Beruflich Dokumente
Kultur Dokumente
s1 ln13106879 1243193637 1939656818Hwf 807831619IdV30516443213106879PDF - HI0001
Hochgeladen von
David BédardOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
s1 ln13106879 1243193637 1939656818Hwf 807831619IdV30516443213106879PDF - HI0001
Hochgeladen von
David BédardCopyright:
Verfügbare Formate
Draft Manuscript Submitted to MHR for Peer Review
Draft Manuscript For Review. Reviewers should submit their review at http://mc.manuscriptcentral.com/molehr
Human chorionic gonadotropin regulates endothelial cell responsiveness to interleukin 1 and amplifies the cytokinemediated effect on cell proliferation, migration and the release of angiogenic factors
Manuscript ID: Manuscript Type: Date Submitted by the Author:
Complete List of Authors:
Key Words:
Note: The following files were submitted by the author for peer review, but cannot be converted to PDF. You must view these files (e.g. movies) online.
Bourdiec et al-B&W Figs.zip
r Fo
Journal:
Molecular Human Reproduction MHR-12-0159 Original Research 19-Sep-2012
Bourdiec, Amlie; Laval University, Ob/gyn Bdard, David; Laval University, Rao, CV; Florida International University, Akoum, Ali; Laval University, Ob/gyn angiogenesis, embryo implantation , endothelial cells , hCG, IL1
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Page 1 of 31
Draft Manuscript Submitted to MHR for Peer Review
Human chorionic gonadotropin regulates endothelial cell responsiveness to interleukin 1 and amplifies the cytokine-mediated effect on cell proliferation, migration and the release of angiogenic factors
Amlie Bourdiec 1, David Bdard1, CV Rao2, Ali Akoum1*
Endocrinologie de la reproduction, Centre de recherche-HSFA, CHUQ, Facult de mdecine,
Universit Laval, Qubec, Canada
r Fo
Re
Herbert Wertheim College of Medicine, Florida International University, Miami, FL, USA
*Corresponding author: Dr Ali Akoum, Centre de recherche, Hpital Saint-Franois dAssise, 10, rue de lEspinay, Qubec, Qubec, Canada, G1L 3L5; Fax. 418-525-4195; E-mail. ali.akoum@crsfa.ulaval.ca
http://molehr.oxfordjournals.org/
vi
ew
On ly
Draft Manuscript Submitted to MHR for Peer Review
Page 2 of 31
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
ABSTRACT
Successful embryonic implantation requires the establishment of an appropriate communication network between the embryo and its near environment within the implantation site. Herein we showed that human chorionic gonadotropin (hCG), the major embryonic signal, targets endothelial cells to amplify their responsiveness to interleukin 1 (IL1), one of the earliest signals released by embryonic cells. Human microvascular endothelial cell (HMVEC) proliferation and migration, as assessed by BrdU incorporation into cell DNA and wound healing, increased in response to IL1 beta (B) and further in the presence of hCG. IL1B increased the expression of the signaling IL1 receptor 1 (IL1R1) and the decoy inhibitory IL1R2, as assessed by real-time PCR, Western blotting and ELISA, while adding hCG further increased IL1R1 and led to a significant downregulation of IL1R2. This translated into a significant upregulation of IL8 mRNA steady-state levels and protein secretion, which was significantly inhibited in cells where IL1R2 was overexpressed by transient cell transfection. These findings revealed a new mechanism by which hCG may directly target endothelial cells to stimulate angiogenesis and favor embryonic growth.
Key words: angiogenesis / embryo implantation / endothelial cells / hCG / IL1
r Fo
Re
vi
ew
On
ly
19
http://molehr.oxfordjournals.org/
Page 3 of 31
Draft Manuscript Submitted to MHR for Peer Review
20 21
INTRODUCTION
22 23 24 25 26 27
Human chorionic gonadotropin (hCG), a glycoprotein hormone mainly produced by the syncytiotrophoblasts in the chorionic villi, plays a critical role in the initiation and maintenance of pregnancy. One of the major and earliest embryonic signals, hCG is detectable in maternal serum as early as one day after the initiation of embryo implantation. Its levels rapidly reach a peak value after about sixty to ninety days of gestation and gradually drop within the second trimester (Jaffe et al. , 1969). Stimulation of progesterone production by ovarian corpus luteal cells and maintenance of embryo implantation were first considered as the main biological activities of hCG (Jaffe, 1983, Strott et al. , 1969). Cumulative evidence showed, however, that hCG is a key growth and differentiation hormone involved in multiple steps of placentation and fetal development (Rao, 2001, Rao and Lei, 2007, Shi et al. , 1993). Functional luteinizing
r Fo
28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43
hormone/choriogonadotropin receptor (LHCGR) was found in nongonadal tissues such as the human endometrium (Bernardini et al. , 1995, Reshef et al. , 1990). hCG was found to directly modulate endometrial functions, thereby promoting endometrial receptivity as well as the implantation and growth of human embryo (Rao, 2002). hCG appeared to promote human endometrial stromal cell decidualization (Tang and Gurpide, 1993) through morphological and functional differentiation resulting in an up-regulation of cyclooxygenase-2 (COX-2) gene expression and increased production of prostaglandin (PG) E2 (Han et al. , 1999). Additional evidence showed hCG involvement in key biological processes underlying embryonic implantation and growth including immunological modulation and angiogenesis (Evans et al. , 2009, Rao and Lei, 2007). Kane and al (Kane et al. , 2009), showed that hCG regulates tissue specific uterine natural killer (uNK) cell proliferation. Decidual NK cells
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Draft Manuscript Submitted to MHR for Peer Review
Page 4 of 31
44 45
produce an array of angiogenic factors, which is essential for the induction of vascular growth and the establishment of an adequate decidua (Mor, 2008). Implantation of human embryo involves a complex process of tissue remodeling, which requires the development of an efficient new blood vessel network and the activation of the host angiogenic responses. Several studies showed that hCG promotes angiogenesis as well as vasculogenesis in the uterine vasculature during pregnancy to ensure maximal blood supply and optimal nutrition to the fetus (Berndt et al. , 2009, Lincoln et al. , 1992, Toth et al. , 1994, Toth et al. , 2001, Zygmunt et al. , 2002). In vivo, in the chicken chorioallantoic membrane assay, hCG induces a neovascularization comparable to the activity of vascular endothelial growth factor (VEGF) (Zygmunt et al., 2002) and promotes the production of angiogenic factors such as VEGF by the placenta (Islami et al. , 2003) and decidual macrophages (Zygmunt et al. , 2003). We have previously demonstrated that hCG directly upregulates the synthesis and secretion of macrophage inhibitory factor (MIF), a multifunctional cytokine with potent immunomodulatory and angiogenic properties (Akoum et al. , 2005). Recently, hCG was shown to possess intrinsic agiogenic effects (Zygmunt et al., 2002). In vitro, hCG significantly increases capillary formation and migration of endothelial cells, thereby suggesting both direct and indirect angiogenic properties.
46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
61 62 63 64 65 66 67 68
Deep morphological, structural and functional changes occurring within the uterus are primarily orchestrated by embryonic factors/signals which coordinate and facilitate embryonic growth and development (Aghajanova et al. , 2008, Licht et al. , 2001). Interleukin 1 (IL1) is one of the earliest embryonic signals. Beside its major immunological effects, IL1 regulates cell growth and angiogenesis (Sheth et al. , 1991) (Apte et al. , 2006). IL1 was shown to increase tumor invasiveness and metastasis by enhancing the expression of adhesion molecules on endothelial and malignant cells (Chirivi et al. , 1996, Vidal-Vanaclocha et al. , 1996). IL1 also stimulates the proliferation of endothelial cells and production of cytokines
r Fo
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Page 5 of 31
Draft Manuscript Submitted to MHR for Peer Review
69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89
(Apte and Voronov, 2008, Dinarello, 1996, Meager, 1999). The addition of exogenous IL1B in IL1B-KO mice partially restored the angiogenic response, whereas the administration of IL1 receptor antagonist (IL1RN) to wild type mice inhibited the growth of blood vessel networks (Apte and Voronov, 2002, Bar et al. , 2004). IL1 has two known receptors. The receptor type 1 (IL1R1) acts as functional signaling receptor that mediates cell activation by IL1 via the recruitment of IL1 accessory protein (IL1RAP) (Greenfeder et al. , 1995). The receptor type 2 (IL1R2) acts as a negative regulator (or antagonist) of IL1 action and has been termed decoy receptor (Colotta et al. , 1993). Our first studies revealed that hCG acts directly on endometrial epithelial cells to downregulate the expression of the decoy antagonist IL1R2, without affecting IL1R1 expression (Herrmann-Lavoie et al. , 2007). These data point to a possible mechanism by which hCG could modulate IL1 receptivity thereby favoring IL1-mediated actions. The main objective of this study was to investigate whether the hCG-mediated modulation of cell receptivity to IL1 can operate in endothelial cells. Our data showed that hCG significantly amplifies the IL1induced stimulation of endothelial cell proliferation and migration, IL8 synthesis and secretion and expression of IL1R1, but it concurrently exerts a inhibitory effect on the IL1induced IL1R2. Such a dual complementary action may amplify endothelial cell responsiveness to IL1 and potentiate the release of angiogenic factors. This points to a possible new mechanism by which hCG stimulates angiogenesis and promotes consequently the development of new blood vessels required for the highly demanding embryonic growth.
