Beruflich Dokumente
Kultur Dokumente
Chroma To
Hochgeladen von
shubham007Originalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Chroma To
Hochgeladen von
shubham007Copyright:
Verfügbare Formate
UNIT-5
Chromatography
no t
Aim
to N be C ER re pu T bl is he d
EXPERIMENT 5.1
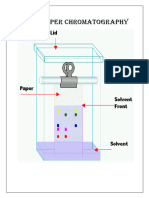
HE technique of chromatography is vastly used for the separation, purification and identification of compounds. According to IUPAC, chromatography is a physical method of separation in which the components to be separated are distributed between two phases, one of which is stationary while the other moves in a definite direction. The stationary phase is usually in the form of a packed column (column chromatography) but may take other forms such as flat sheet or a thin layer adhering to a suitable form of backing material such as glass (thin-layer chromatography). In column chromatography, mobile phase flows through the packed column, while in thin layer chromatography, mobile phase moves by capillary action. In this the thin film stationary phase may be either a liquid or a solid and the mobile phase may be a liquid or a gas. Different possible combinations of these phases give rise to principal techniques of chromatography. Two of these are described below. In partition chromatography, stationary phase is thin film of liquid adsorbed on an essentially inert support. Mobile phase may be a liquid or a gas. Paper chromatography is an example of partition chromatography in which liquid present in the pores of paper is stationary phase and some other liquid is movable phase. Separation depends upon partition of substance between two phases and the adsorption effects of inert support on compounds undergoing chromatographic separation. In adsorption chromatography, the stationary phase is a finely divided solid adsorbent and the mobile phase is usually a liquid. Process of separation depends upon selective adsorption of components of a mixture on the surface of a solid. In chromatography, substance equilibrates between a mobile and a stationary phase. The more the interaction of substance with the stationary phase, slower is its movement. In this unit you will learn about the technique of separating the components of a mixture by using paper chromatography.
Separation of pigments present in the leaves (spinach) and flowers (rose, marigold) by paper chromatography and determination of R f value of components.
CHROMATOGRAPHY
Theory
In paper chromatography, water molecules present in the pores of the filter paper act as the stationary phase and the moving phase can be a solvent like hexane, toluene, acetone or a mixture of solvents such as methanol-water mixture etc. As the moving phase passes through the spot on which sample has been adsorbed, it dissolves the components more or less readily; depending upon the solubility and carries them along with it while moving on the support. At a given temperature and for a given solvent, it is possible to determine the characteristic rate of movement of each substance on the chromotographic paper, as the moving phase moves. This is represented by relative front or retardation factor also called Rf value. Rf values of different compounds are different even if the mobile phase (solvent) is same. Furthermore, Rf value of a compound may be different in different solvents. Rf values can be calculated by using the following expression:
Rf Distan ce travelled by the substance fr om reference line (cm)
Methanol Acetone
Distance travelled by th e solvent fron t from r eference line (cm)
Since solvent front moves faster than the compounds, the Rf value of a substance will always be less than one. Also note that Rf value has no unit. If the compound is coloured then its position on the chromatographic paper may be easily located. However, if the substance is colourless, it may be treated with a reagent, which imparts it a characteristic colour. This reagent is given the name developer. Iodine is the most commonly used developer in paper chromatography. Several other techniques are available for locating the spots.
