of the wrist. Trigger nger is thickening of the tendon, making it difcult for the tendon to move in and out of the sheath during exion. The nger can be locked in exion and requires force to move. Possible causes are degeneration, repeated trauma, or repetitive movements with hand tools. A solid knowledge of ergonomics, along with
288 Pandis et al
American Journal of Orthodontics and Dentofacial Orthopedics September 2007
postural exercises, is the key to the prevention of musculoskeletal problems.135-137
Psychological Problems
Mathias et al,138 who surveyed American Dental Association members, reporting that 9% had psychological problems, investigated the overall depression rate of dentists. They found that female pedodontists and periodontists were more depressed than their male counterparts, and that only 15% of depressed dentists were seeking treatment, an issue that raises concerns about quality of care. Several studies identied issues related to nances and job growth, time and scheduling, dentist-patient relations, and staff and technical problems as stress sources in dentistry.139-142 High levels of occupational stress among dentists are correlated with hypertension,143 coronary artery disease,144 and suicide.145,146 A study of burnout and its causes among dentists in Finland identied psychological fatigue, loss of enjoyment for work, and becoming insensitive toward patients.147 Comparisons of stress and coping in male and female dentists found that stress levels were similar, although the women experienced more personal and domestic problems. Regarding coping style, both sexes responded similarly in most respects, except that the women were more inclined to discuss their problems.148 Brand and Chalmers142 compared stress patterns of dentists of various ages and concluded that older practitioners had less stress than their younger colleagues. However, for some issues related to nance and patient management, both groups were equally affected. Alcohol use among South Australian dentists was investigated in a study, and the authors concluded that stress and hazardous alcohol consumption were both present, although it can be argued that personal vulnerability factors are much stronger predictors of alcohol consumption than stress or burnout.149 The dental community has often been portrayed as having a high suicide rate. Although various studies investigated this issue, consensus is lacking. A study that targeted this parameter was undertaken in Norway in 1960 and lasted until 2000.150 The authors found higher suicide rates among physicians and health care professionals compared with policemen and theologians. Suicide rates were signicantly lower in the 1990s than in the 1980s. Suicide and undetermined deaths for physicians, dentists, registered nurses, attendants in psychiatric care, and auxiliary nurses, were also studied in Sweden after the censuses of 1960, 1970, 1975, and 1980. Male dentists had higher suicide rates after 1960, with
signicantly higher suicide rates during the 1970s than the total male working population. A rapid fall in the number of suicides took place from 1981 to 1985. Female dentists had consistently high suicide rates during the 1970s and 1980s. These suicide trends were related to an increase in the proportion of women in the work force and alterations in family patterns in Sweden during the 1970s.151 On reviewing the literature, we concluded that there is little valid evidence that dentists are more prone to suicide than the general population, although data suggest that female dentists might be more vulnerable.146 According to a report, 20% of dentists on disability were diagnosed with neurological or mental problems, but the percentage of pure psychological problems was not specied.152 A Swedish study found greater job satisfaction, higher self-condence, and less anxiety among specialists compared with general denists.153 With respect to the specic anxiety sources, a survey of Canadian orthodontists reported that the highest scoring stressors in terms of severity were patient dissatisfaction with treatment, working with uncooperative patients, and falling behind schedule; these agreed with the results of similar studies of general dental practitioners.141,154,155 Stressors such as causing pain in patients and when the subject proceeds to a difcult operation, which appear among general dentists, were not found in orthodontists. Issues that were more prominent for orthodontics were unrealistic patient expectations, orthodontic relapse, pressure from patients to terminate treatment, general practitioners questioning orthodontic treatment planning or progress, burn-out cases, and oral hygiene compliance. When the stressors were evaluated from a frequency perspective, the highest ranked ones were adult patient management, late or no-show patients, and poor elastic or headgear cooperation. When severity and frequency of stressors were combined, time management, broken appliances, and poor oral hygiene and decalcication were the highest-ranking ones. This last classication is important because it represents how the greatest number of survey participants felt about the stressing issues. From the above information, it appears that time management is important in reducing occupational stress. Humphris et al156 compared the burn-out rates of various specialists and found the lowest rate for orthodontists, assigning their ndings to better working environment, more exibility and control in managing patients, and more cooperative patients because orthodontic treatment is
American Journal of Orthodontics and Dentofacial Orthopedics Volume 132, Number 3
Pandis et al 289
elective and usually poses no serious threats to a patients health.
CONCLUSIONS
Contemporary orthodontics involves many potentially hazardous factors related to the general setting of practice; to specic materials and tools that expose the operator to vision and hearing risks; to chemical substances with known allergenic, toxic, or irritating actions; to increased microbial counts and silica particles of the aerosols produced during debonding; to ergonomic considerations that might have an impact on the providers muscoleskeletal system; and to psychological stress with proven undesirable sequelae. The identication and elimination of the foregoing risk factors should be incorporated in a standard practice management program as an integral part of orthodontic education. Professional organizations can also assist in informing practitioners of potential hazards and methods to deal with them. We thank Stamatis Balis for reading the section on eye pathology.