90
MATERIAL AND METHODS Cell culture and treatment
r Fo
Re
vi
ew
On
ly
91
http://molehr.oxfordjournals.org/
Draft Manuscript Submitted to MHR for Peer Review
Page 6 of 31
92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107
In this study, we used human microvascular endothelial cell line (HMVEC), which has been established by engineering the human telomerase catalytic protein (hTERT) (Shao and Guo, 2004a). Cells were shown to maintain inherent features of primary endothelial cells and to produce angiogenic response (Shao and Guo, 2004b). HMVEC were cultured in endothelial cell basal medium MCDB 131 (Life Technologies Inc. Burlington, ON, Canada) supplemented with 10% fetal bovine serum (FBS), 10 g/mL epithelial cell growth factor (EGF), 1 g/mL hydrocortisone, 10mM glutamine, 100 U/mL penicillin, 100 g/mL
streptomycin and 0.25 g/mL amphotericin B (complete MCDB 131 medium). Cells were seeded in 6-well plates. At pre-confluence, they were washed twice with MCDB 131 medium and cultured overnight with the same medium containing 10% charcoal-treated FBS. Cells were then washed twice with MCDB 131 and cultured for 24 and 48 h with fresh FBS-free MCDB 131 containing different concentrations of hCG (0-1000 ng/mL, recombinant expressed in mouse cell line, 10,000 IU/mg; Sigma Chemical Co., St. Louis, MO) and IL1B (0-1 ng/mL) (R & D systems Inc., Minneapolis, MN, USA). Culture supernatants were centrifuged to remove particulate debris, cells were trypsinized and they both were stored at 80C until further use.
r Fo
Re
vi
ew
On
108
ly
109
Immunocytofluorescence
110 111 112 113 114 115
Cells were grown on coverslips in 24-well plates in complete MCDB 131 medium. At preconfluence, they were incubated overnight in the same medium containing 10% charcoaltreated FBS, then rinsed in serum-free MCDB 131 medium and fixed in 10% Formalin for 20 min. For IL1R1, IL1R2 and IL1RAP, cultures were washed in PBS/0.1% Tween 20 and incubated for 90 min with a monoclonal mouse anti-human IL1R1 (R&D Systems) [10 g/mL in PBS containing 0.2% BSA and 0.01% Tween 20 (PBS/BSA/Tween 20)], a
http://molehr.oxfordjournals.org/
Page 7 of 31
Draft Manuscript Submitted to MHR for Peer Review
116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136
monoclonal mouse anti-human IL1R2 (R&D Systems) (10 g/mL in PBS/BSA/Tween 20) or a polyclonal goat anti-human IL1RAP (R&D Systems) (10 g/mL in PBS/BSA/Tween 20) for 1 h at room temperature in humid chamber. After washing with PBS containing 0.1% Tween 20 and subsequently with PBS alone, coverslips were incubated for 1 h at room temperature with a biotin-conjugated horse anti-mouse or rabbit anti goat antibody (Vector Laboratories, Burlingame, CA, USA) (1:100 dilution in PBS/BSA/Tween 20). Subsequently culture slides were washed with PBS and incubated with 1% streptavidin-fluorescein isothiocyanate in PBS/BSA/Tween 20 for 1 h at room temperature in humid chamber. For LHCGR, cells were incubated with a polyclonal anti-hLHCGR rabbit antibody (1:300 dilution in PBS/0.1% Tween 20) (primary antibody), rinsed three times with PBS/0.1% Tween 20, and incubated for 90 min at room temperature with a biotin-conjugated goat anti-rabbit IgG (heavy and light chains) (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) (1:1000 dilution in PBS/0.2% BSA/0.1% Tween 20), then for 45 min at room temperature with Alexa 488-labeled streptavidin (1:100 dilution in PBS/0.2% BSA) (Molecular Probes Inc., Eugene, OR). Cells were counterstained with 4,6-diaminido-2-phenyl-indole (1:2000 dilution in PBS/0.1% Tween 20) and covered with mounting medium (Mowiol containing 10% para-phenylenediamine, an antifading agent; Sigma). Cells incubated without the primary antibody were included as negative controls. Slides were observed under a microscope equipped for fluorescence (Olympus BX51; Olympus Corp., Tokyo, Japan) and photographed using Image-Pro Express 5.1 software (Media Cybernetics Inc., Bethesda, MD).
r Fo
Re
vi
ew
On
ly
137
138
Scratch wound assay
http://molehr.oxfordjournals.org/
Draft Manuscript Submitted to MHR for Peer Review
Page 8 of 31
139 140 141 142 143 144 145 146 147 148 149
Cells were seeded into 24-well plates and grown in complete MCDB 131 medium. At preconfluence, they were incubated overnight in the same medium containing 10% charcoaltreated FBS. Cell monolayers were then carefully wounded with a 200 L pipette tip to
generate a cut of ~1 mm in width. After two washing steps with Hank's, cells were incubated for 24h with hCG and IL1 or hCG plus IL1 in MCDB 131 medium. Incubation for 24 h was selected for optimal assessment of cell migration in response to the indicated stimuli, as longer incubation periods led to spontaneous closure of the wound. Cells were then fixed with 10% formalin for 20 min, stained with DAPI and wound healing was determined by measuring scratch width at seven random locations along the wound to examine the extent of closure using fluorescence microscopy. Measures were performed in duplicate and repeated
r Fo
Re
three times.
150
151
DNA synthesis assay bay bromodeoxyuridine (BrdU) incorporation HMVEC were seeded at 1.104cell/well into 96-well microtitre plates and cultured in complete MCDB 131 medium. At preconfluence, they were incubated overnight in the same medium containing 10% charcoal-treated FBS, starved for 48h with 0.2% charcoal-treated FBS in MCDB 131 and incubated for 48 h with hCG and IL1 or hCG plus IL1. Cells were then labeled with 10 M BrdU for 24 h at 37C and cell proliferation was assessed by ELISA according to the manufacturers instructions (GE Healthcare, Mississauga, ON, Canada). Briefly, cells were then fixed and genomic DNA was denatured by adding 200 L/well of blocking reagent (1:10) for 30 min at room temperature. Peroxidase-labeled anti BrdU antibody (1:100) was added (100 L/well) and incubated for 90 min at room temperature. After washing three times, TMB 3,3,5,5-tetramethylbenzidine (TMB) substrate solution was
vi
ew
152 153 154 155 156 157 158 159 160 161
http://molehr.oxfordjournals.org/
On
ly
Page 9 of 31
Draft Manuscript Submitted to MHR for Peer Review
162 163
added (100 L/well) and incubated for 15 min at room temperature. The enzymatic reaction was stopped by the addition of H2SO4 (2M) and the optical density was measured at 450 nm.
164
165
Real Time PCR Total RNA was extracted with Trizol according to the manufacturer's instructions (Life Technologies Inc.). cDNA was synthesized using 100 ng of RNA and 2.5 M random
166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185
hexamers in 20 L of a solution containing 50 mM KCl, 10 mM Tris-HCl, 5 mM MgCl2, 1 mM of each deoxyribonucleotide triphosphate (dNTP), 20 U of RNase inhibitor and 50 U of reverse mRNA expression using Gene Amp PCR Core Kit (Perkin-Elmer, Foster City, CA, USA). The reaction was incubated at 25C for 15 min, 42C for 30 min and 99C for 5 min. Quantitative real212 time PCR was carried out using an ABI 7000 thermal cycler (Applied Biosystems, Foster city, CA, USA). Each standard PCR reaction contained 2.5
product, 0.5 L of each primer (final concentration, 5 pM/L), 12.5 L SYBR Green PCR Master Mix consisting of Taq DNA polymerase reaction buffer, dNTP mix, SYBR Green I, MgCl2 and Taq DNA polymerase. Following a 10 min denaturation at 95C, the reactions were cycled 50 times with 15 s denaturation at 95C and 60 s annealing at 60C. IL1R1 primers (forward, 5'-AGAGGAAAACAAACCCACAAGG-3'; reverse, 5'-
CTGGCCGGTGACATTACAGAT-3'; amplimer size 106 bp), IL1R2 primers (forward, 5'TGGCACCTACGTCTGCACTACT-3'; reverse, 5'-TTGCGGGTATGAGATGAACG- 3'; amplimer size 112 bp), sIL1RAP primers (forward,
5foGAAAGGTAATAGATGCGGTCAGTG-3T; reverse, 5 reverse, GATGCGGTCAGTG-3; amplimer size 122 bp), mbIL1RAP primers (forward, 5forward, rimers GTCAGTG-; reverse, 5 reverse, imers GTCAGTG-3TGAAC; amplimer size 122 bp), IL1RN primers (forward, 5AACAGAAAGCAGGACAAGCG-3; reverse, 5-CCTTCGTCAGGCATATTGGT-3;
r Fo
Re
http://molehr.oxfordjournals.org/
vi
L of RT
ew
On
ly
Draft Manuscript Submitted to MHR for Peer Review
Page 10 of 31
186 187 188 189 190 191 192 193 194 195 196
amplimer size 149 bp), IL8 primers (forward, 5-ATACTCCAAACCTTTCC-3; reverse, 5CTTCTCCACAACCCTC-3; amplimer size CAGGGCTGCTTTTAACTCTGG-3'; reverse, 157 bp) and GAPDH (forward, 5'-
5'-TGGGTGGAATCATATTGGAACA;
amplimer size 102 bp), were designed with Primer expressTM, version 2.0 (Applied Biosystems), spanned intron-exon boundaries to avoid amplification of genomic DNA and selected so as to have compatible Tm values (57-61C). Quantification of IL1R1, IL1R2, mbIL1RAP, sIL1RAP, IL1RN and IL8 mRNA was performed using a relative quantification method. For each experimental sample, IL1R1, IL1R2, mbIL1RAP, sIL1RAP, IL1RN and IL8 mRNA levels were normalized to GAPDH mRNA levels. After each run, melting curve analysis (55-95C) was performed to verify the specificity of the PCR. All samples were tested in duplicate, and each run included no-template and no-reverse transcription controls.