Material Required
no t
Whatmans filter paper No.1 of size 4 cm 17 cm : Gas jar of size 5 cm 20 cm: Rubber cork fixed with hook in the centre : Test tubes :
Procedure
(i) (ii)
Grind flowers/leaves in a mortar and transfer the paste into a test tube. Add small amounts of methanol or acetone in the crushed material. Close the test tube with an appropriate cork and
to N be C ER re pu T bl is he d
Chloroform
Petroleum ether
One One
One As per need
Flower extract and extract of leaves : Distilled water : Methanol/Acetone : Petroleum ether boiling range (6080C) : Chloroform /Acetone :
As per need As per need As per need
As per need As per need
35
LABORATORY MANUAL CHEMISTRY
no t
(a) (b)
Fig. 5.1 : (a) Marked paper; (b) Dipping the filter paper in the solvent; (c) Developing chromatogram; and (d) Developed chromatogram
36
to N be C ER re pu T bl is he d
(c) (d)
shake it well. Filter it and collect the filtrate in a test tube and cork the test tube. (iii) Procure a Whatman filter paper No.1 of size 4 cm 17 cm and mark a line at a distance of 3 cm from one of the ends of the paper with the help of a pencil [Fig. 5.1(a)]. (iv) Using a finely drawn capillary, put one spot a for the extract of leaves and one spot b for the extract of flowers. Allow these spots to dry as shown in Fig. 5.1 (a). (v) Hang the filter paper in a jar containing 20 mL mixture of petroleum ether (boiling range 6080C) and chloroform containing 19 mL petroleum ether and 1 mL chloroform or a mixture of petroleum ether (boiling range 6080C) and acetone in the ratio 9:1 (18 mL petroleum ether + 2 mL acetone) so that the solvent does not touch the reference line as given in Fig. 5.1 (b). (vi) Keep this jar as such till the mobile phase (solvent) rises up to 2/3 of the length of the paper [Fig. 5.1(c)]. (vii) Remove the filter paper from the jar, mark the solvent front, outline the spots with the help of a pencil and allow the filter paper to get dry. (viii) Measure the distance travelled by the solvent front and the centre of different spots with respect to the reference line as given in Fig. 5.1 (d). (x) Ascertain the number of pigments, which are present in the extract of leaves and flowers. (xi) Calculate the R f value of different spots with the help of the expression mentioned earlier.
CHROMATOGRAPHY
(xii)
Record your observations as in Table 5.1.
Table 5.1: Separation of pigments of leaves and flowers Sl. No. Name of the extract 1. 2. 3. 4. Colour of the spot Distance travelled by the components of the spots a or b from the reference line in cm Distance travelled by the solvent from reference line in cm Rf value
Result
(i) (ii)
R f values of components of flower are __________. R f values of components of leaves are __________.
Precautions
(a)
Use good quality pencil for drawing the reference line so that the mark does not dissolve in the solvent in which TLC is run. (b) Dip the paper strip in the solvent in such a way that the spot of the mixture is above the solvent level and the movement of the solvent front is not zig-zag. (c) While spotting the test solution on the paper, do not allow the spots to spread. Use finely drawn capillary to put the spot on the paper. (d) Ensure that the filter paper strip hangs freely in the jar. (e) Once the experiment is set, do not disturb the jar as long as the chromatogram is being developed. (f) Keep the jar covered with the lid when the chromatogram is being developed. (g) Make the paper strip perfectly dry before developing the spots. (h) Handle the organic solvent/solvents, with care.
EXPERIMENT 5.2
Aim
Separation of the constituents of a mixture of inorganic compounds containing two cations, Pb2+ and Cd2+ , using chromatographic technique.
no t
to N be C ER re pu T bl is he d
37
LABORATORY MANUAL CHEMISTRY
Theory
Principle for the separation of cations is same as has been explained in Experiment 5.1. In this case the two cations to be separated are colourless. therefore, a developer is needed. In the present case, ammonium sulphide (NH4) 2S*, can be used to locate the position of these ions on chromatographic paper or plate.
Material Required
Ethanol
Lead nitrate
Cadmium nitrate
no t
* Ammonium sulphide is prepared by passing H2S gas through the mixture containing 100 mL water and 10 mL liquor ammonia for about 45 minutes.
38
to N be C ER re pu T bl is he d
One One One As per need 12% solution of Pb(NO3)2 and Cd(NO3)3 Ehthanol 6.0 M HNO3 : : : As per need As per need As per need
Whatmans filter paper No. 1 of size 4 cm 17 cm : Gas jar of size 5 cm 20 cm : Rubber cork fixed with hook in the centre : Test tubes :
Procedure
(i)
(ii)
(iii) (iv)
(v)
Procure a Whatman No. 1 filter paper of size 4 cm 17 cm. With the help of a pencil, mark a line at a distance of 3 cm from one of the ends of this paper. Put a spot of the mixture on the marked line with the help of a fine capillary. Hang the filter paper in a jar containing a mixture of ethanol, 6.0 M HNO3 and distilled water, in the ratio 8:1:1. Keep the jar as such till the mobile phase (solvent) rises up to two third of the length of the paper. Remove the filter paper from the jar, mark the solvent front.