REFERENCES 1. Simpson JA, Weiner ESC. Oxford English dictionary. Oxford: Clarendon Press; 1989. 2. Jacobsen N, Hensten-Pettersen A. Occupational health problems and adverse patient reactions in orthodontics. Eur J Orthod 1989;11:254-64. 3. Jacobsen N, Hensten-Pettersen A. Changes in occupational health problems and adverse patient reactions in orthodontics from 1987 to 2000. Eur J Orthod 2003;25:591-8. 4. Munksgaard EC, Hansen EK, Engen T, Holm U. Self reported occupational dermatological reactions among Danish dentists. Eur J Oral Sci 1996;104:396-402. 5. Lnnroth EC, Shahnavaz H. Adverse health reactions in skin, eyes, and respiratory tract among dental personnel in Sweden. Swed Dent J 1998;22:33-45. 6. Hamann CP, Rodgers PA, Sullivan KM. Occupational allergens in dentistry. Curr Opin Allergy Clin Immunol 2004;4:403-9. 7. Rubel DM, Watchorn RB. Allergic contact dermatitis in dentistry. Australas J Dermatol 2000;41:63-9. 8. Sinclair NA, Thomson WM. Prevalence of self-reported hand dermatoses in New Zealand dentists. N Z Dent J 2004;100:38-41. 9. Wallenhammar LM, Ortengren U, Andreasson H, Barregard L, Bjorkner B, Karlsson S, et al. Contact allergy and hand eczema in Swedish dentists. Contact Dermatitis 2000;43:192-9. 10. Uveges RE, Grimwood RE, Slawsky LD, Marks JG Jr. Epidemiology of hand dermatitis in dental personnel. Mil Med 1995;160:335-8. 11. Altuna G, Freeman E. The reaction of skin to primers used in the single-step bonding systems. Am J Orthod Dentofacial Orthop 1987;91:105-10. 12. Strobl K, Bahns TL, Willham L, Bishara SE, Stwalley WC. Laser-aided debonding of orthodontic ceramic brackets. Am J Orthod Dentofacial Orthop 1992;101:152-8.
13. Tocchio RM, Williams PT, Mayer FS, Standing KG. Laser debonding of ceramic orthodontic brackets. Am J Orthod Dentofacial Orthop 1993;103:155-62. 14. Rickabaugh JL, Marangoni RD, McCaffrey KK. Ceramic bracket debonding with the carbon dioxide laser. Am J Orthod Dentofacial Orthop 1996;110:388-93. 15. Sarver DM. Principles of cosmetic dentistry in orthodontics: part 1. Shape and proportionality of anterior teeth. Am J Orthod Dentofacial Orthop 2004;126:749-53. 16. Sarver DM, Yanosky M. Principles of cosmetic dentistry in orthodontics: part 2. Soft-tissue laser technology and cosmetic gingival contouring. Am J Orthod Dentofacial Orthop 2005; 127:85-90. 17. Sarver DM, Yanosky M. Principles of cosmetic dentistry in orthodontics: part 3. Laser treatments for tooth eruption and soft-tissue problems. Am J Orthod Dentofacial Orthop 2005; 127:262-4. 18. Arai E. Evaluation of dental equipment based on clinical efciency and eye fatigue. Shikai Tenbo 1983;61:331-40. 19. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002;295: 1070-3. 20. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002;295:1065-70. 21. Menaker M. Circadian rhythms. Circadian photoreception. Science 2003;299:213-4. 22. Wee R, Van Gelder RN. Sleep disturbances in young subjects with visual dysfunction. Ophthalmology 2004;111:297-303. 23. Van Gelder RN. Blue light and the circadian clock. Br J Ophthalmol 2004;88:1353. 24. Pautler EL, Morita M, Beezley D. Hemoprotein(s) mediate blue light damage in the retinal pigment epithelium. Photochem Photobiol 1990;51:599-605. 25. Grimm C, Wenzel A, Williams T, Rol P, Hafezi F, Reme C. Rhodopsin-mediated blue-light damage to the rat retina: effect of photoreversal of bleaching. Invest Ophthalmol Vis Sci 2001;42:497-505. 26. Wihlmark U, Wrigstad A, Roberg K, Nilsson S, Brunk U. Lipofuscin accumulation in cultured retinal pigment epithelial cells causes enhanced sensitivity to blue light irradiation. Free Radical Biol Med 1997;22:1229-34. 27. Abbott A. Restless nights, listless days. Nature 2003;425:896-8. 28. Gasyna KA, Rezaei WF, Mieler KA, Rezai P. Blue light induces apoptosis in human fetal retinal pigment epithelium (abstract 3813). Inv Ophth Visl Sci (IOVS) 2006;47:180. 29. Satrom KD, Morris MA, Crigger LP. Potential retinal hazards of visible-light photopolymerization units. J Dent Res 1987;66: 731-6. 30. Nomoto R, McCabe JF, Hirano S. Comparison of halogen, plasma and LED curing units. Oper Dent 2004;29:287-94. 31. Kanski JJ. Clinical ophthalmology-a systematic approach. London: Butterworth; 1989. 32. Shimmura S, Shimazaki J, Tsubota K. Results of a populationbased questionnaire on the symptoms and lifestyles associated with dry eye. Cornea 1999;18:408-11. 33. Doughty MJ, Fonn D, Richter D, Simpson T, Caffery B, Gordon K. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci 1997;74: 624-31.