r Fo
Re
197
vi
198
Western blot analysis
199 200 201 202 203 204 205 206 207 208 209
Proteins were isolated with Trizol according to the manufacturer's instructions (Life Technologies Inc.). Total protein concentration was determined using Bio-Rad DC Protein Assay (Bio-Rad Laboratories Ltd., Mississauga, Ontario, Canada). Protein extracts (10 g) and culture supernatants (40 L) were then separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred onto 0.45 m nitrocellulose membranes and analyzed by Western blotting as described previously (Akoum et al. , 2007). Briefly, IL1R1, IL1R2 and IL1RAP were detected using specific goat polyclonal antibodies [2 g/mL in PBS containing 5% skim milk and 0.1% Tween 20) (blocking solution)] (R&D systems), IL1RN was detected using a specific rabbit polyclonal antibody (2 g/mL in blocking solution) (Genzyme) and hCG/LH receptor was detected using a rabbit polyclonal antibody raised against the synthetic 15-38 N-terminus amino acid sequence (1:1500 dilution in blocking solution) (Herrmann-
http://molehr.oxfordjournals.org/
ew
On
ly
Page 11 of 31
Draft Manuscript Submitted to MHR for Peer Review
210 211 212 213 214 215 216 217 218 219
Lavoie et al., 2007). Fc-specific peroxidase-labeled rabbit anti-goat antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) (1:10 000 in blocking solution), were then used for IL1R1, IL1R2 and IL1RAP, whereas an Fc-specific peroxidase-labeled goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) (1:150 000 in blocking solution), was used for IL1RN and LHCGR. ECL reagent (GE healthcare, Chalfont St Giles, UK) and exposure to Super RX films (Fuji, Tokyo, Japan) for 530 min for optimal detection (all bands visible but not overexposed). Membranes were stripped and reblotted with a monoclonal antibody specific to -actin (Sigma Chemical Co., St. Louis, MO, USA) (1:50 000 dilution in PBS-0.01% Tween-20) used as internal control for protein loading and transfer.
r Fo
Re
220
221
Enzyme-linked immunosorbent assay (ELISA)
222 223 224 225 226 227 228 229 230 231 232 233
IL8 concentrations in HMVEC supernatants were measured using an ELISA procedure developed in the laboratory. This assay uses a mouse monoclonal anti-human IL8 antibody and a rabbit polyclonal anti-human IL8 antibody (Biosource International). Briefly, 96-well plates were coated overnight at 4C with the mouse monoclonal antibody. Plates were washed four times with PBS containing 0.1% Tween 20 (washing buffer), and recombinant human IL8 (R and D Systems), used at concentrations ranging from 100 pg/mL to 6.4 ng/mL, or samples diluted in serum-free RPMI were then added to the plates. After 60 min incubation at 37C, the plates were washed and incubated for 60 min at 37C with the IL8 polyclonal antibody (1/8000 dilution in PBS containing 0.5% BSA). The plates were then washed and incubated for 60 min at 37C with a goat anti-rabbit IgG peroxidase conjugate (Zymed Laboratories Inc., San Francisco, CA, USA) (1/2000 dilution in PBS/0.5% BSA). After a final wash, 100 L of TMB-peroxidase substrate (Bio-Rad Laboratories Ltd, Mississauga,
http://molehr.oxfordjournals.org/
vi ew On ly
Draft Manuscript Submitted to MHR for Peer Review
Page 12 of 31
234 235 236 237 238
Ontario, Canada) was added to each well, the enzymatic reaction was terminated by the addition of 50 L of 2 N H2SO4 and the optical density was determined at 450 nm. sIL1R2 and IL1RN concentrations in the culture medium were measured using a previously reported sandwich ELISA (Bellehumeur et al. , 2009). IL8, sIL1R2 and IL1RN concentrations were calculated by interpolation from standard curves.
239
240
Cell transfection
241 242 243 244 245 246 247 248 249 250 251 252
HMVEC were seeded into 24-well plates and grown in complete MCDB 131 medium before being incubated overnight in the same medium containing 10% charcoal-treated FBS and transfected at 70% confluence. Cells were transfected with the eukaryotic expression vector pcDNA3 either alone, or containing a cDNA coding for IL1R2 for overexpression of IL1R2 (Bossu et al., 1995). Transfection was performed using Lipofectamine Plus reagent according to manufacturer's instructions (Life Technologies Inc.) and our previously described procedure (Akoum et al., 2007). Before cell stimulation, the culture medium was replaced by a serum-free medium for 24 h. Cells were then exposed or not for 24 h to hCG, IL1 or hCG plus IL1. The culture supernatants were collected and kept in small aliquots at -80C until use by ELISA. Cells were recovered by trypsinization and RNA was extracted, as described
earlier, and kept at -80C until assay.
253
Statistical analysis All analyses were performed with GraphPad Software Prism 4.0 (GraphPad Software, Inc., San Diego, CA, USA). Data followed a parametric distribution and were expressed as means SEM. The significance of statistical differences was determined using one way analysis of
r Fo
Re
vi
ew
On
ly
254 255 256
http://molehr.oxfordjournals.org/
Page 13 of 31
Draft Manuscript Submitted to MHR for Peer Review
257 258
variance (ANOVA) followed by the Boferroni test post hoc, for multiple analyses, and the Student's t-test for the comparison of two groups.
259 260 261 262 263 264 265
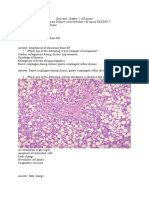
RESULTS Immunocytofluorescence analysis first showed that HMVECs express hCG and IL1 receptors. A positive immunostaining for LHCGR, IL1R1, IL1R2 and IL1RAP was observed (Fig. 1A, C, E and G). No immunostaining in control cells incubated with rabbit (LHCGR), mouse (IL1R1 and IL1R2) or goat (IL1RAP) IgGs was noted, instead of the respective primary antibodies (Fig. 1B, D, F and H).
266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281
Further studies showed that hCG and IL1B can induce HMVEC proliferation and migration. HMVEC cultures were exposed to different concentrations of hCG (0-1000 ng/mL), IL1B (0-1 ng/mL) or a combination of hCG and IL1. As shown in Fig. 2A and B, a dose-dependent increase in BrdU incorporation into DNA was observed in HMVECs in response to these stimuli, which was significant at 100 and 1000 ng/mL hCG (P < 0.01 and P < 0.001, respectively), and 0.1 and 1 ng/mL IL1B (P < 0.05 and P < 0.001, respectively). Interestingly, BrdU incorporation into HMVEC DNA was further increased in cells stimulated with a combination of hCG (100 ng/ml) and IL1B (0.1 ng/mL) (P < 0.001) as compared the control medium, as well as to each of these stimuli alone (P < 0.01), thus suggesting a synergistic stimulatory action on HMVEC proliferation (Fig. 2C). Assessment of cell migration using scratch wound assay showed a significant increase in response to hCG (100 ng/mL) and IL1B (0.1 ng/mL) (P < 0.001). However, in cells simultaneously incubated with equivalent concentrations of hCG and IL1B, HMVEC migration was significantly increased as compared to cells incubated with the control medium (P < 0.001), but also as compared to hCG or IL1B (P < 0.05), suggesting here too a synergistic stimulatory effect on HMVEC migration (Fig. 2D).
r Fo
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Draft Manuscript Submitted to MHR for Peer Review
Page 14 of 31
282 283 284 285 286 287 288 289
The above-described data suggest that HMVEC responsiveness is increased following exposure to hCG and IL1B. In order to determine whether this is related to any change in hCG and/or IL1 receptors and antagonists, we further investigated IL1s functional activating receptor and its accessory protein (IL1R1 and mbIL1RAP) together with the cytokines specific inhibitors (IL1R2, sIL1RAP and IL1RN) and LHCGR. Cell cultures were exposed to hCG (100 ng/mL), IL1B (0.1 ng/mL) or hCG plus IL1B at similar concentrations for 24 and 48 h, as optimal cell proliferation and cell migration assays described earlier required 48 and 24 h, respectively.
290 291 292 293 294 295 296 297 298 299 300
Quantitative real-time PCR analysis showed that at 24 and 48h, IL1R1 mRNA steadystate levels were significantly increased by IL1B (P < 0.05) and further by a combination of hCG and IL1B (P < 0.05 and P < 0.01, respectively), but hCG has no statistically significant effect (Fig. 3A and B). IL1R2 mRNA levels in HMVECs were also increased by IL1B at 24 and 48h (P < 0.05), but, interestingly, they were significantly down-regulated by co-incubating HMVECs with hCG and IL1B (P < 0.05 and P < 0.01, respectively) (Fig. 3C and D). Meanwhile, no significant effect of hCG and/or IL1B on mbIL1RAP, sIL1RAP, IL1RN or LHCGR mRNA expression was noted (Fig. 3E, G, I and K) at 24 h. However, a significant stimulation of mbIL1RAP in cells exposed for 48h to IL1B and hCG/IL1B was observed (P < 0.05) (Fig. 3F), which in view of mbIL1RAP role in IL1 signaling suggests that 48h stimulation is more suitable for HMVEC stimulation with IL1B or ILB/hCG .
