Beruflich Dokumente
Kultur Dokumente
Brain Injury 2
Hochgeladen von
seigelysticOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Brain Injury 2
Hochgeladen von
seigelysticCopyright:
Verfügbare Formate
BRAIN INJURY: INCREASED INTRACRANIAL PRESSURE AND HERNIATION MEDICALVILLAGE.BLOGSPOT.
COM SPECIAL MEDICAL REPORT PART 2
Increased Intracranial Pressure (ICP).
The brain is enclosed in the rigid confines of the skull, or cranium, making it particularly susceptible to increases in ICP. Increased ICP is a common pathway for brain injury from different types of insults and agents. Excessive ICP obstruct cerebral blood flow, destroy brain cells, displace brain tissue as in herniation, and otherwise damage delicate brain structures. The cranial cavity contains blood (approximately 10%), brain tissue (approximately 80%), and CSF (approximately 10%) in the rigid confines of a nonexpendable skull. Each of these volumes contributes to the ICP, which normally is maintained within a range of 0 to 15 mm Hg when measured in the lateral ventricles. The volumes of each of these components can vary slightly without causing marked changes in ICP. This is because small increases in the volume of the component can be compensated for a decrease in the volume of one or both of the other two components. This association is called the Monro-Kellie hypothesis. Normal fluctuation in ICP occur with respiratory movements and activities of daily living such as straining, coughing, and sneezing. Abnormal variation in intracranial volume with subsequent changes in ICP can be caused by a volume change in any of the three intracranial components. For example, an increase in tissue volume can result from a brain tumor, brain edema, or bleeding into brain tissue. An increase in blood volume develops when there is vasodilation of cerebral vessels or obstruction of venous outflow. Excess production, decrease absorption, or obstructed circulation of CSF affords the potential for an increase in the CSF component. When the change in volume is caused by a brain tumor, it tends to occur slowly and usually is localized to the immediate area, whereas the increase resulting from head injury usually develops rapidly. According to the modified Monro-Kellie hypothesis, reciprocal compensation occurs among the three intracranial compartments. Of the three intracranial volumes, tissue volume is relatively restricted in its ability to udergo change; CSF and blood volume are best able to compensate for changes in ICP. Initial increases in ICP are buffered by a translocation of CSF to the spinal subarachnoid space and increased reabsorption of CSF. The compensatory ability of the blood compartment is limited by a small amount of blood that is in the cerebral circulation. The cerebral blood vessels contain less than 10% of the intracranial volume, most of which is contained in the low-pressure venous system. As the volume-buffering capacity of this compartment becomes exhausted, venous pressure increases, and cerebral blood volume and ICP rise. Also, cerebral blood flow is highly controlled by autoregulatory mechanisms, which affects its compensatory capacity. Conditions such as ischemia and elevated partial pressure of carbon dioxide (PCO2) in the blood produce a compensatory vasodilation of the cerebral blood vessels. A decrease in PCO2 has the opposite effect; for this reason, hyperventilation, which results in a decrease in PCO2 levels, is somes used in the treatment of ICP.
Cerebral Compliance and the Impact of Intracranial Pressure. The impact of increases in blood, brain tissue, or CSF volumes on ICP varies among individuals and depends on the amount of increase that occurs, the effectiveness of compensatory mechanisms, and the compliance of brain tissue. Compliance represents the ratio of change in volume to the resulting change in pressure (compliance = change in volume / change in pressure). The effects of intracranial volume changes (horizontal axis) on ICP changes (vertical axis are depicted in Figure 1.3. The shape of the curve demonstrates effects of intracranial volume changes on ICP. The ICP remains constrant from point a to point B when volume is added to the intracranial space. Because the compensatory mechanism are adequate, compliance is high in this area of the curve, and there is little change in ICP. From points B to C, the compensatory mechanisms becomes less efficient; compliance decreases and ICP begins to rise. At points C to D, the compensatory mechanisms have been exceeded such that even small changes in volume produce large changes in ICP. Impact of Intracranial Pressure on Cerebral Perfusion. The cerebral perfusion pressure (CPP), which represents the difference between the mean arterial blood pressure (MAB) and the ICP (CPP = MABP ICP), is the pressure perfusing the brain. CPP is determined by the pressure gradient between the internal carotid artery and subarachnoid veins. The MABP and ICP are monitored frequently in persons with brain conditions that increase ICP and impair brain perfusion. Normal CPP ranges from 70 to 100 mmHg. Brain ischemia develops at levels below 40 mmHg. When the pressure in the cranial cavity approaches or exceeds the MABP, tissue perfusion becomes inadequate, cellular hypoxia results, and if the pressure is maintained, neuronal death may occur. The highly specialized cortical neurons are the most sensitive to oxygen deficit; a decrease in the level of consciousness is one of the earliest and most reliable signs of increased ICP. The continued cellular hypoxia leads to general neurologic deterioration; the level of consciousness may deteriorate from alertness through confusion, lethargy, obtundation, stupor, and coma.