290 Pandis et al
American Journal of Orthodontics and Dentofacial Orthopedics September 2007
34. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol 2003;136:318-26. 35. Sahai A, Malik P. Dry eye: prevalence and attributable risk factors in a hospital-based population. Indian J Ophthalmol 2005;53:87-91. 36. Nichols KK, Begley CG, Caffery B, Jones LA. Symptoms of ocular irritation in patients diagnosed with dry eye. Optom Vis Sci 1999;76:838-44. 37. Doughty MJ, Blades KA, Ibrahim N. Assessment of the number of eye symptoms and the impact of some confounding variables for ofce staff in non-air-conditioned buildings. Ophthalmic Physiol Opt 2002;22:143-55. 38. Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 2000;118:1264-8. 39. Katz J. Handbook of clinical audiology. Baltimore: Williams and Wilkins; 1985. 40. OSHA. Department of Labor occupational noise exposure. 1983; CFR29, 1910.95. Available at: http://www.OSHA.gov. Accessed 8/16/2006. 41. Sampaio Fernandes JC, Carvalho AP, Gallas M, Vaz P, Matos PA. Noise levels in dental schools. Eur J Dent Educ 2006;10: 32-7. 42. Berger EH, Neitzel R, Kladden C. Noise navigatorTM sound level database with over 1700 measurement values. University of Washington, Department of Environmental & Occupational Health Sciences, Seattle. Avarilable at http:// www.e-a-r.com/pdf/hearingcons/NoiseNav.xls. Accessed 8/20/2006. 43. Sorainen E, Rytkonen E. High-frequency noise in dentistry. AIHA J 2002;63:231-3. 44. Bono S. Are there hazardous auditory effects of high-frequency turbines and ultrasonic dental scalers on dental professionals? (thesis). St Louis: Washington University; 2006. 45. Praml GJ, Sonnabend E. Noise-induced hearing loss caused by dental turbines. Dtsch Zahnarztl Z 1980;35:400-6. 46. Setcos JC, Mahyuddin A. Noise levels encountered in dental clinical and laboratory practice. Int J Prosthodont 1998;11: 150-7. 47. Wilson JD, Darby ML, Tolle SL, Sever JC Jr. Effects of occupational ultrasonic noise exposure on hearing of dental hygienists: a pilot study. J Dent Hyg 2002;76:262-9. 48. Morarasu C, Burlui V, Borta C, Ignat L, Borta B, Morarasu G. The evaluation of sound level in dental practice. Rev Med Chir Soc Med Nat 2001;105:785-9. 49. Szymanska J. Work-related noise hazards in the dental surgery. Ann Agric Environ Med 2000;7:67-70. 50. Starck J, Toppila E, Pyykko I. Smoking as a risk factor in sensory neural hearing loss among workers exposed to occupational noise. Acta Otolaryngol 1999;119:302-5. 51. Hyson JM Jr. The air turbine and hearing loss: are dentists at risk? J Am Dent Assoc 2002;133:1639-42. 52. Altinoz HC, Gokbudak R, Bayraktar A, Belli S. A pilot study of measurement of the frequency of sounds emitted by high-speed dental air turbines. J Oral Sci 2001;43:189-92. 53. Barek S, Adam O, Motsch JF. Large band spectral analysis and harmful risks of dental turbines. Clin Oral Investig 1999;3: 49-54. 54. Wilson CE, Vaidyanathan TK, Cinotti WR, Cohen SM, Wang SJ. Hearing-damage risk and communication interference in dental practice. J Dent Res 1990;69:489-93. 55. Siegelaub AB, Friedman GD, Kedar A, Oakland C, Selzer CC. Hearing loss in adults. Relation to age, sex exposure to loud
56.
57.
58.
59.
60.
61.
62.
63.
64. 65.
66.
67.
68. 69. 70. 71.
72.
73.
74.
75.
76.
noise, and cigarette smoking. Arch Environ Health 1974;29:107-9. Siew C, Chang S, Gruninger SE, Verrusio AC, Neidle EA. Self-reported percutaneous injuries in dentists. J Am Dent Assoc 1992;123:37-44. Cleveland JL, Lockwood SA, Gooch BF, Mendekson MH, Chamberland ME, Valauri DV, et al. Percutaneous injuries in dentistry: an observational study. J Am Dent Assoc 1995;126: 745-51. Siew C, Gruninger SE, Chang S, Neidle EA. Percutaneous injuries in practicing dentists. J Am Dent Assoc 1995;126: 1227-34. Bagramian RA, McNamara JA Jr. A prospective survey of percutaneous injuries in orthodontists. Am J Orthod Dentofacial Orthop 1998;114:654-8. McNamara JA Jr, Bagramian RA. A prospective survey of percutaneous injuries in orthodontic assistants. Am J Orthod Dentofacial Orthop 1999;115:72-6. Sims AP, Roberts-Harry TJ, Roberts-Harry DP. The incidence and prevention of ocular injuries in orthodontic practice. Br J Orthod 1993;20:339-43. Liss GM, Sussman GL. Latex sensitization: occupational versus general population prevalence rates. Am J Ind Med 1999;35: 196-200. Ownby DR, Ownby HE, McCullough J, Shafer AW. The prevalence of anti-latex IgE antibodies in 1000 volunteer blood donors. J Allergy Clin Immunol 1996;97:1188-92. Turjanmaa K. Incidence of immediate allergy to latex gloves in hospital personnel. Contact Dermatitis 1987;17:270-5. Tarlo SM, Sussman GL, Holness DL. Latex sensitivity in dental students and staff: a cross-sectional study. J Allergy Clin Immunol 1997;99:396-401. Safadi GS, Safadi TJ, Terezhalmy GT, Taylor JS, Battisto JR, Melton AL Jr. Latex hypersensitivity: its prevalence among dental professionals. J Am Dent Assoc 1996;127:83-8. Hamann CP, Turjanmaa K, Rietschel R, Siew C, Owensby D, Gruninger SE, et al. Natural rubber latex hypersensitivity: incidence and prevalence of type I allergy in the dental profession. J Am Dent Assoc 1998;129:43-54. Berky ZT, Luciano WJ, James WD. Latex glove allergy. A survey of the US Army Dental Corps. JAMA 1992;268;2695-7. Cullinnan P. Latex allergy. CPD Bull Immunol Allergy 2004; 3:82-4. Latex allergy. Available at: http://www.OSHA.gov. Accessed August 10, 2006. Wrangsjo K, Wallenhammar LM, Ortengren U, Barregard L, Andreasson H, Bjorkner B, et al. Protective gloves in Swedish dentistry: use and side-effects. Br J Dermatol 2001;145:32-7. Heese A, Hintzenstern J, Peters KP, Koch HU, Hornstein OP. Allergic and irritant reactions to rubber gloves in medical health services. J Am Acad Dermatol 1991;25:831-9. Siew C, Hamann CP, Gruninger SE, Rodgers P, Sullivan KM. Type I latex allergic reactions among dental professionals, 1996-2001 (abstract). J Dent Res 2003;82:1718. Charous BL, Schuenemann PJ, Swanson MC. Passive dispersion of latex aeroallergen in a healthcare facility. Ann Allergy Asthma Immunol 2000;85:285-90. Levy DA, Allouache S, Chabane MH, Leynadier F, Burney P. Powder-free protein poor natural rubber latex gloves and latex sensitisation. JAMA 1999;281:988. Tomazic VJ, Shampaine EL, Lamanna A, Withrow TJ, Adkinson NF Jr, Hamilton RG. Cornstarch powder on latex products is an allergen carrier. J Allergy Clin Immunol 1994;93:751-8.