301 302 303 304 305 306
Then, we assessed the effect of hCG and/or IL1B on IL1Rs and LHCGR protein expression. Western blot analysis showed that hCG reduced the IL1-induced expression of IL1R2 at the protein level as well, but had no noticeable effect on that of ILR1, mbIL1RAP, sIL1RAP or IL1RN (Fig. 4A). Measurement of soluble IL1Rs and IL1RN in the culture medium further showed that sIL1R2 levels were significantly up-regulated in cells stimulated with IL1B as compared to non-stimulated cells (P < 0.001), but, co-incubation with hCG and IL1B led in
r Fo
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Page 15 of 31
Draft Manuscript Submitted to MHR for Peer Review
307 308 309 310
contrast to a significant downregulation of the IL1B-induced sIL1R2 secretion (P < 0.01) (Fig. 4B). No significant effect of hCG and/or IL1B on sIL1RAP was noted (Fig. 4C). However, IL1B significantly increased IL1RN secretion (P < 0.05) and such an effect was not observed in cells co-incubated with IL1B and hCG (Fig. 4D). In order to investigate the impact of the hCG-mediated downregulation of IL1R2 in endothelial cells, we assessed its effect on the synthesis and secretion levels of IL8. Quantitative real time PCR and ELISA analyses showed a significant increase in IL8 mRNA and protein level in response to IL1B (P < 0.05) and further in response to IL1B and hCG together (P < 0.01), whereas hCG had no statistically significant effect. Furthermore, a significant increase of IL8 secretion in cultures simultaneously treated with hCG and IL1B compared to IL1B alone was seen (P < 0.05) (Fig. 5 A and B). Overexpression of IL1R2 in HMVECs by cell transfection with the pcDNA3 expression vector containing IL1R2 cDNA, significantly inhibited IL8 secretion following exposure to IL1B alone or in combination with hCG (P < 0.05 and P < 0.01, respectively). It is noteworthy that sIL1R2 concentrations were increased in the culture medium of endothelial cells transfected with IL1R2 cDNA than in the culture media of pcDNA3 vector-transfected cells (Fig. 6 A and B).
311 312 313 314 315 316 317 318 319 320 321 322
r Fo
Re
vi
ew
On
323
ly
324
DISCUSSION
325 326 327 328 329 330
Normal reproductive functions require highly coordinated interactions between the embryo and the maternal endometrium via an intricate network of signaling and communication. Only less than 24 hours are required for the embryo to breakdown the uterine endometrial epithelial lining and implant within the maternal uterine endometrium. We believe that appropriate interactions with the different types of surrounding endometrial cells are crucial for the implanting embryo to modulate maternal endometrial cell receptivity and favor its own growth. Herein we show that
http://molehr.oxfordjournals.org/
Draft Manuscript Submitted to MHR for Peer Review
Page 16 of 31
331 332 333 334
hCG, the major embryonic signal, acts directly on endothelial cells to up-regulate the receptivity of these cells to IL1, one of the earliest embryonic signals, which taking into account the immune-modulatory, growth-promoting and tissue remodeling effects of IL1, may represent a possible mechanism underlying embryonic implantation and development. Actually, our data first showed that hCG amplifies HMVEC proliferation and migration in response to IL1B. Analysis of IL1 receptors and antagonist further revealed that the IL1Binduced expression of IL1R2, the decoy inhibitory receptor of IL1 (Colotta et al., 1993), is significantly down-regulated in the presence of hCG, whereas the ILB-induced expression of the functional activating IL1R1 (Colotta et al., 1993) is rather up-regulated by this hormone. These data strongly suggest that hCG profoundly modulates endothelial cell receptivity to IL1 via intriguing selective bidirectional regulatory mechanisms, which may amplify endothelial cell responsiveness to IL1 and promote angiogenesis. Interestingly, the data of the present study showed a significant increase of IL8 synthesis and secretion in cells co-exposed to IL1B and hCG and point to a possible synergistic effect. Furthermore, overexpressing IL1R2 in HMVEC significantly inhibited IL8 secretion in response to hCG and IL1B, thereby supporting a role for IL1R2 in the regulation of the ILB- and hCG/IL1B-induced IL8 secretion in HMVEC. First reported as a specific factor for mononuclear cells chemoattraction and activation, IL8 is now known for promoting a wide variety of biological effects, including tissue remodeling, cell invasion and angiogenesis (Kayisli et al. , 2002). Thus, IL8 has been shown to promote angiogenic responses in endothelial cells, inducing the proliferation, survival, and migration of vascular endothelial cells (Waugh and Wilson, 2008) and to influence endometrial differentiation, survival and tissue remodeling (Gazvani et al. , 2002, Mulayim et al. , 2004). Other studies showed that IL8 stimulates trophoblast secretion of progesterone, which plays an important role in maintaining successful pregnancy (Tsui et al. , 2004). According to more recent data, IL1B stimulates migration and survival of first-trimester villous cytotrophoblast cells by inducing IL8
335 336 337 338 339 340 341 342 343 344 345 346 347 348 349 350 351 352 353 354 355
r Fo
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Page 17 of 31
Draft Manuscript Submitted to MHR for Peer Review
356 357 358 359 360 361 362 363 364 365 366 367
secretion in endometrial cells (Hirota et al. , 2009). This is interesting as IL8 expression in the endometrium was found to be enhanced during early pregnancy (Milne et al. , 1999) and IL8 receptor (IL8R) expression was detected in trophoblastic cells of the developing embryo (Dame and Juul, 2000). Furthermore, NK cells, which are the most abundant leukocytes during early pregnancy, were found to produce IL8 and choriodecidual cells from early pregnancy tissues were also shown to produce considerable amounts of IL8 (Fluhr et al. , 2009). Chemokines and chemoattractant cytokines mediate leukocyte migration and are known to play a role in endometrial function, embryo implantation and recruitment of the pregnancy-associated leukocytes (Jones et al. , 2004). In view of the above-indicated findings, the IL1B-induced production of IL8 by endothelial cells may not only stimulate angiogenesis and modulate the immune environment at the implantation site, but may also directly promote embryo invasion and implantation.
368 369 370 371 372 373 374 375 376 377 378 379
The available literature supports a role for IL1 in embryo implantation. The expression of IL1B appeared to increase prior to the initiation of blastocyst implantation in the mouse (Kruessel et al. , 1997, Takacs and Kauma, 1996) and might be an initiator of conceptus-uterine cross-talk during pregnancy in the human (Lindhard et al. , 2002). IL1B was shown to increase the expression of integrin 3 in human endometrial epithelial cells (Simon et al. , 1997), to upregulate IL1R1 (Simon et al. , 1994) and prostaglandin-endoperoxide synthase 2 (PTGS2) (Huang et al. , 1998) expression in human endometrial stromal cells and to induce pro-matrix metalloproteinase (MMP)-3 synthesis in primate stromal decidual cells (Strakova et al. , 2003). Previous studies also suggest a possible role for IL1B and hCG in the inhibition of apoptosis and the development of an immunotolerant environment (Strakova et al. , 2005). According to more recent studies, the presence of serum IL1B can predict ongoing pregnancy, and positively affect the implantation rate (Bonetti et al. , 2010).
r Fo
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Draft Manuscript Submitted to MHR for Peer Review
Page 18 of 31
380 381 382 383 384 385 386 387 388 389 390 391
Tight control of IL1 effects is exerted via various mechanisms involving the regulation of IL1 receptors and receptor antagonist. Known for being involved in IL1 signaling via IL1R1, mbIL1RAP has recently been shown to possess a soluble form, the sIL1RAP, which downregulates IL1 effects through its capability to increase the cytokines affinity for the inhibitory sIL1R2 (Sims and Smith, 2003). IL1RN is rather endowed with potent IL1R antagonistic effects, as it competes with IL1 for binding to IL1R1 (Sims and Smith, 2010). Our present data showed that hCG had no significant effects on mbIL1RAP or sIL1RAP. However, it down-regulated the inhibitory IL1R2 and IL1RN and concomitantly up-regulated the activating IL1R1. The mechanisms underlying such opposite, but functionally complementary, effects are not yet known and further investigations will be necessary to examine the possible involved signaling pathways. The induction of IL1R1 expression was found to be PI3K/Akt-dependent (Teshima et al. , 2004), but nothing is known about IL1R2 and IL1RN signaling pathways. Investigations are underway to assess the impact of hCG on human uterine endothelial cells. Nevertheless, our current data using a human microvascular endothelial cell line, together with our previous data using human primary endometrial epithelial (Boucher et al. , 2001) and stromal (Bourdiec et al. , 2012) cells, make plausible that the embryo can directly target, interact with and modulate the receptivity of different endometrial cell types. The IL1B/hCG synergy shown in the present study and exerted at the level of endothelial cells points to a novel mechanism that may underlie the capability of the embryo to modulate maternal endometrial cell receptivity and promote angiogenesis. This is relevant to physiology of embryo implantation, as IL1B and hCG are both produced by embryos, and broadens the spectrum of hCGs impact on early embryonic growth and development.
r Fo
Re
vi
392 393 394 395 396 397 398 399 400 401
ew
On
ly
402
403
AUTHORS ROLES
http://molehr.oxfordjournals.org/
Page 19 of 31
Draft Manuscript Submitted to MHR for Peer Review
404 405 406 407 408 409 410 411 412 413 414 415 416
A.B and D.V conducted experiments, generated data, contributed to data analysis and wrote the first draft of the manuscript; N.B. conducted experiments; C.V.R provided reagents and precious collaboration to set up the study; AA set up design and concepts, supervised experiments and data analysis and reviewed, corrected and finalized the manuscript.
