Beruflich Dokumente
Kultur Dokumente
Case Studies in Bonding and Structure
Hochgeladen von
Doc_CrocOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Case Studies in Bonding and Structure
Hochgeladen von
Doc_CrocCopyright:
Verfügbare Formate
F321 Structure Case Studies Case Studies in Bonding and Structure
(q) describe structures as: (i) giant ionic lattices, with strong ionic bonding, ie as in NaCl, (ii) giant covalent lattices, ie as in diamond and graphite, (iii) giant metallic lattices, (iv) simple molecular lattices, ie as in I2 and ice;
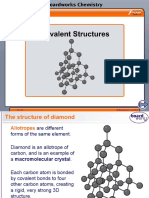
Diamond a typical giant covalent lattice In diamond each carbon atom is covalently bonded to four other carbon atoms in a tetrahedral arrangement. Since all four outer shell electrons in carbon are used for bonding there are no charge carriers in diamond, so it does not conduct electricity. The extremely high melting and boiling point of diamond, the extreme strength of the substance, and its lack of solubility in any solvents are all due to the very strong covalent bonds throughout the lattice. These must be broken, requiring a lot of energy, before the structure will melt, break or dissolve . Graphite an unusual giant covalent lattice In graphite each carbon atom is covalently bonded to thee others forming a hexagonal layer structure with 120 bond angles. Each layer has further layers above and below. The one remaining electron in the outer shell of each carbon atom is not used in bonding. This becomes delocalized and can move throughout the lattice carrying electrical charge, so graphite conducts electricity like a metal even though it is not a metal. Graphite is a soft substance. The layers are held one to another by weak Van der Waals forces, which means that the layers can easily slide over each other making graphite slippery and good for use as a lubricant. Pencils leave their marks on paper because the graphite 'lead' in the pencil leaves layers of graphite stuck to the paper.
F321 Structure Case Studies Graphite has a very high melting point (and boiling point) and is insoluble. To melt or dissolve graphite would require the layers to be broken down, and this means large numbers of very strong covalent bonds have to be broken requiring lots of energy. Sodium Chloride a typical giant ionic lattice Sodium chloride has a 3D structure in which sodium ions and chloride ions alternate. The electrostatic attraction between the ions is nondirectional so every sodium ion attracts chloride ions all around it above, below and on each side. Similarly each chloride ion attracts six sodium ions. The formula NaCl does not indicate that sodium and chloride ions "go around in pairs", but that there is a ratio of one sodium ion for every chloride ion in the lattice it is an empirical formula. Because the electrostatic attractions between the oppositely-charged ions run throughout the lattice, sodium chloride is hard and brittle. These attractions are strong, and must be overcome before the lattice can be broken down to form a liquid, which requires a lot of energy. Sodium chloride thus has a high melting and boiling point. Sodium chloride dissolves in polar solvents such as water. The energy needed to break down the lattice so the ions can be in solution is offset by the energy released when bonds are formed between the ions and the water molecules. Once in solution (or melted), the sodium and chloride ions are free to move around. They are charge carriers, so in these states sodium chloride is able to conduct electricity. When solid the ions are locked in place in the lattice and although they are charged, they are unable to move and carry the electrical charge. This means that solid sodium chloride does not conduct electricity.
F321 Structure Case Studies Iodine a simple molecular lattice Iodine has a simple molecular structure. Iodine atoms are bonded in pairs by covalent bonds, so the formula is I2. In the solid state, the weak intermolecular forces between the iodine molecules hold them together in a 3D lattice structure. This is a simple molecular lattice. There are no delocalized electrons in iodine, and no ions, so there are no charge carriers and iodine cannot conduct electricity. Iodine has the unusual property that when heated it goes directly from being solid to being a gas. This property is called sublimation. It does this at a low temperature (114C) because only weak intermolecular forces hold the iodine molecules together and these take little energy to overcome. Iodine vapour still consists of I2 molecules no bonds have been broken in turning iodine into a gas. Being non-polar iodine dissolves readily in most non-polar organic solvents such as hexane or trichloromethane. It is almost completely insoluble in water because to dissolve in water the hydrogen bonds between the water molecules have to be broken, and there are no new bonds formed between iodine and the water so the energy cannot be offset by any released energy. Ice another simple molecular lattice Ice consists water molecules held in a 3D lattice by a network of hydrogen bonds. The two lone pairs on the oxygen atom in each water molecule form hydrogen bonds to the hydrogen atoms of two other water molecules forming a 3D network of hydrogen bonds. The hydrogen bonds do not take anywhere as much energy to overcome as covalent bonds would, so the temperature required to break down the simple molecular lattice in ice is much lower than the temperature required to melt giant covalent lattices.