One of the late reflexes seen with a marked increase in ICP is the CNS ischemic response, which triggered by ischemia of the vasomotor center in the brain stem. Neurons in the vasomotor center respond directly to ischemia by producing a marked increase in MABP, sometimes to levels as high as 270 mmHg, accompanied by a widening of the pulse pressure and reflex slowing of the heart rate. These three signs, sometimes called the Cushing reflex, are important but late indicators of increased ICP. The ischemic reflex is a last-ditch effort by the nervous system to maintain cerebral circulation. The Cushing reflex seldom is seen in modern clinical settings since the advent of ICP monitoring.
Brain Herniation
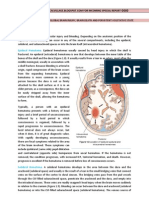
The brain is protected by the nonexpendable skull and supporting septa, the falx cerebri and the tentorium cerebelli, that divide the intracranial cavity into fossae or compartments that normally protect against excessive movement. The falx cerebri is a sickle shaped septum that separates the two hemispheres. The tentorium cerebelli divides the cranial cavity into anterior and posterior fossae (Fig. 1.4A). this inflexible dual sheath extends posteriorly from the bony petrous ridges and the anterior to the clinoid process, sloping downward and outward from its medial edge to attach laterally to the occipital bone. Extending posteriorly into the center of the tentorium is a large semicircular opening called the incisures or tentorial notch. The temporal lobe rests on the tentorial incisura, and the midbrain occupies the anterior portion of the tentorial notch. The cerebellum is closely opposed to the dorsum of the midbrain and fills the posterior part of the notch. Other important anatomic associations exists among the anterior cerebral, internal carotid, and posterior communication, and posterior and superior cerebellar arteries, and the incisures (see Fig. 1.4.B). The oculomotor nerve (cranial nerve III) emerges from the mediolateral surfaces of each peduncle just caudal to the tentorium.
Brain herniation represents a displacement of brain tissue under the falx cerebri or through the tentorial notch or incisures of the tentorium cerebelli. It occurs when an elevated ICP in one of brain comparments causes displacement of the cerebral tissue toward an area of lower ICP. The different types of herniation syndromes are based on the area of the brain that has herniated and the structure under which it has been pushed. (see Figure 1.4C). they commonly are divided into two broad categories, supratentorial and infratentorial, based on whether they are located above or below the tentorium.
Supratentorial Herniations. Three major patterns of supratentorial herniation were describe by Plum and Posner in their classic work: cingulate, central tranastentorial, and uncal tentorial. Table 1.2 describes the key structures and clinical signs of these three types of herniations. Of the three, cingulate herniation poses the less serious threat in terms of clinical outcomes. Transtentorial herniation result in two distinct syndomes: an uncal syndrome and a central syndrome. Clinically, they display distinct patterns early in their course, but both merge in a similar pattern once they begin to involve the midbrain level and below (brain stem structures). Cingulate herniation involves displacement of the cingulate gyrus and hemisphere beneath the sharp edges of the falx cerebri to the opposite side of the brai. Displacement of the falx can compress the local brain tissue and blood supply from the anterior cerebral artery, causing ischemia and edema, which further increase ICP levels. Unilateral or bilateral leg weakness is an early sign of impending cingulate herniation. Central transtentorial herniation involves the downward displacement of the cerebral hemispheres, basal ganglia, diencephalon, and midbrain through the tentorial incisura. The diencephalon, may be compressed tightly against the midbrain with such force that edema and haemorrhage result. It may or may not be associated with uncal or lateral herniation. In the early diencephalic stage, there is clouding of consciousness, bilaterally small pupuls (approximately 2 mm in diameter) with a full range of constriction, and motor responses to pain that are purposeful or semipurposeful (localizing) and often a first sign of central herniations, is caused by pressure on the reticular activating system (RAS) in the upper midbrain, which is responsible for wakefulness. As the herniation progresses to the late diencephalic stage, painful