ACKNOWLEDGEMENTS We thank Mrs. Nathalie Bourcier and Dr M. Al-Akoum for technical assistance and Dr. Rong Shao, Pioneer Valley Life Sciences Institute, University of Massachusetts Amherst, Springfield, MA, USA, for providing HMVECs.
r Fo
Re vi ew
FUNDING
NSERC Discovery Grant to A.A., Chercheur National, "Fonds de la Recherche en Sant du Qubec ".
417 418 419 420 421 422 423
On
CONFLICT OF INTEREST None declared.
http://molehr.oxfordjournals.org/
ly
Draft Manuscript Submitted to MHR for Peer Review
Page 20 of 31
424
REFERENCES
425 426 427 428 429 430 431 432 433 434 435 436 437 438 439 440 441 442 443 444 445 446
Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol. 2008;19:20411. Akoum A, Lawson C, Herrmann-Lavoie C, Maheux R. Imbalance in the expression of the activating type I and the inhibitory type II interleukin 1 receptors in endometriosis. Hum Reprod. 2007;22:1464-73. Akoum A, Metz CN, Morin M. Marked increase in macrophage migration inhibitory factor synthesis and secretion in human endometrial cells in response to human chorionic gonadotropin hormone. J Clin Endocrinol Metab. 2005;90:2904-10. Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387-408. Apte RN, Voronov E. Interleukin-1--a major pleiotropic cytokine in tumor-host interactions. Semin Cancer Biol. 2002;12:277-90.
Apte RN, Voronov E. Is interleukin-1 a good or bad 'guy' in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222:222-41.
Bar D, Apte RN, Voronov E, Dinarello CA, Cohen S. A continuous delivery system of IL-1 receptor antagonist reduces angiogenesis and inhibits tumor development. Faseb J. 2004;18:161-3. Bellehumeur C, Blanchet J, Fontaine JY, Bourcier N, Akoum A. Interleukin 1 regulates its own receptors in human endometrial cells via distinct mechanisms. Hum Reprod. 2009;24:2193-204.
r Fo
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Page 21 of 31
Draft Manuscript Submitted to MHR for Peer Review
447 448 449 450 451 452 453 454 455 456 457 458 459 460 461 462 463 464 465 466 467 468 469
Bernardini L, Moretti-Rojas I, Brush M, Rojas FJ, Balmaceda JP. Status of hCG/LH receptor and G proteins in human endometrium during artificial cycles of hormone replacement therapy. J Soc Gynecol Investig. 1995;2:630-5. Berndt S, Blacher S, Perrier d'Hauterive S, Thiry M, Tsampalas M, Cruz A, Pequeux C, Lorquet S, Munaut C, Noel A et al. Chorionic gonadotropin stimulation of angiogenesis and pericyte recruitment. J Clin Endocrinol Metab. 2009;94:4567-74. Bonetti TC, Salomao R, Brunialti M, Braga DP, Borges E, Jr., Silva ID. Cytokine and hormonal profile in serum samples of patients undergoing controlled ovarian stimulation: interleukin-1beta predicts ongoing pregnancy. Hum Reprod. 2010;25:2101-6. Boucher A, Kharfi A, Al-Akoum M, Bossu P, Akoum A. Cycle-dependent expression of interleukin-1 receptor type II in the human endometrium. Biol Reprod. 2001;65:890-8. Bourdiec A, Shao R, Rao CV, Akoum A. Human Chorionic Gonadotropin Triggers Angiogenesis via the Modulation of Endometrial Stromal Cell Responsiveness to Interleukin 1: A New Possible Mechanism Underlying Embryo Implantation. Biol Reprod. 2012. Chirivi RG, Chiodoni C, Musiani P, Garofalo A, Bernasconi S, Colombo MP, Giavazzi R. IL1alpha gene-transfected human melanoma cells increase tumor-cell adhesion to endothelial cells and their retention in the lung of nude mice. Int J Cancer. 1996;67:856-63. Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472-5. Dame JB, Juul SE. The distribution of receptors for the pro-inflammatory cytokines interleukin (IL)-6 and IL-8 in the developing human fetus. Early Hum Dev. 2000;58:25-39. Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095-147.
r Fo
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Draft Manuscript Submitted to MHR for Peer Review
Page 22 of 31
470 471 472 473 474 475 476 477 478 479 480 481 482 483 484 485 486 487 488 489 490 491 492
Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. Faseb J. 2009;23:2165-75. Fluhr H, Sauter G, Steinmuller F, Licht P, Zygmunt M. Nonapoptotic effects of tumor necrosis factor-related apoptosis-inducing ligand on interleukin-6, leukemia inhibitory factor, interleukin-8, and monocyte chemoattractant protein 1 vary between undifferentiated and decidualized human endometrial stromal cells. Fertil Steril. 2009;92:1420-3. Gazvani R, Smith L, Fowler PA. Effect of interleukin-8 (IL-8), anti-IL-8, and IL-12 on endometrial cell survival in combined endometrial gland and stromal cell cultures derived from women with and without endometriosis. Fertil Steril. 2002;77:62-7. Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, Ju G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem.
1995;270:13757-65.
Han SW, Lei ZM, Rao CV. Treatment of human endometrial stromal cells with chorionic gonadotropin promotes their morphological and functional differentiation into decidua. Mol Cell Endocrinol. 1999;147:7-16.
Herrmann-Lavoie C, Rao CV, Akoum A. Chorionic gonadotropin down-regulates the expression of the decoy inhibitory interleukin 1 receptor type II in human endometrial
epithelial cells. Endocrinology. 2007;148:5377-84.
Hirota Y, Osuga Y, Hasegawa A, Kodama A, Tajima T, Hamasaki K, Koga K, Yoshino O, Hirata T, Harada M et al. Interleukin (IL)-1beta stimulates migration and survival of firsttrimester villous cytotrophoblast cells through endometrial epithelial cell-derived IL-8. Endocrinology. 2009;150:350-6.
r Fo
Re
http://molehr.oxfordjournals.org/
vi ew On ly
Page 23 of 31
Draft Manuscript Submitted to MHR for Peer Review
493 494 495 496 497 498 499 500 501 502 503 504 505 506 507 508 509 510 511 512 513 514 515
Huang JC, Liu DY, Yadollahi S, Wu KK, Dawood MY. Interleukin-1 beta induces cyclooxygenase-2 gene expression in cultured endometrial stromal cells. J Clin Endocrinol Metab. 1998;83:538-41. Islami D, Bischof P, Chardonnens D. Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol Hum Reprod. 2003;9:395-8. Jaffe RB. Fetoplacental endocrine and metabolic physiology. Clin Perinatol. 1983;10:669-93. Jaffe RB, Lee PA, Midgley AR, Jr. Serum gonadotropins before, at the inception of, and following human pregnancy. J Clin Endocrinol Metab. 1969;29:1281-3. Jones RL, Hannan NJ, Kaitu'u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89:6155-67. Kane N, Kelly R, Saunders PT, Critchley HO. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor.
Endocrinology. 2009;150:2882-8.
Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47:213-21.
Kruessel JS, Huang HY, Wen Y, Kloodt AR, Bielfeld P, Polan ML. Different pattern of interleukin-1 beta-(IL-1 beta), interleukin-1 receptor antagonist- (IL-1ra) and interleukin-1 receptor type I- (IL-1R tI) mRNA-expression in single preimplantation mouse embryos at various developmental stages. J Reprod Immunol. 1997;34:103-20. Licht P, Russu V, Wildt L. On the role of human chorionic gonadotropin (hCG) in the embryo-endometrial microenvironment: implications for differentiation and implantation. Semin Reprod Med. 2001;19:37-47.
r Fo
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Draft Manuscript Submitted to MHR for Peer Review
Page 24 of 31
516 517 518 519 520 521 522 523 524 525 526 527 528 529 530 531 532 533 534 535 536 537 538 539 540
Lincoln SR, Lei ZM, Rao CV, Yussman MA. The expression of human chorionic gonadotropin/human luteinizing hormone receptors in ectopic human endometrial implants. J Clin Endocrinol Metab. 1992;75:1140-4. Lindhard A, Bentin-Ley U, Ravn V, Islin H, Hviid T, Rex S, Bangsboll S, Sorensen S. Biochemical evaluation of endometrial function at the time of implantation. Fertil Steril. 2002;78:221-33. Meager A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev. 1999;10:27-39. Milne SA, Critchley HO, Drudy TA, Kelly RW, Baird DT. Perivascular interleukin-8 messenger ribonucleic acid expression in human endometrium varies across the menstrual cycle and in early pregnancy decidua. J Clin Endocrinol Metab. 1999;84:2563-7. Mor G. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci. 2008;1127:121-8.
Mulayim N, Savlu A, Guzeloglu-Kayisli O, Kayisli UA, Arici A. Regulation of endometrial stromal cell matrix metalloproteinase activity and invasiveness by interleukin-8. Fertil Steril. 2004;81 Suppl 1:904-11.
Rao CV. An overview of the past, present, and future of nongonadal LH/hCG actions in reproductive biology and medicine. Semin Reprod Med. 2001;19:7-17.