American Journal of Orthodontics and Dentofacial Orthopedics Volume 132, Number 3
Pandis et al 291
77. Beezhold D, Beck WC. Surgical glove powders bind latex antigens. Arch Surg 1992;127:1354-7. 78. Amin A, Palenik CJ, Cheung SW, Burke FJ. Latex exposure and allergy: a survey of general dental practitioners and dental students. Dent J 1998;48:77-83. 79. Field EA. The use of powdered gloves in dental practice: a cause for concern? J Dent 1997;25:209-14. 80. Pisati G, Barufni A, Bernabeo F, Stanizzi R. Bronchial provocation testing in the diagnosis of occupational asthma due to latex surgical gloves. Eur Respir J 1994;7:332-6. 81. Vandenplas O, Delwiche JP, Evrard G, Aimont P, van der Brempt X, Jamart J, et al. Prevalence of occupational asthma due to latex among hospital personnel. Am J Respir Crit Care Med 1995;151:54-60. 82. Rego A, Roley L. In-use barrier integrity of gloves: latex and nitrile superior to vinyl. Am J Infect Control 1999;27:405-10. 83. Lonnroth EC. Toxicity of medical glove materials: a pilot study. Int J Occup Saf Ergon 2005;11:131-9. 84. Geukens S, Goossens A. Occupational contact allergy to (meth)acrylates. Contact Dermatitis 2001;44:153-9. 85. Ortengren U. On composite resin materials. Degradation, erosion and possible adverse effects in dentists. Swed Dent J 2000;141 (Suppl):1-61. 86. Lonnroth EC, Dahl JE. Cytotoxicity of liquids and powders of chemically different dental materials evaluated using dimethylthiazol diphenyltetrazolium and neutral red tests. Acta Odontol Scand 2003;6:52-6. 87. Lonnroth EC, Dahl JE, Shahnavaz H. Evaluating the potential occupational hazard of handling dental polymer products using the HET-CAM technique. Int J Occup Saf Ergon 1999;5:43-57. 88. Lonnroth EC, Eystein Ruyter I. Resistance of medical gloves to permeation by methyl methacrylate (MMA), ethylene glycol dimethacrylate (EGDMA), and 1,4-butanediol dimethacrylate (1,4-BDMA). Int J Occup Saf Ergon 2003;9:289-99. 89. Yoshii E. Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J Biomed Mater Res 1997;37:517-24. 90. Lonnroth EC, Ruyter IE. Permeability of medical gloves to mono- and dimethacrylate monomers in dental restorative materials. Int J Occup Saf Ergon 2002;8:497-509. 91. Lonnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci 2003;111:440-6. 92. Roberta T, Federico M, Federica B, Antonietta CM, Sergio B, Ugo C. Study of the potential cytotoxicity of dental impression materials. Toxicol In Vitro 2003;17:657-62. 93. Ravis SM, Shaffer MP, Shaffer C, Shaffer CL, Dehkhaghani S, Belsito DV. Glutaraldehyde-induced and formaldhyde-induced allergic contact dermatitis among dental hygienists and dental assistants. J Am Dent Assoc 2003;134:1072-8. 94. Hamann C, Werner RA, Franzblau A, Rodgers PA, Siew C, Gruninger S. Prevalence of carpal tunnel syndrome and median mononeuropathy among dentists. J Am Dent Assoc 2001;132: 163-70. 95. Shaffer MP, Belsito DV. Allergic contact dermatitis from glutaraldehyde in health-care workers. Contact Dermatitis 2000;43:150-6 96. AGFA. Material safety data sheets. Available at: www.agfa. com. Accessed 8/20/2006. 97. Bolyard EA, Tablan OC, Williams WW, Pearson ML, Shapiro CN, Deitchman SD. Hospital Infection Control Practices Advisory Committee. Guideline for infection control in health care personnel, 1998. Am J Infect Control 1998;26:289-354.
