Beruflich Dokumente
Kultur Dokumente
Aquatic Chemistry
Hochgeladen von
Petromania MuthayalaOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Aquatic Chemistry
Hochgeladen von
Petromania MuthayalaCopyright:
Verfügbare Formate
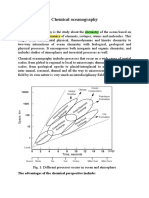
Aquatic chemistry Scope of aquatic chemistry Aquatic chemistry is concerned with the chemical reactions and processes affecting
the distribution and circulation of chemical species in natural waters. The objectives include the development of a theoretical basis for the chemical behavior of ocean waters, estuaries, rivers, lakes, ground waters, and soil water systems, as well as the description of processes involved in water technology. Aquatic chemistry draws primarily on the fundamentals of chemistry, but it is also by other sciences, especially geology and biology. A theme of this book is the fundamental principles of physical chemistry can be used to identify the pertinent variables that determine the composition of natural water systems. The student of chemistry is perhaps not fully aware that the well known laws of physical chemistry not only apply in the chemical laboratory but also regulate the course of reactions taking place in nature. During the hydrological cycle, water interacts continuously with the earth. Thus a progressive differentiation of geological material is achieved by processes of weathering, soil erosion, and soil and sediment formation. These processes accomplished by nature on large scale have been likened to sequence of separations carried out during the course of a chemical analysis. The basic processes- dissolution and precipitation, oxidation and reduction, acid-base and complexation interactions are the same in the nature of the laboratory. Silen likened the evolution of the earths atmosphere ocean system to a set of gigantic, coupled acid-base and oxidant-reductant titrations in which volatile acids from the interior of the earth were titrated by the bases of the rocks, and the reduced volatiles were titrated by the oxygen of the evolving atmosphere-biosphere system. While this book treats several topics similar to those found in an analytical chemistry text, it endevours to consider the spatial and temporal scales of the reactions in nature as distinctly different from those of the laboratory. For example, in chemical analysis, precipitates (frequently metastable and active compounds) are formed from strongly oversaturated solutions , whereas in the natural water systems, the solid phase is often formed under conditions of slight supersaturation; often crystal growth and aging may continue over geological time spans. Interfacial phenomena are particularly important because chemical processes of significance often occur only at phase discontinuities. Natural systems typically consists of numerous mineral assemblages and often include gas phase in addition to the aqueous phase; they almost always involve a portion of the biosphere. Hence natural aqueous habitats are characterized by a complexity seldom encountered in the laboratory. Inorder to select the pertinent variables out of sometimes bewildering number of possible ones, it is advantageous to compare the real systems with their idealized counterparts. Figure 1.1 shows in a very general way the kinds of natural water environments of interest to aquatic chemistry. The cycle of water links the elemental cycles of the atmospheric with those of the sediments. Thus atmospheric chemistry, water chemistry, sediment geochemistry, soil chemistry, and groundwater