Rao CV. [LH receptors: follicle and endometrium]. J Gynecol Obstet Biol Reprod (Paris). 2002;31:1S7-1S11. Rao CV, Lei ZM. The past, present and future of nongonadal LH/hCG actions in reproductive biology and medicine. Mol Cell Endocrinol. 2007;269:2-8. Reshef E, Lei ZM, Rao CV, Pridham DD, Chegini N, Luborsky JL. The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua. J Clin Endocrinol Metab. 1990;70:421-30.
r Fo
Re
http://molehr.oxfordjournals.org/
vi ew On ly
Page 25 of 31
Draft Manuscript Submitted to MHR for Peer Review
541 542 543 544 545 546 547 548 549 550 551 552 553 554 555 556 557 558 559 560 561 562 563 564 565
Shao R, Guo X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem Biophys Res Commun. 2004a;321:788-94. Shao R, Guo X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem Biophys Res Commun. 2004b;321:788-94. Sheth KV, Roca GL, al-Sedairy ST, Parhar RS, Hamilton CJ, al-Abdul Jabbar F. Prediction of successful embryo implantation by measuring interleukin-1-alpha and immunosuppressive factor(s) in preimplantation embryo culture fluid. Fertil Steril. 1991;55:952-7. Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132:1387-95. Simon C, Gimeno MJ, Mercader A, O'Connor JE, Remohi J, Polan ML, Pellicer A. Embryonic regulation of integrins beta 3, alpha 4, and alpha 1 in human endometrial epithelial cells in vitro. J Clin Endocrinol Metab. 1997;82:2607-16. Simon C, Piquette GN, Frances A, el-Danasouri I, Irwin JC, Polan ML. The effect of interleukin-1 beta (IL-1 beta) on the regulation of IL-1 receptor type I messenger ribonucleic acid and protein levels in cultured human endometrial stromal and glandular cells. J Clin Endocrinol Metab. 1994;78:675-82.
Sims JE, Smith DE. Regulation of interleukin-1 activity is enhanced by cooperation between the interleukin-1 receptor type II and interleukin-1 receptor accessory protein. Eur Cytokine Netw. 2003;14:77-81. Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89102. Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, Brudney A, Knight O, Fazleabas AT. In vivo infusion of interleukin-1beta and chorionic gonadotropin induces
r Fo
Re
http://molehr.oxfordjournals.org/
vi
ew
On
ly
Draft Manuscript Submitted to MHR for Peer Review
Page 26 of 31
566 567 568 569 570 571 572 573 574 575 576 577 578 579 580 581 582 583 584 585 586 587 588 589 590
endometrial changes that mimic early pregnancy events in the baboon. Endocrinology. 2005;146:4097-104. Strakova Z, Szmidt M, Srisuparp S, Fazleabas AT. Inhibition of matrix metalloproteinases prevents the synthesis of insulin-like growth factor binding protein-1 during decidualization in the baboon. Endocrinology. 2003;144:5339-46. Strott CA, Yoshimi T, Ross GT, Lipsett MB. Ovarian physiology: relationship between plasma LH and steroidogenesis by the follicle and corpus luteum; effect of HCG. J Clin Endocrinol Metab. 1969;29:1157-67. Takacs P, Kauma S. The expression of interleukin-1 alpha, interleukin-1 beta, and interleukin1 receptor type I mRNA during preimplantation mouse development. J Reprod Immunol.
r Fo
Re
1996;32:27-35.
Tang B, Gurpide E. Direct effect of gonadotropins on decidualization of human endometrial stroma cells. J Steroid Biochem Mol Biol. 1993;47:115-21. Teshima S, Nakanishi H, Nishizawa M, Kitagawa K, Kaibori M, Yamada M, Habara K, Kwon AH, Kamiyama Y, Ito S et al. Up-regulation of IL-1 receptor through PI3K/Akt is essential for the induction of iNOS gene expression in hepatocytes. J Hepatol. 2004;40:616-
vi
ew
On
23.
Toth P, Li X, Rao CV, Lincoln SR, Sanfilippo JS, Spinnato JA, 2nd, Yussman MA. Expression of functional human chorionic gonadotropin/human luteinizing hormone receptor gene in human uterine arteries. J Clin Endocrinol Metab. 1994;79:307-15. Toth P, Lukacs H, Gimes G, Sebestyen A, Pasztor N, Paulin F, Rao CV. Clinical importance of vascular LH/hCG receptors--a review. Reprod Biol. 2001;1:5-11. Tsui KH, Chen LY, Shieh ML, Chang SP, Yuan CC, Li HY. Interleukin-8 can stimulate progesterone secretion from a human trophoblast cell line, BeWo. In Vitro Cell Dev Biol Anim. 2004;40:331-6.
http://molehr.oxfordjournals.org/
ly
Page 27 of 31
Draft Manuscript Submitted to MHR for Peer Review
591 592 593 594 595 596 597 598 599 600 601 602
Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello CA. Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst. 1996;88:198-205. Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:673541. Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290-6. Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. European journal of obstetrics, gynecology, and reproductive biology. 2003;110 Suppl 1:S10-8.
r Fo
Re
vi
603
ew
604
605
On
606
ly
607
608
609
610
611
http://molehr.oxfordjournals.org/
Draft Manuscript Submitted to MHR for Peer Review
Page 28 of 31
612
FIGURE LEGENDS
613
614 615 616 617 618 619 620
Figure 1. Immunocytofluorescence analysis of LHCGR, IL1Rs and IL1RN in HMVECs. The receptors were detected using antibodies specific to LHCGR (A), IL1R1 (C), IL1R2 (E) and IL1RAP (G). Streptavidin-fluorescein isothiocyanate (green) was used for detection of IL1Rs receptors and Alexa 488 (red) for LHCGR. Cells were counterstained with 4,6-diaminido-2phenyl-indole (blue). Cells incubated without the primary antibody were included as negative controls (B, D, F, H). Data are representative of experiments from 3 different cultures.
621 622 623 624 625 626 627 628 629 630 631 632
Figure 2. hCG and IL1B trigger HMVEC proliferation and migration. For cell proliferation (A, B and C), HMVEC (104 cells/well in 96-well microtiter plates) were cultured overnight, starved in the endothelial cell basal medium (MCDB 131) containing 0.2% FBS and incubated in quadricate for 48h with this medium alone (control medium) or supplemented with hCG, IL1 and hCG/IL1 at the indicated concentrations. Cell proliferation was assessed by ELISA using BrdU incorporation into HMVEC DNA. Data are from 4 different cultures and expressed as % of control. For cell migration, confluent HMVEC monolayers were wounded by scraping and incubated in duplicate for 24 h with hCG, IL1B and hCG/IL1B at the indicated concentrations. Cell migration to the wound surface was quantified by microscopy at magnification X4. Data are from 3 different cultures and expressed as % of closure. *P < 0.05, **P < 0.01, ***P < 0.001 relative to the control medium. P < 0.05, P < 0.01 relative to equivalent hCG dose; +P < 0.05,
+++
P < 0.001 relative to equivalent IL1B dose.
r Fo
Re
vi
ew
On
ly
633
634 635
Figure 3. hCG modulates IL1 receptors mRNA expression in HMVEC. Confluent cell cultures were starved in the endothelial cell basal medium (MCDB 131) containing 0.2% FBS and
http://molehr.oxfordjournals.org/
Page 29 of 31
Draft Manuscript Submitted to MHR for Peer Review
636 637 638 639 640 641 642 643 644 645 646
incubated in duplicate with this medium alone (control medium) or supplemented with hCG, IL1B or hCG/IL1B at the indicated concentrations for 24 (A, C, E, G, I, K) and 48 (B, D, F, H, J, L) h. Total RNA was extracted and reverse transcribed. IL1R1, IL1R2, mbIL1RAP, sIL1RAP, IL1RN, LHCGR and GAPDH (internal control) mRNA levels were quantified by real-time PCR as described in Materials and Methods. IL1R1 (A, B), IL1R2 (C, D), mbIL1RAP (E, F), sIL1RAP (G, H), IL1RN (I, J) and LHCGR (K, L) mRNA ratio was then determined following normalization to GAPDH mRNA. Data were from 8 different cultures and expressed as % of control (ratio of each of IL1Rs, IL1RN or LHCGR mRNA levels found in cells incubated with IL1B, hCG or hCG/ILB to those found in cells incubated with the control medium for an equivalent period of time). *P < 0.05, **P < 0.01 relative to the control medium; P < 0.05, P < 0.01 relative to equivalent IL1B dose.
r Fo
Re
647
648 649 650 651 652 653 654 655 656 657 658
Figure 4. hCG modulates IL1 receptors protein expression in HMVEC. Confluent cell cultures were starved in the endothelial cell basal medium (MCDB 131) containing 0.2% FBS and incubated in duplicate with this medium alone (control medium) or supplemented with hCG, IL1B or hCG/IL1B at the indicated concentrations for 48 h. Cells were recovered to extract total proteins and analyze IL1Rs, IL1RN and LHCGR by Western blotting (A), and culture supernatants were collected to measure sIL1R2 (B), sIL1RAP (C) and IL1RN (D) as described in Materials and Methods. Data were from 6 (sIL1R2 and sIL1RAP) and 4 (IL1RN) different cultures and expressed as % of control (ratio of sIL1R2 or IL1RN levels found in cells incubated with IL1B, hCG or hCG/ILB to those found in cells incubated with the control medium for an equivalent period of time). *P < 0.05, *** P < 0.001 to the control medium; P < 0.01 relative to equivalent IL1B dose.