98. Barbeau J, Tanguay R, Faucher E, Avegard C, Trudel L, Cote L. Multiparametric analysis of waterline contamination in dental units. Appl Environ Microbiol 1996;62:3954-9. 99. Atlas RM, Williams JF, Huntington MK. Legionella contamination of dental-unit waters. Appl Environ Microbiol 1995;61: 1208-13. 100. Kelstrup J, Funder-Nielsen T, Theilade J. Microbial aggregate contamination of water lines in dental equipment and its control. Acta Pathol Microbiol Scand [B] 1977;85:177-83. 101. Challacombe SJ, Fernandes LL. Detecting Legionella pneumophila in water systems: a comparison of various dental units. J Am Dent Assoc 1995;126:603-8. 102. Santiago JI. Microbial contamination of dental unit waterlines: short and long term effects of ushing. Gen Dent 1994;42: 528-35. 103. Crawford JJ, Broderius C. Control of cross-infection risks in the dental operatory: prevention of water retraction by bur cooling spray systems. J Am Dent Assoc 1988;116:685-7. 104. Mills SE, Kuehne JC, Bradley DV Jr. Bacteriological analysis of high-speed handpiece turbines. J Am Dent Assoc 1993;124: 59-62. 105. Lewis DL, Arens M, Appleton SS, Nakashima K, Ryu J, Boe RK, et al. Cross-contamination potential with dental equipment. Lancet 1992;340:1252-4. 106. Lewis DL, Boe RK. Cross-infection risks associated with current procedures for using high-speed dental handpieces. J Clin Microbiol 1992;30:401-6. 107. Checchi L, Montebugnoli L, Samaritani S. Contamination of the turbine air chamber: a risk of cross infection. J Clin Periodontol 1998;25:607-11. 108. Epstein JB, Rea G, Sibau L, Sherlock CH, Le ND. Assessing viral retention and elimination in rotary dental instruments. J Am Dent Assoc 1995;126:87-92. 109. Barbeau J, ten Bokum L, Gauthier C, Prevost AP. Crosscontamination potential of saliva ejectors used in dentistry. J Hosp Infect 1998;40:303-11. 110. Mann GL, Campbell TL, Crawford JJ. Backow in low-volume suction lines: the impact of pressure changes. J Am Dent Assoc 1996;127:611-5. 111. Watson CM, Whitehouse RL. Possibility of cross-contamination between dental patients by means of the saliva ejector. J Am Dent Assoc1993;124:77-80. 112. Sofou A, Larsen T, Fiehn NE, Owall B. Contamination level of alginate impressions arriving at a dental laboratory. Clin Oral Invest 2002;6:161-5. 113. McNeill MR, Coulter WA, Hussey DL. Disinfection of irreversible hydrocolloid impressions: a comparative study. Int J Prosthodont 1992;5:563-7. 114. Gerhardt DE, Sydiskis RJ. Impression materials and virus. J Am Dent Assoc 1991;122:51-4. 115. Leung RL, Schonfeld SE. Gypsum casts as a potential source of microbial cross-contamination. J Prosthet Dent 1983;49:210-1. 116. Huizing KL, Palenik CJ, Setcos JC, Sheldrake MA, Miller CH. Method of evaluating the antimicrobial abilities of disinfectantcontaining gypsum products. QDT Yearbook 1994;17:172-6. 117. Toroglu MS, Bayramoglu O, Yarkin F, Tuli A. Possibility of blood and hepatitis contamination through aerosols generated during debonding procedures. Angle Orthod 2003;73:571-8. 118. Toroglu MS, Haytac MC, Koksal F. Evaluation of aerosol contamination during debonding procedures. Angle Orthod 2001;71:299-306.
292 Pandis et al
American Journal of Orthodontics and Dentofacial Orthopedics September 2007
119. Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings2003. MMWR 2003;52(No. RR-17):1-61. Accessed August 10, 2006. 120. Logothetis DD, Martinez-Welles JM. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc 1995;126:1634-9. 121. Ireland AJ, Moreno T, Price R. Airborne particles produced during enamel cleanup after removal of orthodontic appliances. Am J Orthod Dentofacial Orthop 2003;124:683-6. 122. Miller DJ, Shugars DA, The health of the dental professional. J Am Dent Assoc 1987;114:515-8. 123. Lehto TU, Helenius HY, Alaranta HT. Musculoskeletal symptoms of dentists assessed by a multidisciplinary approach. Community Dent Oral Epidemiol 1991;19:38-44. 124. Rucker LM, Sunell S. Ergonomic risk factors associated with clinical dentistry. J Calif Dent Assoc 2002;30:139-48. 125. Kerosuo E, Kerosuo H, Kanerva L. Self-reported health complaints among general dental practitioners, orthodontists, and ofce employees. Acta Odontol Scand 2000;58:207-12. 126. Brown PN. Whats ailing us? Prevalence and type of long-term disabilities among an insured cohort of orthodontists. Am J Orthod Dentofacial Orthop 2004;125:3-7. 127. Lalumandier JA, McPhee SD, Parrott CB, Vendemia M. Musculoskeletal pain: prevalence, prevention, and differences among dental ofce personnel. Gen Dent 2001;49:160-6. 128. Rundcrantz BL. Pain and discomfort in the musculo-skeletal system in dentists. Swed Dent J 1991;76 (Suppl):101-2. 129. Oleske DM, Neelakantan J, Andersson GB, Hinrichs BG, Lavender SA, Morrissey MJ, et al. Factors affecting recovery from work-related, low back disorders in autoworkers. Arch Phys Med Rehabil 2004;85:1362-4. 130. Rundcrantz BL, Johnsson B, Moritz U. Occupational cervicobrachial disorders among dentists. Analysis of ergonomics and locomotor functions. Swed Dent J 1991;15:105-15. 131. Krishnan SG, Hawkins RJ. Rotator cuff and impingement lesions in adult and adolescent athletes. In: DeLee JC, Drez D Jr, editors. DeLee and Drezs orthopaedic sports medicine: principles and practice. Philadelphia: Saunders; 2003. p. 106595. 132. Apley G. Apleys system of orthopaedics and fractures. London: Butterworth-Heinemann; 1993. 133. Fish DR, Morris-Allen DM. Muscoloskeletal disorders in dentists. N Y State Dent J 1998;64:44-8. 134. Ariyan S. The hand book. New York: McGraw-Hill; 1989. 135. Rundcrantz BL, Johnsson B, Moritz U, Roxendal G. Cervicobrachial disorders in dentists. A comparison between two kinds of physiotherapeutic interventions. Scand J Rehab Med 1991; 23:11-7. 136. Frost H, Lamb SE, Doll HA, Carver PT, Stewart-Brown S. Randomised controlled trial of physiotherapy compared with advice for low back pain. BMJ 2004;329:708. 137. Murphy DC. Ergonomics and the dental care worker. American Public Health Association. Washington, DC: United Book Press; 1998.