stimulation results in decorticate posturing, which may be asymmetric (Figure 2.5), and there is waxing and waning of respirations with periods of apnea (Cheyne-Stokes respirations). With midbrain involvement, the pupils are fixed and midsize (approximately 5 mm in diameter), and reflexes adduction of the eyes are impaired; pain elicits cerebrate posturing; and respirations change from Cheyne-Stokes to neurogenic hyperventilation, in which the frequency may exceed 40 breaths per minute because of uninhibited stimulation of the inspiratory and expiratory centers. Progression to involve the lower pons and upper medulla produces fixed, midpoint (3 to 5 mm) pupils with loss of reflex abduction and adduction of the eyes, and absence of motor responses or only leg flexion or painful stimuli. Once the area of herniation has progressed beyond the diencephalon and into the midbrain and brain stem, the process is generally irreversible and the prognosis is poor. TABLE 2.2 Herniation Syndrome Cingulate Central transtentorial Key Structures and Clinical Signs of Cingulate, Central andUncal Herniation Key Structures Involved Key Clinical Signs Anterior cerebral artery Leg weakness Reticular activating system Altered level of consciousness Corticospinal tract Decorticate posturing Rostral-caudal deterioration Cerebral peduncle Hemiparesis Oculomotor nerve Ipsilateral pupil dilatation Posterior cerebral artery Visual field loss Cerebellar tonsil Respiratory center Respiratory arrest
Uncal
Uncal herniation occurs when a lateral mass pushes the brain tissue centrally and forces the medial aspect of the temporal lobe, which contains the uncus and hippocampal gyrus, under the edge of the tentorial incisura, into the posterior fossa. As a result, the diencephalon and midbrain are compressed and displaced laterally to the opposite side of the tentorium. Cranial nerve III (oculomotor nerve) and the posterior cerebral artery frequently are caught between the uncus and the tentorium. The oculomotor nerve controls pupillary constriction; entrapment of this nerve results in ipsilateral pupillary dilatation, which usually is an early sign of uncal herniation. Consciousness may be impaired because the RAS has not yet been affected. However, after any signs of herniation or brain stem compression appear, deterioration may proceed rapidly making it important to recorgnize the distinguishing early features of uncal herniations. As uncal herniation progress, there are changes in motor strength and coordination of voluntary movements because of compression of the descending motor pathways. It is not unusual for initial changes in motor function to occur on the side of the damage because of compression of contralateral cerebral peduncles. This may result in a false localizing sign of hemiparesis on the same side as cranial nerve III, rather than on the opposite side, where the motor nerves have crossed over, as would be expected. As the condition progresses, bilateral positive Babinski responses and respiratory changes (e.g., Cheyne-Stokes respirations, ataxic pattern) occur. Decorticate and decerebrate posturing develop, followed by dilated, fixed pupils, flaccidity, and respiratory arrest. Infratentorial Herniation. Infratentorial herniation results from increased pressure in the infratentorial compartment. It often progress rapidly and can cause death because it is likely to involve the lower brain stem centers that control vital cardiopulmonary functions. Herniation may occur superiorly (upward) through the tentorial incisura or inferiorly (downward) through the foramen magnum. Upward displacement of brain tissue can cause blockage of the aqueduct of Sylvius and lead to hydrocephalus and coma. Downward displacement of the midbrain through the tentorial notch or the cerebellar tonsils through the foramen magnum can interfere with medullary functioning and can cause cardiac or respiratory arrest. In cases of pre-existing ICP elevations, herniation may occur when the pressure is released from below, such as in lumbar puncture. If the CSF pathway is blocked, and fluid cannot leave the ventricles, the volume expands, and fluid is displaced downward through the tentorial notch. The expanding volume causes all function at the given level to cease as destruction progresses in a rostral-caudal direction. The result of this displacement is brain stem ischemia and haemorrhage extending from the diencephalon to the pons. If the lesions expands rapidly, displacement and obstruction occur quickly, leading to irreversible infarction and haemorrhage.