vi
ew
On
ly
659
http://molehr.oxfordjournals.org/
Draft Manuscript Submitted to MHR for Peer Review
Page 30 of 31
660 661 662 663 664 665 666 667 668 669 670 671
Figure 5. hCG enhances IL8 expression in HMVEC in response to ILB. Confluent cell cultures were starved in the endothelial cell basal medium (MCDB 131) containing 0.2% FBS and incubated in duplicate with this medium alone (control medium) or supplemented with hCG, IL1B or hCG/IL1B at the indicated concentrations for 48h. Total cell RNA was extracted and reverse transcribed. IL8 and GAPDH were quantified by real-time PCR as described in Materials and Methods. IL8 mRNA (A) levels were then determined following normalization to GAPDH mRNA levels. The culture supernatants were recovered for determination of IL8 secretion by ELISA (B). Data are from 5 different HMVEC cultures and expressed as % of control (ratio of IL8 mRNA levels or protein secretion in cells incubated with IL1B, hCG or IL1B/hCG to those in cells incubated with the control medium for an equivalent period of time). *P < 0.05, **P < 0.01 relative to the control medium; P < 0.05 relative to IL1B control dose.
r Fo
Re
vi
672
673 674 675 676 677 678 679 680 681
Figure 6. IL1R2 is a possible target for the hCG-mediated modulation of endothelial cell responsiveness to IL1B. HMVECs were transiently transfected with pcDNA3-IL1R2 or with the control vector pcDNA3, as described in Materials and Methods. Transfected and nontransfected cells were incubated in triplicate and for 48h with the endothelial cell basal medium (MCDB 131) containing 0.2% FBS (control medium) or supplemented with hCG, IL1B or hCG/IL1B at the indicated concentrations. IL8 (A) and sIL1R2 (B) concentrations were measured in the culture supernatant by ELISA. *P < 0.05, ***P < 0.001 relative to the control medium; P < 0.05 relative to equivalent IL1B dose; +P < 0.05, ++P < 0.01 relative to equivalent IL1B or hCG/IL1B dose.
http://molehr.oxfordjournals.org/
ew
On
ly
Page 31 of 31
Draft Manuscript Submitted to MHR for Peer Review
r Fo Re
Fig.1 82x125mm (300 x 300 DPI)
http://molehr.oxfordjournals.org/
vi ew On ly
Das könnte Ihnen auch gefallen
- Fournier 2011Dokument9 SeitenFournier 2011Nofi NurinaNoch keine Bewertungen
- Endometrial Receptivity BiomarkersDokument19 SeitenEndometrial Receptivity BiomarkersEndriana Gak Endut EndutNoch keine Bewertungen
- Interleukins, Interferons, and Establishment of Pregnancy in PigsDokument12 SeitenInterleukins, Interferons, and Establishment of Pregnancy in PigsDaveMartoneNoch keine Bewertungen
- E443.fullDokument14 SeitenE443.fullMarianaafiatiNoch keine Bewertungen
- Comparative Gene Expression Analysis of Genital TubercleDokument29 SeitenComparative Gene Expression Analysis of Genital TubercleAnonymous rprdjdFMNzNoch keine Bewertungen
- Condrocyte HypertrophyDokument9 SeitenCondrocyte HypertrophyLasa SiahaanNoch keine Bewertungen
- Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human PrimatesDokument21 SeitenPhysiologic Events of Embryo Implantation and Decidualization in Human and Non-Human PrimatesAnh Vũ Hồ Ngọc100% (1)
- Angiogenic FactorsDokument2 SeitenAngiogenic FactorsNaomy OdhiamboNoch keine Bewertungen
- P.gingivalis Na HaecDokument20 SeitenP.gingivalis Na HaecMarta KabacińskaNoch keine Bewertungen
- 17-Maternal Thyroid Dysfunction Affects Placental Profile of Inflammatory Mediators and The Intrauterine Trophoblast Migration KineticsDokument14 Seiten17-Maternal Thyroid Dysfunction Affects Placental Profile of Inflammatory Mediators and The Intrauterine Trophoblast Migration KineticschimansqmNoch keine Bewertungen
- Modulate Endothelial Function and Coagulation Bacterial Lipoprotein TLR2 Agonists BroadlyDokument13 SeitenModulate Endothelial Function and Coagulation Bacterial Lipoprotein TLR2 Agonists Broadlynandhus2227Noch keine Bewertungen
- Hu 2021Dokument10 SeitenHu 2021Carlos ParraNoch keine Bewertungen
- Cytokines and Myometrial Signalling in Human Labour S P Sivarajasingam, N Imami, MR JohnsonDokument53 SeitenCytokines and Myometrial Signalling in Human Labour S P Sivarajasingam, N Imami, MR JohnsonMBMNoch keine Bewertungen
- Chapter 2 - Immunological Changes in Pregnancy and - 2021 - Covid 19 InfectionsDokument15 SeitenChapter 2 - Immunological Changes in Pregnancy and - 2021 - Covid 19 InfectionsWalter MendozaNoch keine Bewertungen
- Park Et Al., 2004 EGF-Like Growth Factors As Mediators of LH Action in The Ovulatory FollicleDokument4 SeitenPark Et Al., 2004 EGF-Like Growth Factors As Mediators of LH Action in The Ovulatory FollicleLudimilaNoch keine Bewertungen
- Endometrial Inflammation and Effect On Implantation - Granot - 2012Dokument8 SeitenEndometrial Inflammation and Effect On Implantation - Granot - 2012Marco MPNoch keine Bewertungen
- Angiogenic Growth FactorsDokument9 SeitenAngiogenic Growth FactorsArchana Kumari ShawNoch keine Bewertungen
- Ni Hms 808507Dokument24 SeitenNi Hms 808507Vivien WuNoch keine Bewertungen
- Extragonadal Actions of Chorionic GonadotropinDokument16 SeitenExtragonadal Actions of Chorionic GonadotropinАнатолий КомогорцевNoch keine Bewertungen
- Graduate Student Literature Review: Effects of Human ChorionicDokument10 SeitenGraduate Student Literature Review: Effects of Human ChorionicMargarita MujicaNoch keine Bewertungen
- The Time Interval Between HCG Priming and Oocyte Retrieval in ART Program: A Meta-AnalysisDokument10 SeitenThe Time Interval Between HCG Priming and Oocyte Retrieval in ART Program: A Meta-AnalysisErvan SuryaNoch keine Bewertungen
- 3rd PaperDokument9 Seiten3rd PaperNajat MohammedNoch keine Bewertungen
- v1 457252 PDFDokument41 Seitenv1 457252 PDFAbhishek YadavNoch keine Bewertungen
- NIH Public AccessDokument13 SeitenNIH Public AccessYenifer Quispe PalominoNoch keine Bewertungen
- Hormones and Embryonic Development: Advances in the BiosciencesVon EverandHormones and Embryonic Development: Advances in the BiosciencesG. RaspéNoch keine Bewertungen
- 1 s2.0 S0301211523003202 main (科研通 ablesci.com)Dokument28 Seiten1 s2.0 S0301211523003202 main (科研通 ablesci.com)Carlos ParraNoch keine Bewertungen
- Smitz 2006Dokument12 SeitenSmitz 2006Ahmed GhanimNoch keine Bewertungen
- VEGF Expression During Socket Healing in Diabetic RatsDokument6 SeitenVEGF Expression During Socket Healing in Diabetic Ratswanda oktariaNoch keine Bewertungen
- (17417899 - Reproduction) Mitochondrial Humanin Peptide Acts As A Cytoprotective Factor in Granulosa Cell SurvivalDokument11 Seiten(17417899 - Reproduction) Mitochondrial Humanin Peptide Acts As A Cytoprotective Factor in Granulosa Cell SurvivalLeilane GlienkeNoch keine Bewertungen
- Binding of The Fap2 Protein of Fusobacterium Nucleatum To Human Inhibitory Receptor TIGIT Protects Tumors From Immune Cell Attack 2015 ImmunityDokument13 SeitenBinding of The Fap2 Protein of Fusobacterium Nucleatum To Human Inhibitory Receptor TIGIT Protects Tumors From Immune Cell Attack 2015 ImmunityMatthew MacowanNoch keine Bewertungen
- Li 2018Dokument26 SeitenLi 2018Berkah Tania Sawitri PasaribuNoch keine Bewertungen
- Middle East Fertility Society Journal: Maryam Eftekhar, Elham Naghshineh, Nosrat Neghab, Robabe HosseinisadatDokument4 SeitenMiddle East Fertility Society Journal: Maryam Eftekhar, Elham Naghshineh, Nosrat Neghab, Robabe HosseinisadatM AbhiNoch keine Bewertungen
- DisxussionDokument2 SeitenDisxussionGERA KAYE BUNCALANNoch keine Bewertungen
- Defic I Enc I A MultipleDokument10 SeitenDefic I Enc I A MultipleEnrique GonzalvusNoch keine Bewertungen
- 1471 2431 11 29 PDFDokument6 Seiten1471 2431 11 29 PDFCeleste SantiNoch keine Bewertungen
- Mathematical Analysis of A Model For The Growth of The Bovine Corpus Luteum JMB 2013Dokument32 SeitenMathematical Analysis of A Model For The Growth of The Bovine Corpus Luteum JMB 2013api-244962164Noch keine Bewertungen
- OncogeneDokument5 SeitenOncogenejohn.geek2014Noch keine Bewertungen