Spray ammonium sulphide solution on the chromatography paper to obtain spots of yellow and black colour. Mark the position of spots with a pencil and allow the paper to dry. (vii) Measure the distance moved by the solvent front and the different spots of the cations with respect to the reference line. This distance is the shortest distance between the reference line and the centre of different spots. (viii) Record the observations in tabular form as in Table 5.2. Calculate the R f value for each cation.
(vi)
CHROMATOGRAPHY
Table 5.2 : Separation of Pb2+ and Cd2+ ions by paper chromatography Sl. Colour of No. the spot 1. 2. 3. Distance travelled by components from reference line/cm Distance travelled by the solvent from reference line/cm Rf value
Result
(i) (ii)
R f values of Pb2+ ions is __________. 2+ R f values of Cd ions is __________.
Precautions
(a)
Use good quality pencil for drawing the reference line so that the mark does not dissolve in the solvent in which TLC is run. (b) Dip the paper strip in the solvent in such a way that the spot of the mixture is above the solvent level and movement of solvent front is not zig-zag. (c) While spotting the test solution on the paper, do not allow the spots to spread. Use finely drawn capillary to put the spot on the paper. (d) Ensure that the filter paper strip hangs freely in the jar. (e) Once the experiment is set, do not disturb the jar as long as the chromatogram is being developed. (f) Keep the jar covered with the lid when the chromatogram is being developed. (g) Make the paper strip perfectly dry before developing the spots. (h) Handle the organic solvent/solvents, with care.
Discussion Questions
(i)
(ii) (iii)
no t
What is a chromatogram? Explain the principle on which the technique of chromatography is based. What are the essential characteristics of the substance used as a developer?
How is the phenomenon of adsorption applied in the separation of compounds by chromatography?
to N be C ER re pu T bl is he d
39
Das könnte Ihnen auch gefallen
- Selected Readings in Chromatography: The Commonwealth and International Library: Selected Readings in Analytical ChemistryVon EverandSelected Readings in Chromatography: The Commonwealth and International Library: Selected Readings in Analytical ChemistryR. J. MageeNoch keine Bewertungen
- Exercise 4 (Chromatography)Dokument6 SeitenExercise 4 (Chromatography)Wendell Kim LlanetaNoch keine Bewertungen
- A Practical Handbook of Preparative HPLCVon EverandA Practical Handbook of Preparative HPLCBewertung: 3 von 5 Sternen3/5 (3)
- Exercise 4 (Chromatography)Dokument6 SeitenExercise 4 (Chromatography)fangirlton0% (1)
- Experiment 2 ChromatographyDokument4 SeitenExperiment 2 ChromatographySuryansh MalikNoch keine Bewertungen
- Introduction & Analysis (TLC)Dokument5 SeitenIntroduction & Analysis (TLC)Syaza Izzah Athirah Bt SpaieeNoch keine Bewertungen
- Thermometric Titrimetry: International Series of Monographs in Analytical ChemistryVon EverandThermometric Titrimetry: International Series of Monographs in Analytical ChemistryNoch keine Bewertungen
- 5.1 5.2 ChromatographyDokument3 Seiten5.1 5.2 Chromatographyrubishaasokan015Noch keine Bewertungen
- ChromatographyDokument18 SeitenChromatographyYerram Raju BeharaNoch keine Bewertungen