Das könnte Ihnen auch gefallen
- Alphabetical Reasons To Celebrate My Alphabet YearDokument1 SeiteAlphabetical Reasons To Celebrate My Alphabet YearseigelysticNoch keine Bewertungen
- Prehospital Trauma CareDokument5 SeitenPrehospital Trauma CareseigelysticNoch keine Bewertungen
- Surgical InfectionDokument5 SeitenSurgical InfectionseigelysticNoch keine Bewertungen
- Prehospital Trauma Care 3Dokument8 SeitenPrehospital Trauma Care 3seigelysticNoch keine Bewertungen
- Medical Surgical Nursing Review SeriesDokument23 SeitenMedical Surgical Nursing Review SeriesseigelysticNoch keine Bewertungen
- Obstetric Gynecology Review SeriesDokument12 SeitenObstetric Gynecology Review Seriesseigelystic100% (1)
- Prehospital Trauma Care 2Dokument5 SeitenPrehospital Trauma Care 2seigelysticNoch keine Bewertungen
- Diabetes Drug Could Treat AlzheimerDokument2 SeitenDiabetes Drug Could Treat AlzheimerseigelysticNoch keine Bewertungen
- Brain Injury 3Dokument5 SeitenBrain Injury 3seigelysticNoch keine Bewertungen
- Brain Injury 3Dokument5 SeitenBrain Injury 3seigelysticNoch keine Bewertungen
- Weekly 5 Qa4Dokument2 SeitenWeekly 5 Qa4seigelysticNoch keine Bewertungen
- Brain Injury 4Dokument7 SeitenBrain Injury 4seigelysticNoch keine Bewertungen
- Brain InjuryDokument6 SeitenBrain InjuryseigelysticNoch keine Bewertungen
- POEADokument1 SeitePOEAseigelysticNoch keine Bewertungen
- Reference Values For Laboratory TestsDokument3 SeitenReference Values For Laboratory TestsseigelysticNoch keine Bewertungen
- Weekly 5 QA3Dokument2 SeitenWeekly 5 QA3seigelysticNoch keine Bewertungen
- Birth Control Pill For Men Is On The WayDokument1 SeiteBirth Control Pill For Men Is On The WayseigelysticNoch keine Bewertungen
- Clinical Case Study Wk2Dokument1 SeiteClinical Case Study Wk2seigelysticNoch keine Bewertungen
- Next Stop: Down Under, Australia - SCC LabourDokument2 SeitenNext Stop: Down Under, Australia - SCC LabourseigelysticNoch keine Bewertungen
- Ten ThingsDokument3 SeitenTen ThingsseigelysticNoch keine Bewertungen
- MV DVTDokument6 SeitenMV DVTseigelysticNoch keine Bewertungen
- Clinical Case Study Wk1Dokument1 SeiteClinical Case Study Wk1seigelysticNoch keine Bewertungen
- The Hippocratic OathDokument1 SeiteThe Hippocratic OathseigelysticNoch keine Bewertungen
- One in 100 UK ChildrenDokument1 SeiteOne in 100 UK ChildrenseigelysticNoch keine Bewertungen
- Good Day RecruitmentDokument1 SeiteGood Day RecruitmentseigelysticNoch keine Bewertungen
- Patent Ductus ArteriosusDokument4 SeitenPatent Ductus ArteriosusseigelysticNoch keine Bewertungen
- Archangel Global Solutions IncorporatedDokument1 SeiteArchangel Global Solutions IncorporatedseigelysticNoch keine Bewertungen
- Mega Manpower CorporationDokument2 SeitenMega Manpower CorporationseigelysticNoch keine Bewertungen
- MV Acute GlumerolunephritisDokument4 SeitenMV Acute GlumerolunephritisseigelysticNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Vietnamese Alphabet and PronounDokument10 SeitenVietnamese Alphabet and Pronounhati92Noch keine Bewertungen
- Language EducationDokument33 SeitenLanguage EducationLaarni Airalyn CabreraNoch keine Bewertungen
- Gender and Patriarchy: Crisis, Negotiation and Development of Identity in Mahesh Dattani'S Selected PlaysDokument6 SeitenGender and Patriarchy: Crisis, Negotiation and Development of Identity in Mahesh Dattani'S Selected Playsতন্ময়Noch keine Bewertungen