chemistry of the elements are all connected, on a range of time scales. Chemical models aim to capture the most important variables of the complex natural water systems
Das könnte Ihnen auch gefallen
- Hydrogeochemistry Fundamentals and Advances, Mass Transfer and Mass TransportVon EverandHydrogeochemistry Fundamentals and Advances, Mass Transfer and Mass TransportNoch keine Bewertungen
- Updated FROR 3203 LimnologyDokument35 SeitenUpdated FROR 3203 Limnologyaminu saboNoch keine Bewertungen
- Chemical OceanographyDokument8 SeitenChemical OceanographyHalima akterNoch keine Bewertungen
- Makalah Self PurificationDokument12 SeitenMakalah Self PurificationFatimah Zuhra100% (1)
- Presentation 1Dokument51 SeitenPresentation 1AfeworkNoch keine Bewertungen
- Phase Interactions in Aquatic ChemistryDokument33 SeitenPhase Interactions in Aquatic ChemistryPeter John Paul TarimanNoch keine Bewertungen
- Water Pollution ControlVon EverandWater Pollution ControlSuresh T. NesaratnamNoch keine Bewertungen
- The Tragedy of the Commodity: Oceans, Fisheries, and AquacultureVon EverandThe Tragedy of the Commodity: Oceans, Fisheries, and AquacultureNoch keine Bewertungen
- Course Title: Environmental Chemistry by Dr. Sajid MahmoodDokument31 SeitenCourse Title: Environmental Chemistry by Dr. Sajid MahmoodSajid Mahmood MayoNoch keine Bewertungen
- Literature Review On Ocean AcidificationDokument7 SeitenLiterature Review On Ocean Acidificationfdnmffvkg100% (1)
- Global Environment: Water, Air, and Geochemical Cycles - Second EditionVon EverandGlobal Environment: Water, Air, and Geochemical Cycles - Second EditionBewertung: 5 von 5 Sternen5/5 (1)
- Betsy EVS Internals Term 1 2021Dokument11 SeitenBetsy EVS Internals Term 1 2021Betsy BetsyNoch keine Bewertungen
- Hydrogeochemistry Fundamentals and Advances, Groundwater Composition and ChemistryVon EverandHydrogeochemistry Fundamentals and Advances, Groundwater Composition and ChemistryNoch keine Bewertungen
- Environmental ChemistryDokument39 SeitenEnvironmental ChemistryErika EludoNoch keine Bewertungen
- Dynamics of Marine Ecosystems: Biological-Physical Interactions in the OceansVon EverandDynamics of Marine Ecosystems: Biological-Physical Interactions in the OceansNoch keine Bewertungen
- Study of River EcosystemDokument16 SeitenStudy of River EcosystemIshanShikarkhane40% (5)
- 2014 Book GeochemistryAtTheEarthSSurfaceDokument327 Seiten2014 Book GeochemistryAtTheEarthSSurfaceMax Garzon100% (3)
- CE 6327-Class Note-1-April-2023Dokument18 SeitenCE 6327-Class Note-1-April-2023Sabbir Hasan MonirNoch keine Bewertungen
- Chemical Fate and Transport in the EnvironmentVon EverandChemical Fate and Transport in the EnvironmentBewertung: 2.5 von 5 Sternen2.5/5 (1)
- The Ripple Effect Unraveling The Psychology of Social ResponsibilityVon EverandThe Ripple Effect Unraveling The Psychology of Social ResponsibilityNoch keine Bewertungen
- VIII. The Sea As A Biological EnvironmentDokument17 SeitenVIII. The Sea As A Biological EnvironmentJastina NguyenNoch keine Bewertungen
- Fluid Inclusions in Sedimentary and Diagenetic SystemsDokument35 SeitenFluid Inclusions in Sedimentary and Diagenetic SystemsJuan Diego CollazosNoch keine Bewertungen
- Lecture 01Dokument11 SeitenLecture 01Hilmi HasaniNoch keine Bewertungen
- Fluid Systems: Fluids in The CrustDokument6 SeitenFluid Systems: Fluids in The CrustAkoSiMambuconOtitNoch keine Bewertungen
- Introduction to the Physics of Cohesive Sediment Dynamics in the Marine EnvironmentVon EverandIntroduction to the Physics of Cohesive Sediment Dynamics in the Marine EnvironmentNoch keine Bewertungen