138. Mathias S, Koerber A, Fadavi S, Punwani I. Specialty and sex as predictors of depression in dentists. J Am Dent Assoc 2005;136:1388-95. 139. Myers HL, Myers LB. Its difcult being a dentist: stress and health in the general dental practitioner. Br Dent J 2004 24;197:89-93. 140. Moller AT, Spangenberg JJ. Stress and coping amongst South African dentists in private practice. J Dent Assoc S Afr 1996;51:347-57. 141. OShea RM, Corah NL, Ayer WA. Sources of dentists stress. J Am Dent Assoc 1984;109:48-51. 142. Brand AA, Chalmers BE. Age differences in the stress patterns of dentists. J Dent Assoc S Afr 1990;45:461-5. 143. Cutright DE, Carpenter WA, Tsaknis PG, Lyon TC. Survey of blood pressures of 856 dentists. J Am Dent Assoc 1977;94:918-9. 144. Russek HI. Emotional stress and coronary heart disease in American physicians, dentists and lawyers. Am J Med Sci 1962;243:716-25. 145. Simpson R, Beck J, Jakobsen J, Simpson J. Suicide statistics of dentists in Iowa, 1968 to 1980. J Am Dent Assoc 1983;107: 441-3. 146. Alexander RE. Stress-related suicide by dentists and other health care workers. Fact or folklore? J Am Dent Assoc 2001;132:786-94. 147. Murtomaa H, Haavio-Mannila E, Kandolin I. Burnout and its causes in Finnish dentists. Community Dent Oral Epidemiol 1990;18:208-12. 148. Rankin JA, Harris MB. Comparison of stress and coping in male and female dentists. J Dent Pract Adm 1990;7:166-72. 149. Winwood PC, Wineeld AH, Lushington K. The role of occupational stress in the maladaptive use of alcohol by dentists: a study of South Australian general dental practitioners. Aust Dent J 2003;48:102-9. 150. Hem E, Haldorsen T, Aasland OG, Tyssen R, Vaglum P, Ekeberg O. Suicide rates according to education with a particular focus on physicians in Norway 1960-2000. Psychol Med 2005;35:873-80. 151. Stefansson CG, Wicks S. Health care occupations and suicide in Sweden 1961-1985. Soc Psychiatry Psychiatr Epidemiol 1991; 26:259-64. 152. CDSPI (Canadian Dental Service Plans Inc) Report. High stress related claims hurt everyone. J Can Dent Assoc 1994;60:387-8. 153. Rundcrantz BL, Johnsson B, Moritz U, Roxendal G. Occupational cervico-brachial disorders among dentists. Psychosocial work environment, personal harmony and life satisfaction. Scand J Soc Med 1991;19:174-80. 154. Bourassa M, Baylard JF. Stress situations in dental practice. J Can Dent Assoc 1994;60:65-7,70-1. 155. Freeman R, Main JR, Burke FJ. Occupational stress and dentistry: theory and practice. Part I. Recognition. Br Dent J 1995;178:214-7. 156. Humphris G, Lilley J, Kaney S, Broomeld D. Burnout and stress-related factors among junior staff of three dental hospital specialties. Br Dent J 1997;183:15-21.
Das könnte Ihnen auch gefallen
- HazzaredDokument5 SeitenHazzaredعلي موسى مهديNoch keine Bewertungen
- The Toxicant Induction of Irritant Asthma, Rhinitis, and Related ConditionsVon EverandThe Toxicant Induction of Irritant Asthma, Rhinitis, and Related ConditionsNoch keine Bewertungen
- Lec 8Dokument5 SeitenLec 8مؤمل رياض سعد كاظمNoch keine Bewertungen
- Occupational Hazards Part 1Dokument94 SeitenOccupational Hazards Part 1Eshan VermaNoch keine Bewertungen
- Occupational Hazard in DentistryDokument21 SeitenOccupational Hazard in DentistryAisha DewiNoch keine Bewertungen
- 188 ArticleText 561 1 10 20191029Dokument7 Seiten188 ArticleText 561 1 10 20191029khaled alahmadNoch keine Bewertungen
- Onehealth AssighnmentDokument37 SeitenOnehealth AssighnmentYasir IsrarNoch keine Bewertungen
- Management of Medically Compromised Orthodontic PatientsDokument64 SeitenManagement of Medically Compromised Orthodontic Patientsakshi1947Noch keine Bewertungen
- ZZ 1Dokument11 SeitenZZ 1Cornerstone OrganizationNoch keine Bewertungen
- Occupational Health Hazard in ProsthodonticsDokument5 SeitenOccupational Health Hazard in ProsthodonticsPradeepNoch keine Bewertungen
- Occupational Health & Safety Workshop Reference Materials: Practical 1Dokument22 SeitenOccupational Health & Safety Workshop Reference Materials: Practical 1Eduardo Coldibeli Bianchi0% (1)
- Experiment 3 Standard PrecautionDokument3 SeitenExperiment 3 Standard PrecautionJanson SarmientoNoch keine Bewertungen
- Sources and Effects of Chemical Hazards-1Dokument4 SeitenSources and Effects of Chemical Hazards-1نرمين سميرNoch keine Bewertungen
- Topic 7Dokument12 SeitenTopic 7Bharathi Sneha PeriasamyNoch keine Bewertungen
- Occupational HazardsDokument24 SeitenOccupational HazardsMahesh Boopathy100% (1)
- Occupational Hazzard in Dental Office PDFDokument3 SeitenOccupational Hazzard in Dental Office PDFtifaniNoch keine Bewertungen
- Girne American University: Health Care Management DepartmentDokument38 SeitenGirne American University: Health Care Management DepartmentMetehan ÖlçerNoch keine Bewertungen
- Epidemiology of Noncommunicable DiseasesDokument57 SeitenEpidemiology of Noncommunicable DiseasessanchitasinhaNoch keine Bewertungen