- Gingival Crevice FluidDokument3 SeitenGingival Crevice FluidMohammed NabeelNoch keine Bewertungen
- TVT CaninoDokument8 SeitenTVT CaninoLuis Alfredo Bernal LopezNoch keine Bewertungen
- Causes and consequences of variation in human cytokine productionDokument9 SeitenCauses and consequences of variation in human cytokine productionLluis GomezNoch keine Bewertungen
- Fendo 13 1107903Dokument3 SeitenFendo 13 1107903puspitaNoch keine Bewertungen
- Angiogenesis of Endocrine Gland Tumours - New Molecular Targets in Diagnostics and TherapyDokument9 SeitenAngiogenesis of Endocrine Gland Tumours - New Molecular Targets in Diagnostics and Therapyexavier123Noch keine Bewertungen
- Reproductive Biology and Endocrinology: Factors Involved in The Inflammatory Events of Cervical Ripening in HumansDokument17 SeitenReproductive Biology and Endocrinology: Factors Involved in The Inflammatory Events of Cervical Ripening in HumansPriyaaNoch keine Bewertungen
- Wang 2011Dokument8 SeitenWang 2011Nicolas Riffo LepeNoch keine Bewertungen
- Il-17 32 Print Anatomi 3Dokument11 SeitenIl-17 32 Print Anatomi 3a f indra pratamaNoch keine Bewertungen
- Reduced FSH and LH Action: Implications For Medically Assisted ReproductionDokument12 SeitenReduced FSH and LH Action: Implications For Medically Assisted ReproductionKhoirunisah Dwi HartantiNoch keine Bewertungen
- Fetal-Specific CD8+ Cytotoxic T Cell Responses Develop During Normal Human Pregnancy and Exhibit Broad Functional CapacityDokument10 SeitenFetal-Specific CD8+ Cytotoxic T Cell Responses Develop During Normal Human Pregnancy and Exhibit Broad Functional Capacity石一Noch keine Bewertungen
- Journal Pone 0046483Dokument11 SeitenJournal Pone 0046483Candra BumiNoch keine Bewertungen
- Zhang 2017Dokument25 SeitenZhang 2017hayNoch keine Bewertungen
- HHS Public Access: Nutrition and fasting diets prevent autoimmune diseasesDokument20 SeitenHHS Public Access: Nutrition and fasting diets prevent autoimmune diseasesANANoch keine Bewertungen
- Leptin Receptor Is Induced in Endometriosis and Leptin Stimulates The Growth of Endometriotic Epithelial Cells Through The JAK2/STAT3 and ERK PathwaysDokument9 SeitenLeptin Receptor Is Induced in Endometriosis and Leptin Stimulates The Growth of Endometriotic Epithelial Cells Through The JAK2/STAT3 and ERK PathwaysDesyHandayaniNoch keine Bewertungen
- Research ArticleDokument7 SeitenResearch ArticleElena IancuNoch keine Bewertungen
- Differences in Inflammatory Markers Between NullipDokument8 SeitenDifferences in Inflammatory Markers Between Nulliponline videoNoch keine Bewertungen
- Nilsson 2017Dokument18 SeitenNilsson 2017نذير الشرعبيNoch keine Bewertungen
- Translational Glycobiology: Patient-Oriented Glycoscience ResearchDokument25 SeitenTranslational Glycobiology: Patient-Oriented Glycoscience ResearchCamron DaviesNoch keine Bewertungen
- Growth Factor ConcentraeDokument7 SeitenGrowth Factor ConcentraeHossam BarghashNoch keine Bewertungen
- 2007-3.9-Differential Role of Gonadotropin-Releasing Hormone On HumanDokument10 Seiten2007-3.9-Differential Role of Gonadotropin-Releasing Hormone On Humanshidis1028Noch keine Bewertungen
- Feocromocitomul in VHLDokument6 SeitenFeocromocitomul in VHLkaeyaNoch keine Bewertungen
- Introduction to Clinical Reproductive EndocrinologyVon EverandIntroduction to Clinical Reproductive EndocrinologyBewertung: 1 von 5 Sternen1/5 (1)
- Oppenheim J.J., Feldmann M. - Introduction To The Role of Cytokines in Innate Host Defense and Adaptive Immunity (2000) PDFDokument18 SeitenOppenheim J.J., Feldmann M. - Introduction To The Role of Cytokines in Innate Host Defense and Adaptive Immunity (2000) PDFHesbon MomanyiNoch keine Bewertungen
- Cellular and Molecular Mechanisms of Asthma and COPDDokument18 SeitenCellular and Molecular Mechanisms of Asthma and COPDMihaela BerindeieNoch keine Bewertungen
- IL6 Interleukin 6 Gene in HumansDokument13 SeitenIL6 Interleukin 6 Gene in HumansMUHAMMAD SENDINoch keine Bewertungen
- Acute Inflammation: Rino Pattiata Dept. Patologi Anatomik FKUI JakartaDokument48 SeitenAcute Inflammation: Rino Pattiata Dept. Patologi Anatomik FKUI JakartaRaja DarmawanNoch keine Bewertungen
- In Vitro Biocompatibility of Titanium Alloy Discs Made Using Direct MetalDokument8 SeitenIn Vitro Biocompatibility of Titanium Alloy Discs Made Using Direct MetalAngelNoch keine Bewertungen
- Stenotrophomona Maltophilia y Bulkorelia Cepacia. Mnadell 2009Dokument8 SeitenStenotrophomona Maltophilia y Bulkorelia Cepacia. Mnadell 2009Laura López Del Castillo LalydelcaNoch keine Bewertungen
- Pathology, Chapter 3, Inflammation (Slides)Dokument187 SeitenPathology, Chapter 3, Inflammation (Slides)Ali Al-Qudsi100% (29)
- Acute Inflammatory Response - : VPM 152 Winter 2006 Inflammation and Repair General Pathology 18Dokument19 SeitenAcute Inflammatory Response - : VPM 152 Winter 2006 Inflammation and Repair General Pathology 18ANI REHMNoch keine Bewertungen
- Respiratory Syncytial Virus Infection in CattleDokument10 SeitenRespiratory Syncytial Virus Infection in CattleDayanna MorenoNoch keine Bewertungen
- Vape 3Dokument9 SeitenVape 3Iskandar PakayaNoch keine Bewertungen
- Inflammation, Immunity and Potential Target Therapy of SARS-COV-2 ADokument23 SeitenInflammation, Immunity and Potential Target Therapy of SARS-COV-2 AShukr Wesman BlbasNoch keine Bewertungen
- CAYRAPF – MED II, PULMONOLOGY - FEBRUARY 2017, 2ND SEMESTERDokument8 SeitenCAYRAPF – MED II, PULMONOLOGY - FEBRUARY 2017, 2ND SEMESTERMiguel Cuevas DolotNoch keine Bewertungen
- Cytoquine Storm and SepsisDokument12 SeitenCytoquine Storm and SepsisEduardo ChanonaNoch keine Bewertungen
- Biodegradable Metals PDFDokument202 SeitenBiodegradable Metals PDFvw5ohgjr100% (1)
- Cytokines PosterDokument2 SeitenCytokines PosterGregorio AndresNoch keine Bewertungen
- CANINE-Pathophysiology of Organ Failure in Severe Acute Pancreatitis in DogsDokument10 SeitenCANINE-Pathophysiology of Organ Failure in Severe Acute Pancreatitis in Dogstaner_soysurenNoch keine Bewertungen
- BUUN Immunomodulatory Effects of Massage in Skeletal MuscleDokument133 SeitenBUUN Immunomodulatory Effects of Massage in Skeletal Muscledavidescu5costinNoch keine Bewertungen
- Damage Control Orthopaedics, EvolvingDokument17 SeitenDamage Control Orthopaedics, EvolvingAzmi Farhadi100% (1)
- Macrofagos Asociados A TumoresDokument9 SeitenMacrofagos Asociados A Tumoresjuan ramon zepeda tejedaNoch keine Bewertungen
- A Role For Macrophages Under Cytokine Control in Mediating Resistance To ADI PEG20 (Pegargiminase) in ASS1 Deficient MesotheliomaDokument15 SeitenA Role For Macrophages Under Cytokine Control in Mediating Resistance To ADI PEG20 (Pegargiminase) in ASS1 Deficient MesotheliomaAlexander SergeevichNoch keine Bewertungen
- Art:10.1186/1939 4551 1 S3 A1 PDFDokument335 SeitenArt:10.1186/1939 4551 1 S3 A1 PDFYunus wanendaNoch keine Bewertungen
- Moura Et Al. (2011)Dokument8 SeitenMoura Et Al. (2011)Callum BromleyNoch keine Bewertungen
- Immune Regulation by GlucocorticoidsDokument35 SeitenImmune Regulation by GlucocorticoidsSyifa SariNoch keine Bewertungen
- Tea Tree Oil As A Novel Antipsoriasis Weapon: Letter To The EditorDokument2 SeitenTea Tree Oil As A Novel Antipsoriasis Weapon: Letter To The EditorBIOMEMNoch keine Bewertungen
- Kvir 13 2090071Dokument12 SeitenKvir 13 2090071Familia PipekNoch keine Bewertungen
- WO2015169811A2Dokument141 SeitenWO2015169811A2Muhamad Alif Bin Che NordinNoch keine Bewertungen
- IF4.02 Molecular Pathogenesis of Klebsiella Pneumoniae PDFDokument36 SeitenIF4.02 Molecular Pathogenesis of Klebsiella Pneumoniae PDFdindaNoch keine Bewertungen
- Pathology QuizesDokument13 SeitenPathology Quizesსალომე მუმლაძე “Slay” TMANoch keine Bewertungen
- Draft CPG Management of AcneDokument63 SeitenDraft CPG Management of AcneAl Mutarabbi100% (1)
- From Pathogenesis of Acne Vulgaris To Anti-Acne AgentsDokument13 SeitenFrom Pathogenesis of Acne Vulgaris To Anti-Acne AgentsNazihan Safitri AlkatiriNoch keine Bewertungen