- Bo 201 - TLC Paper CDokument2 SeitenBo 201 - TLC Paper CEzety TermiziNoch keine Bewertungen
- DocumentDokument21 SeitenDocumentcᴘcтԍᴀмιɴԍ YTNoch keine Bewertungen
- Experiment 1 ChromatographyDokument2 SeitenExperiment 1 ChromatographyNandita KrishnanNoch keine Bewertungen
- Exp 7 ChromatographyDokument10 SeitenExp 7 ChromatographyNur AidaNoch keine Bewertungen
- Exercise No.2 Paper ChromatographyDokument5 SeitenExercise No.2 Paper ChromatographyMary Jane YepesNoch keine Bewertungen
- Lab 3 - Separation of PH Indicators Using Paper ChromatographyDokument6 SeitenLab 3 - Separation of PH Indicators Using Paper ChromatographyJesiann SmithNoch keine Bewertungen
- Chemistry Project Paper ChromatographyDokument20 SeitenChemistry Project Paper ChromatographyAmrita SNoch keine Bewertungen
- Experiment 2Dokument3 SeitenExperiment 2Ushna Asif BSCHE-ENoch keine Bewertungen
- Samriddhi Chromatography ProjectDokument17 SeitenSamriddhi Chromatography Projectcᴘcтԍᴀмιɴԍ YTNoch keine Bewertungen
- Exercise 4 (Chromatography)Dokument6 SeitenExercise 4 (Chromatography)Cherryl SurigaoNoch keine Bewertungen
- Chemistry ProjectDokument28 SeitenChemistry ProjectVikramjeet Singh82% (11)
- Technical Paper Lab Report: Paper Chromatography: John Michael E. Lunar 12 - Stem HonestyDokument6 SeitenTechnical Paper Lab Report: Paper Chromatography: John Michael E. Lunar 12 - Stem HonestyJezra MajabaNoch keine Bewertungen
- Bioc 211Dokument6 SeitenBioc 211Femina ArgonzaNoch keine Bewertungen
- Separation and Identification of Plant Pigments by TLC MainDokument5 SeitenSeparation and Identification of Plant Pigments by TLC MainnaomiNoch keine Bewertungen
- SEMESTER 3 Practical Science 2 Experiment 8 TopicDokument6 SeitenSEMESTER 3 Practical Science 2 Experiment 8 Topickat tunNoch keine Bewertungen
- HrmtograhiDokument8 SeitenHrmtograhiALJOREY LAZARITONoch keine Bewertungen
- Activity 5 Paper ChromDokument5 SeitenActivity 5 Paper ChromYma FeelNoch keine Bewertungen
- Lab 5: Paper ChromatographyDokument3 SeitenLab 5: Paper ChromatographySyaf AronzNoch keine Bewertungen
- B.tech. Biotechnology NotesDokument2 SeitenB.tech. Biotechnology NotesMudit MisraNoch keine Bewertungen
- Results and Discussions 6Dokument4 SeitenResults and Discussions 6Lucile BronzalNoch keine Bewertungen
- Unit 6Dokument42 SeitenUnit 6Nur Amelia AmirNoch keine Bewertungen
- ChromatographyDokument112 SeitenChromatographyprateeksinghal26961Noch keine Bewertungen
- Exp 5 - Paper Chrom. (Methods and Appendices)Dokument6 SeitenExp 5 - Paper Chrom. (Methods and Appendices)Chali HaineNoch keine Bewertungen
- Experiment 4 - Lab Manual - B32b752bc8c4ca86579ffad85f - 230417 - 122136Dokument5 SeitenExperiment 4 - Lab Manual - B32b752bc8c4ca86579ffad85f - 230417 - 122136Retardo Meal O'sNoch keine Bewertungen
- Chromatography, M&M, Chromatography PaperDokument5 SeitenChromatography, M&M, Chromatography PaperAljoša TimaracNoch keine Bewertungen
- Analysis of Ink by TLCDokument7 SeitenAnalysis of Ink by TLCkeshavNoch keine Bewertungen
- Paper Chromatography Model and Practical LabDokument4 SeitenPaper Chromatography Model and Practical Labdebbie bongNoch keine Bewertungen
- Chromatographic Techniques 1200328060603035050Dokument12 SeitenChromatographic Techniques 1200328060603035050KIRAN ALLUNoch keine Bewertungen