- Rules of Bursa Malaysia Securities Clearing 2019Dokument11 SeitenRules of Bursa Malaysia Securities Clearing 2019Evelyn SeethaNoch keine Bewertungen
- 2015 Grade 4 English HL Test MemoDokument5 Seiten2015 Grade 4 English HL Test MemorosinaNoch keine Bewertungen
- Submitted By: S.M. Tajuddin Group:245Dokument18 SeitenSubmitted By: S.M. Tajuddin Group:245KhurshidbuyamayumNoch keine Bewertungen
- Madam Shazia PaperDokument14 SeitenMadam Shazia PaperpervaizhejNoch keine Bewertungen
- Learning Spanish - 1dDokument23 SeitenLearning Spanish - 1dChima C. Ugwuegbu100% (1)
- Service Manual: NISSAN Automobile Genuine AM/FM Radio 6-Disc CD Changer/ Cassette DeckDokument26 SeitenService Manual: NISSAN Automobile Genuine AM/FM Radio 6-Disc CD Changer/ Cassette DeckEduardo Reis100% (1)
- Industry GeneralDokument24 SeitenIndustry GeneralilieoniciucNoch keine Bewertungen
- Dr. Babasaheb Ambedkar Technological UniversityDokument3 SeitenDr. Babasaheb Ambedkar Technological UniversityalfajNoch keine Bewertungen
- Venere Jeanne Kaufman: July 6 1947 November 5 2011Dokument7 SeitenVenere Jeanne Kaufman: July 6 1947 November 5 2011eastendedgeNoch keine Bewertungen
- Manual de Utilizare HUMAX DIGI TV RDSDokument116 SeitenManual de Utilizare HUMAX DIGI TV RDSenamicul50Noch keine Bewertungen
- Color Coding Chart - AHGDokument3 SeitenColor Coding Chart - AHGahmedNoch keine Bewertungen
- Thesis Statement On Corporate Social ResponsibilityDokument5 SeitenThesis Statement On Corporate Social Responsibilitypjrozhiig100% (2)
- Technical Data - Tad1342veDokument9 SeitenTechnical Data - Tad1342veRachid SmailiNoch keine Bewertungen
- Pinterest or Thinterest Social Comparison and Body Image On Social MediaDokument9 SeitenPinterest or Thinterest Social Comparison and Body Image On Social MediaAgung IkhssaniNoch keine Bewertungen
- PUBLIC - Axie Origins Changelogs - Season 4Dokument2 SeitenPUBLIC - Axie Origins Changelogs - Season 4Alef CarlosNoch keine Bewertungen
- Technology in Society: SciencedirectDokument10 SeitenTechnology in Society: SciencedirectVARGAS MEDINA ALEJANDRANoch keine Bewertungen
- 20 Ijrerd-C153Dokument9 Seiten20 Ijrerd-C153Akmaruddin Bin JofriNoch keine Bewertungen
- Cultural AnthropologyDokument12 SeitenCultural AnthropologyTRISH BOCANoch keine Bewertungen
- Tushnet - An Essay On RightsDokument43 SeitenTushnet - An Essay On RightslarisamannNoch keine Bewertungen
- Week 1-2 Module 1 Chapter 1 Action RseearchDokument18 SeitenWeek 1-2 Module 1 Chapter 1 Action RseearchJustine Kyle BasilanNoch keine Bewertungen
- Lab Activity 5Dokument5 SeitenLab Activity 5Jasmin CeciliaNoch keine Bewertungen
- Reviewer CSCDokument22 SeitenReviewer CSCChristopher CocalNoch keine Bewertungen
- Security Policy 6 E CommerceDokument6 SeitenSecurity Policy 6 E CommerceShikha MehtaNoch keine Bewertungen
- Boeing SWOT AnalysisDokument3 SeitenBoeing SWOT AnalysisAlexandra ApostolNoch keine Bewertungen
- First Aid Transportation of The InjuredDokument30 SeitenFirst Aid Transportation of The InjuredMuhammad Naveed Akhtar100% (1)
- Using MonteCarlo Simulation To Mitigate The Risk of Project Cost OverrunsDokument8 SeitenUsing MonteCarlo Simulation To Mitigate The Risk of Project Cost OverrunsJancarlo Mendoza MartínezNoch keine Bewertungen
- High Intermediate 2 Workbook AnswerDokument23 SeitenHigh Intermediate 2 Workbook AnswernikwNoch keine Bewertungen