- Physiochemical Properties of WaterDokument32 SeitenPhysiochemical Properties of WaterKori Environmental Research ConsultancyNoch keine Bewertungen
- Biogeochemistry: An Analysis of Global ChangeVon EverandBiogeochemistry: An Analysis of Global ChangeBewertung: 4.5 von 5 Sternen4.5/5 (4)
- Water PollutionDokument21 SeitenWater PollutionyekarlNoch keine Bewertungen
- Ocean Acidification Literature ReviewDokument6 SeitenOcean Acidification Literature Reviewafmzodjhpxembt100% (1)
- Edmunds2009 ReviewDokument16 SeitenEdmunds2009 ReviewYashashavi LadhaNoch keine Bewertungen
- Water Quality and Water Quality Management in AquacultureDokument20 SeitenWater Quality and Water Quality Management in AquacultureAhmed Rashid100% (1)
- Introduction To Freshwater Ecology: Key Words: Ecosystem, Food Web, Lakes, RiversDokument17 SeitenIntroduction To Freshwater Ecology: Key Words: Ecosystem, Food Web, Lakes, RiverssheriefmuhammedNoch keine Bewertungen
- Water Purification in Natural SystemsDokument3 SeitenWater Purification in Natural SystemsColdWinterKid100% (1)
- Hydraulics and Hydrology - Lecture 1 (Introduction - The - Hydrologic - and - Carbon - Cycle)Dokument29 SeitenHydraulics and Hydrology - Lecture 1 (Introduction - The - Hydrologic - and - Carbon - Cycle)joelkyeswa97Noch keine Bewertungen
- AppendixDokument4 SeitenAppendixAbby FaizNoch keine Bewertungen
- Enviromental ChemistryDokument216 SeitenEnviromental ChemistryzockawNoch keine Bewertungen
- Envi. ScienceDokument2 SeitenEnvi. ScienceTricia Maxine DomingoNoch keine Bewertungen
- BCE 3102 Hydrology and Hydraulics - Lecture 2 (The Hydrologic and Carbon Cycle)Dokument29 SeitenBCE 3102 Hydrology and Hydraulics - Lecture 2 (The Hydrologic and Carbon Cycle)Philip TibenderanaNoch keine Bewertungen
- SCE 201 (Marine Science)Dokument37 SeitenSCE 201 (Marine Science)allanlopez_2009100% (1)
- Sea As Biological EnvironmentDokument19 SeitenSea As Biological EnvironmentAamir Khan0% (1)
- Elements MatterDokument15 SeitenElements MatterUsama JavaidNoch keine Bewertungen
- Chem 6Dokument24 SeitenChem 6Adi SoNoch keine Bewertungen
- Environmental Effects of SurfactantsDokument11 SeitenEnvironmental Effects of Surfactantstalready69Noch keine Bewertungen
- Teacher Background: Biogeochemical CyclesDokument5 SeitenTeacher Background: Biogeochemical CycleswonuNoch keine Bewertungen
- McGuire - 2012 Ecosystem Element CyclingDokument5 SeitenMcGuire - 2012 Ecosystem Element CyclingstaldNoch keine Bewertungen
- Chemical Exchange Between Water and SedimentsDokument21 SeitenChemical Exchange Between Water and Sedimentsshiwasish swarNoch keine Bewertungen
- Ocean Acidification ThesisDokument12 SeitenOcean Acidification Thesisevkrjniig100% (1)
- Biogeochemical CycleDokument16 SeitenBiogeochemical CycleBojana ZdrNoch keine Bewertungen
- Water Purification in Natural SystemsDokument24 SeitenWater Purification in Natural Systemsnyan nyan nyanNoch keine Bewertungen
- Freshwater Ecology Synthesis PaperDokument5 SeitenFreshwater Ecology Synthesis PaperDeasserei TatelNoch keine Bewertungen
- 1) Water Purification in Natural SystemsDokument29 Seiten1) Water Purification in Natural SystemsJanice OmadtoNoch keine Bewertungen
- Ecological SystemsDokument18 SeitenEcological Systemsanum fatimaNoch keine Bewertungen
- Oscar Marquez-Martinez - CH 07Dokument8 SeitenOscar Marquez-Martinez - CH 07api-478637931Noch keine Bewertungen
- Biodiversidad Ecologia MarinaDokument8 SeitenBiodiversidad Ecologia MarinaJuan TrianaNoch keine Bewertungen
- Chapter 1 IntroductionDokument19 SeitenChapter 1 IntroductionAbdanur JihadNoch keine Bewertungen
- Chem101 Ho8Dokument17 SeitenChem101 Ho8Claire TaborNoch keine Bewertungen