- Lab Report 2 320Dokument3 SeitenLab Report 2 320AeeshaNoch keine Bewertungen
- TLC Chromotography Inv 2015Dokument3 SeitenTLC Chromotography Inv 2015John OsborneNoch keine Bewertungen
- ChromatographyDokument3 SeitenChromatographyAashiNoch keine Bewertungen
- Class 12Dokument21 SeitenClass 12Sujatha SridharaNoch keine Bewertungen
- Paper ChromatographyDokument17 SeitenPaper ChromatographyWhy I am not VIRAT KOHLINoch keine Bewertungen
- 083 - Chromatography and Its Uses in BiologyDokument3 Seiten083 - Chromatography and Its Uses in Biologylastjoe71Noch keine Bewertungen
- Chapter 18 - ChromatographyDokument16 SeitenChapter 18 - ChromatographyJames Miller100% (1)
- CHEMDokument13 SeitenCHEMMohamed MusthapaNoch keine Bewertungen
- Lab 1Dokument4 SeitenLab 1Michelle ChicaizaNoch keine Bewertungen
- In Chemical AnalysisDokument5 SeitenIn Chemical AnalysisVarad GhugeNoch keine Bewertungen
- BEP1021 - Group 3 Experiment 4Dokument16 SeitenBEP1021 - Group 3 Experiment 4Tasmea sultanaNoch keine Bewertungen
- TLC Chromotography Inv Year 11Dokument2 SeitenTLC Chromotography Inv Year 11John OsborneNoch keine Bewertungen
- Air Force School Ambala CanttDokument16 SeitenAir Force School Ambala Canttsimran ranaNoch keine Bewertungen
- Chem Lab Project Paper ChromatographyDokument14 SeitenChem Lab Project Paper ChromatographyFarah Kharuddin100% (1)
- Paper ChromatographyDokument6 SeitenPaper ChromatographyMuslimah Anggun100% (1)
- INK ChromatographyDokument10 SeitenINK ChromatographyRaymond Godfrey Dagwasi100% (1)
- Practice 3 ChromatographyDokument2 SeitenPractice 3 Chromatographymel bien GarduñoNoch keine Bewertungen
- Chromatography Lab #Dokument7 SeitenChromatography Lab #Rhea FrancisNoch keine Bewertungen
- Plant ChromatographyDokument17 SeitenPlant ChromatographyYujin SeoNoch keine Bewertungen
- Chromatography P1eeaoqbpea91bc5e2b1cc84Dokument91 SeitenChromatography P1eeaoqbpea91bc5e2b1cc84Asif AliNoch keine Bewertungen
- 18.01 Supplementary Notes, Exercises, and SolutionsDokument1 Seite18.01 Supplementary Notes, Exercises, and SolutionsAyush K. SharmaNoch keine Bewertungen
- © Ncert Not To Be Republished: LectrochemistryDokument4 Seiten© Ncert Not To Be Republished: LectrochemistryrajatguptNoch keine Bewertungen
- Math Research - Reflections - Intellectual ExperienceDokument2 SeitenMath Research - Reflections - Intellectual ExperienceAyush K. SharmaNoch keine Bewertungen
- Lelm 103Dokument11 SeitenLelm 103ArvindVishakNoch keine Bewertungen
- Lelm 102Dokument11 SeitenLelm 102RishiNoch keine Bewertungen
- © Ncert Not To Be Republished: T A (R R)Dokument9 Seiten© Ncert Not To Be Republished: T A (R R)rajatguptNoch keine Bewertungen
- 09Dokument4 Seiten09ashuNoch keine Bewertungen
- Lelm 110 OkioDokument10 SeitenLelm 110 OkioAMAN10344Noch keine Bewertungen
- Title/ Chemistry Lab Manual 12thDokument10 SeitenTitle/ Chemistry Lab Manual 12thAyush K. Sharma50% (2)
- Lab Manual Class 12Dokument7 SeitenLab Manual Class 12Aravind MuralidharanNoch keine Bewertungen
- Distinction Test (Organic Chemistry)Dokument14 SeitenDistinction Test (Organic Chemistry)Ashish Kumar100% (3)
- Projects (Corrected On 18-08-08)Dokument7 SeitenProjects (Corrected On 18-08-08)raja_r_sNoch keine Bewertungen