- Epidemiology of Non-Communicable DiseasesDokument57 SeitenEpidemiology of Non-Communicable Diseasessarguss1492% (12)
- 3391-Article Text-6014-1-10-20240112Dokument19 Seiten3391-Article Text-6014-1-10-20240112Asmaa MahmoudNoch keine Bewertungen
- Allergies 3Dokument5 SeitenAllergies 3Noor MuhammadNoch keine Bewertungen
- Pharmaceutical Industry Hazards and SafetyDokument23 SeitenPharmaceutical Industry Hazards and SafetyfadliNoch keine Bewertungen
- How Toxicology Impact Other ScienceDokument6 SeitenHow Toxicology Impact Other SciencePurwita NurwidyastutiNoch keine Bewertungen
- 1.introduction To Occupational Hazards, Work, HealthDokument34 Seiten1.introduction To Occupational Hazards, Work, HealthShintonial Ozile100% (1)
- 2023 TFOS Lifestyle Report - Environmental ConditionDokument52 Seiten2023 TFOS Lifestyle Report - Environmental Conditionrisingsun9823Noch keine Bewertungen
- Lecture 9Dokument28 SeitenLecture 9Justin JannatiNoch keine Bewertungen
- Chapter Four NRDokument9 SeitenChapter Four NRnuru jumaNoch keine Bewertungen
- Practice Occupational Health and Safety Procedures: Definition of TermsDokument15 SeitenPractice Occupational Health and Safety Procedures: Definition of Termsmico alilayaNoch keine Bewertungen
- Occupational DiseaseDokument10 SeitenOccupational DiseaseSyafezan SuzaimeNoch keine Bewertungen
- Pharmaco in NanoDokument3 SeitenPharmaco in Nanoftprimo100% (1)
- Antioxidant Micronutrient Impact On Hearing Disorders: Concept, Rationale, and EvidenceDokument7 SeitenAntioxidant Micronutrient Impact On Hearing Disorders: Concept, Rationale, and EvidenceFongmeicha Elizabeth MargarethaNoch keine Bewertungen
- Pneumoconiosis. Silicosis. Silicatosis. Vibration DiseaseDokument130 SeitenPneumoconiosis. Silicosis. Silicatosis. Vibration DiseaseAlina Boghiu-CisleanuNoch keine Bewertungen
- Submitted by Submitted To: TOPIC: "Industrial Hazards and Safety Precautions in Pharmaceutical Industry"Dokument9 SeitenSubmitted by Submitted To: TOPIC: "Industrial Hazards and Safety Precautions in Pharmaceutical Industry"saymaNoch keine Bewertungen
- Chronic Laryngitis in Glassblowers: Industrial Health May 2005Dokument7 SeitenChronic Laryngitis in Glassblowers: Industrial Health May 2005VidinikusumaNoch keine Bewertungen
- Occupational Disease 151016142735 Lva1 App6892Dokument36 SeitenOccupational Disease 151016142735 Lva1 App6892annNoch keine Bewertungen
- Hazards and Risk in The Workplace Tle8Dokument8 SeitenHazards and Risk in The Workplace Tle8Arabelle MorilloNoch keine Bewertungen
- Occupational Hazards in Dentistry: January 2017Dokument14 SeitenOccupational Hazards in Dentistry: January 2017Mustafa AmmarNoch keine Bewertungen
- Environmental Safety Part 1 RMBLDokument58 SeitenEnvironmental Safety Part 1 RMBLLANSANGAN RHIZZA MAE B.Noch keine Bewertungen
- 4 Chemical SafetyDokument35 Seiten4 Chemical SafetyVanessa EspinoNoch keine Bewertungen
- Fire Research Identified 57 Toxins With Polystyrene CombustionDokument7 SeitenFire Research Identified 57 Toxins With Polystyrene CombustionbubisharbiNoch keine Bewertungen
- Industrial Hygiene-Labordo, M.E.-4GNDokument19 SeitenIndustrial Hygiene-Labordo, M.E.-4GNeric labordoNoch keine Bewertungen
- Biological Considerations in Use of Dental Impression MaterialsDokument6 SeitenBiological Considerations in Use of Dental Impression MaterialsNiranjanaNoch keine Bewertungen
- Radiation Exposures in Medicine: Biological and Public Health SignificanceDokument13 SeitenRadiation Exposures in Medicine: Biological and Public Health SignificanceFernando SperandioNoch keine Bewertungen
- Industrial Safety: Omar Mustafa Hussein Al-KubaisiDokument25 SeitenIndustrial Safety: Omar Mustafa Hussein Al-KubaisimarwanNoch keine Bewertungen
- Industrial Safety: Omar Mustafa Hussein Al-KubaisiDokument11 SeitenIndustrial Safety: Omar Mustafa Hussein Al-KubaisimarwanNoch keine Bewertungen
- Practice Occupational Health and Safety ProceduresDokument16 SeitenPractice Occupational Health and Safety ProceduresLourdes D. Delos SantosNoch keine Bewertungen
- DERMATITEe URTICARIAocupacionaisDokument15 SeitenDERMATITEe URTICARIAocupacionaisYgor AlbuquerqueNoch keine Bewertungen
- Ocular InjuryDokument6 SeitenOcular InjuryfidelisdentalNoch keine Bewertungen
- 41-Article Text-153-1-10-20201217Dokument6 Seiten41-Article Text-153-1-10-20201217نورالدين عبدالخالقNoch keine Bewertungen
- Ocular Adverse Effects After Facial Cosmetic Procedures: A Review of Case ReportsDokument7 SeitenOcular Adverse Effects After Facial Cosmetic Procedures: A Review of Case ReportsLucas HoldereggerNoch keine Bewertungen
- 66 Allery and OrthodonticDokument5 Seiten66 Allery and OrthodonticShruthi KamarajNoch keine Bewertungen
- Unit Iii Industrial Health HazardDokument14 SeitenUnit Iii Industrial Health HazardVignesh KNoch keine Bewertungen