- River Geochemistry 1Dokument36 SeitenRiver Geochemistry 1anon-732369100% (1)
- Revised Research ZoomDokument51 SeitenRevised Research ZoomAubrey Unique EvangelistaNoch keine Bewertungen
- Application Bright Ideas Education Grant Program For TeachersDokument6 SeitenApplication Bright Ideas Education Grant Program For Teachersapi-320983699Noch keine Bewertungen
- Validation For A Login PageDokument2 SeitenValidation For A Login PageAmal RajNoch keine Bewertungen
- Original Research PapersDokument13 SeitenOriginal Research Papersrikaseo rikaNoch keine Bewertungen
- Online Preboard Math PDFDokument4 SeitenOnline Preboard Math PDFmarkos0% (1)
- 3-Storeyed 31-3-2015-Schedule PDFDokument1 Seite3-Storeyed 31-3-2015-Schedule PDFSi Thu AungNoch keine Bewertungen
- Advanced Laser Al170: Instruction ManualDokument35 SeitenAdvanced Laser Al170: Instruction ManualJuan Camilo100% (1)
- Vocology For The Singing Voice PDFDokument120 SeitenVocology For The Singing Voice PDFNathalia Parra Garza100% (2)
- PASSAGE ONE (Questions 1-4)Dokument5 SeitenPASSAGE ONE (Questions 1-4)Vian LonkzeerNoch keine Bewertungen
- pm2 5 Sensor 201605Dokument6 Seitenpm2 5 Sensor 201605Vennela NandikondaNoch keine Bewertungen
- IMG - 0009 Thermodynamic Lecture MRCDokument1 SeiteIMG - 0009 Thermodynamic Lecture MRCBugoy2023Noch keine Bewertungen
- Lubricants - McMaster-CarrDokument8 SeitenLubricants - McMaster-CarrjeanyoperNoch keine Bewertungen
- PienaDokument1 SeitePienaMika Flores PedroNoch keine Bewertungen
- Submission Letter To LBUDokument46 SeitenSubmission Letter To LBUramesh bajracharyaNoch keine Bewertungen
- Travel Advertisement RubricDokument2 SeitenTravel Advertisement Rubricapi-316353024Noch keine Bewertungen
- CHAPTER 2 - ALGEBRA (Latest)Dokument41 SeitenCHAPTER 2 - ALGEBRA (Latest)FirdausNoch keine Bewertungen
- Conquering College The Most Fun You Can Have Learning The Things You Need To Know NodrmDokument144 SeitenConquering College The Most Fun You Can Have Learning The Things You Need To Know NodrmVithorNoch keine Bewertungen
- Report Writing: IBS HyderabadDokument35 SeitenReport Writing: IBS HyderabadHarita SudhirNoch keine Bewertungen
- The Aerodynamics of ParachutesDokument78 SeitenThe Aerodynamics of Parachutesstevehuppert50% (2)
- Capital Budgeting and Capital Budgeting and Risk Analysis Risk AnalysisDokument16 SeitenCapital Budgeting and Capital Budgeting and Risk Analysis Risk AnalysisHaris FendiarNoch keine Bewertungen
- VSR Trans. PPT3Dokument16 SeitenVSR Trans. PPT3VSR TRANSNoch keine Bewertungen
- Staad 4Dokument37 SeitenStaad 4saisssms9116100% (2)
- Occupational Therapy Examination Review Guide 4th Edition Ebook PDFDokument57 SeitenOccupational Therapy Examination Review Guide 4th Edition Ebook PDFrobert.campbell485Noch keine Bewertungen
- A Brief Tutorial On Studio MonitorsDokument18 SeitenA Brief Tutorial On Studio MonitorsCurtis O'BrienNoch keine Bewertungen
- Bibliography and FootnotesDokument2 SeitenBibliography and FootnotesHannah de VeraNoch keine Bewertungen
- Animal Defenses TestDokument3 SeitenAnimal Defenses TestNermine MouallemNoch keine Bewertungen
- Sect. 4 Tech Docum PC7 AutoLube - 1209 PDFDokument46 SeitenSect. 4 Tech Docum PC7 AutoLube - 1209 PDFAlexis MikeNoch keine Bewertungen
- Palmiye Leaflet 2015 enDokument4 SeitenPalmiye Leaflet 2015 ensaraju_felixNoch keine Bewertungen
- Pepperl KFD2 STC4 EX1.20 DatasheetDokument2 SeitenPepperl KFD2 STC4 EX1.20 DatasheetAhmed HusseinNoch keine Bewertungen
- Pearson Letter To ParentsDokument2 SeitenPearson Letter To ParentsPatricia WillensNoch keine Bewertungen