- Metal Hypersensitivity in Orthodontic PatientsDokument5 SeitenMetal Hypersensitivity in Orthodontic PatientsvivigaitanNoch keine Bewertungen
- Occupational Hazards and Risk Management in Nursing PracticeDokument27 SeitenOccupational Hazards and Risk Management in Nursing PracticeMelly SumardiNoch keine Bewertungen
- Metal Hypersensitivity in Orthodontic PatientsDokument5 SeitenMetal Hypersensitivity in Orthodontic PatientsMartha Lia Castaño EcheverryNoch keine Bewertungen
- Segurança Da OzonioterapiaDokument13 SeitenSegurança Da OzonioterapiaLeonardoMdCNoch keine Bewertungen
- Toxicology StacyDokument633 SeitenToxicology StacyRevi RinaldiNoch keine Bewertungen
- Occupational Health Hazards: Raymond Gomez BlancoDokument113 SeitenOccupational Health Hazards: Raymond Gomez BlancoAviects Avie JaroNoch keine Bewertungen
- Chemistry (Laboratory)Dokument14 SeitenChemistry (Laboratory)invalidNoch keine Bewertungen
- SampleDokument10 SeitenSampleDhiren SoniNoch keine Bewertungen
- Full TextDokument12 SeitenFull TextDhiren SoniNoch keine Bewertungen
- Learning Style InventoryDokument2 SeitenLearning Style InventoryDhiren SoniNoch keine Bewertungen
- Clinic Human GeneticsDokument8 SeitenClinic Human GeneticsDhiren SoniNoch keine Bewertungen
- Analisis Biomecanico de La Tecnica GYAKU TSUKI de Karate de Un Equipo Donde Un Karateca Presenta Lesion de RodillaDokument14 SeitenAnalisis Biomecanico de La Tecnica GYAKU TSUKI de Karate de Un Equipo Donde Un Karateca Presenta Lesion de Rodillajesusisasis21Noch keine Bewertungen
- 2013 High School Football PreviewDokument24 Seiten2013 High School Football PreviewThe DispatchNoch keine Bewertungen
- 1.1 Bone Function: 2 OsteoarchaeologyDokument5 Seiten1.1 Bone Function: 2 OsteoarchaeologyKeila MenaNoch keine Bewertungen
- Black & Decker Pv1210Dokument8 SeitenBlack & Decker Pv1210KalygulyNoch keine Bewertungen
- Physical AssessmentDokument19 SeitenPhysical Assessmentsilentscream0618Noch keine Bewertungen
- B230 Printer Service ManualDokument364 SeitenB230 Printer Service ManualxorbartosNoch keine Bewertungen
- Legal Notice - Waseem AkramDokument18 SeitenLegal Notice - Waseem AkramMuhammad Asif BhattiNoch keine Bewertungen
- EponymsDokument30 SeitenEponymsNovaatackNoch keine Bewertungen
- Fundamentals of Pediatric Surgery Second EditionDokument893 SeitenFundamentals of Pediatric Surgery Second EditionPatricia Beznea100% (3)
- Vasyl Stefanyk - A STONE CROSSDokument5 SeitenVasyl Stefanyk - A STONE CROSSandreusDADANoch keine Bewertungen
- Tutorial: MODULE: Musculoskeletal System IIDokument3 SeitenTutorial: MODULE: Musculoskeletal System IIrishit20% (5)
- Muscles of Facial ExpressionDokument63 SeitenMuscles of Facial Expressionraphaelyohana140Noch keine Bewertungen
- Injuries StudentsDokument101 SeitenInjuries StudentsBnB UsmleNoch keine Bewertungen
- Blue Writing Is What I Added To These Notes: RadiographyDokument46 SeitenBlue Writing Is What I Added To These Notes: Radiographybjpalmer100% (2)
- Kru PS: Crystal Arome Model #398 Crystal Arome Time Model #458Dokument12 SeitenKru PS: Crystal Arome Model #398 Crystal Arome Time Model #458JOSEACOSTANoch keine Bewertungen
- Introduction To Prolotherapy PRP and Stem Cell TherapyDokument6 SeitenIntroduction To Prolotherapy PRP and Stem Cell TherapyAna TodorovaNoch keine Bewertungen
- Human Anatomy 8th Edition Marieb Solutions Manual DownloadDokument14 SeitenHuman Anatomy 8th Edition Marieb Solutions Manual DownloadMary Brown100% (23)
- HIRADokument57 SeitenHIRAAnonymous Uc25fP83% (6)
- Pelvic FractureDokument18 SeitenPelvic FractureTantyaNoch keine Bewertungen
- Basic First Aid TrainingDokument64 SeitenBasic First Aid TrainingJohn Lexter PayumoNoch keine Bewertungen
- Gait Normalabnormal 120622041834 Phpapp02Dokument102 SeitenGait Normalabnormal 120622041834 Phpapp02citaNoch keine Bewertungen
- Petitioner vs. vs. Respondents: en BancDokument6 SeitenPetitioner vs. vs. Respondents: en BancJessica Magsaysay CrisostomoNoch keine Bewertungen
- Combat Life SaverDokument35 SeitenCombat Life SaverYujal Man Singh100% (1)
- 2.literary of BurnDokument45 Seiten2.literary of BurnKrishnaNoch keine Bewertungen
- PYD Asanas V3 - 1Dokument33 SeitenPYD Asanas V3 - 1aryakushalNoch keine Bewertungen
- 1,000 Questions To Help You Pass The Emergency Medicine BoardsDokument338 Seiten1,000 Questions To Help You Pass The Emergency Medicine Boardsduvayozare100% (1)
- Module 7 FinalDokument14 SeitenModule 7 FinalPATRICK DEGAMONoch keine Bewertungen
- Contemporary Periodontal Surgery: 2. Surgical Practice: PeriodontologyDokument6 SeitenContemporary Periodontal Surgery: 2. Surgical Practice: PeriodontologybkprosthoNoch keine Bewertungen
- Polaris 2002 Owners Manual (9917128) 03 PDFDokument146 SeitenPolaris 2002 Owners Manual (9917128) 03 PDFCorey BarkerNoch keine Bewertungen
- Appendicular SkeletonDokument10 SeitenAppendicular SkeletonJoshua DanielNoch keine Bewertungen