Das könnte Ihnen auch gefallen
- Covalent StructuresDokument11 SeitenCovalent StructuresjtNoch keine Bewertungen
- Clays and Clay Minerals: Proceedings of the Fourteenth National Conference, Berkeley, CaliforniaVon EverandClays and Clay Minerals: Proceedings of the Fourteenth National Conference, Berkeley, CaliforniaS. W. BaileyNoch keine Bewertungen
- Water As A Polar MoleculeDokument13 SeitenWater As A Polar MoleculeAyaan Asim KhanNoch keine Bewertungen
- Elements in Period 3Dokument13 SeitenElements in Period 3FAthiyah Abdul RahimNoch keine Bewertungen
- Properties of Sea WaterDokument30 SeitenProperties of Sea WaterHalima akterNoch keine Bewertungen
- Primary and Secondary CellsDokument20 SeitenPrimary and Secondary CellsVinayKumarNoch keine Bewertungen
- Edexcel IGCSE Chemistry Topic 2: Inorganic ChemistryDokument4 SeitenEdexcel IGCSE Chemistry Topic 2: Inorganic ChemistryVenant HakizimanaNoch keine Bewertungen
- 10-Reactivity of Metals and Displacement ReactionsDokument3 Seiten10-Reactivity of Metals and Displacement ReactionsNkemzi Elias NzetengenleNoch keine Bewertungen
- Bonding in SolidsDokument31 SeitenBonding in SolidsReddyvari VenugopalNoch keine Bewertungen
- The Periodic TableDokument33 SeitenThe Periodic TableIra MunirahNoch keine Bewertungen
- Halogens Information SheetDokument4 SeitenHalogens Information Sheetmallika29Noch keine Bewertungen
- Metals and NonmetalsDokument37 SeitenMetals and NonmetalsLeila PascuaNoch keine Bewertungen
- Voltaic CellDokument32 SeitenVoltaic CellMahijar Jarullhayati Hassan50% (2)
- Metals and Their Properties - Physical and ChemicalDokument5 SeitenMetals and Their Properties - Physical and Chemicalcourtz911Noch keine Bewertungen
- CHM 221 Lecture Note 2022-2023Dokument18 SeitenCHM 221 Lecture Note 2022-2023Olanrewaju Omowunmi GraceNoch keine Bewertungen
- Group 1 - The Alkali Metals Worksheet: 4li(s) + O (G) 2li O(s)Dokument3 SeitenGroup 1 - The Alkali Metals Worksheet: 4li(s) + O (G) 2li O(s)Vaida MatulevičiūtėNoch keine Bewertungen
- Chapter 6b Electrolysis of Aqueous SolutionDokument16 SeitenChapter 6b Electrolysis of Aqueous SolutionKavitha ThayagarajanNoch keine Bewertungen
- Introduction to MO theory for simple moleculesDokument4 SeitenIntroduction to MO theory for simple moleculesBheim LlonaNoch keine Bewertungen
- 11 Introduction To Engineering MaterialsDokument20 Seiten11 Introduction To Engineering MaterialsomkardashetwarNoch keine Bewertungen
- Ionic Bonding 4. Bonding: Evidence For The Existence of IonsDokument9 SeitenIonic Bonding 4. Bonding: Evidence For The Existence of IonsAnastasia ErshNoch keine Bewertungen
- Models of The AtomDokument13 SeitenModels of The AtomAbhinav TripathiNoch keine Bewertungen
- Energi Kisi Dan Born HaberDokument31 SeitenEnergi Kisi Dan Born HaberNovi CherlyNoch keine Bewertungen
- Structure and BondingDokument12 SeitenStructure and BondingNisha JodhanNoch keine Bewertungen
- GCSE Chemistry Guide to Chemical BondingDokument9 SeitenGCSE Chemistry Guide to Chemical BondingSabsNoch keine Bewertungen
- Chapter 19 Review QuestionsDokument49 SeitenChapter 19 Review QuestionshihiorigamipandaNoch keine Bewertungen
- Patterns in Period 3 ElementsDokument18 SeitenPatterns in Period 3 ElementsDania Dobbs100% (2)
- Group IV A CompleteDokument64 SeitenGroup IV A Completeshazi5250Noch keine Bewertungen
- Chemistry Form 4 Chapter 9Dokument23 SeitenChemistry Form 4 Chapter 9Ng Wan LinNoch keine Bewertungen
- Kirkendall Effect, Importance, and Theory, Its Use: HistoryDokument2 SeitenKirkendall Effect, Importance, and Theory, Its Use: HistoryAbu HurairaNoch keine Bewertungen
- CY1001 Exam and Course DetailsDokument18 SeitenCY1001 Exam and Course DetailsutkarshNoch keine Bewertungen
- YT Structure of The AtomDokument72 SeitenYT Structure of The AtomAbhishek Kumar100% (1)
- Ch-27.1 Basic Concepts On Structure of SolidsDokument39 SeitenCh-27.1 Basic Concepts On Structure of SolidsVinit Khaiwal100% (1)
- 4 Group 17 Elements UpdatedDokument8 Seiten4 Group 17 Elements Updatedkarim100% (1)
- Primary and Secondary Cells PPDokument12 SeitenPrimary and Secondary Cells PPJavier DavisNoch keine Bewertungen
- Year 10 Physics LDRs and Thermistors PDF v2Dokument12 SeitenYear 10 Physics LDRs and Thermistors PDF v2sweet.miyuxNoch keine Bewertungen
- 6 4IonizEnergyDokument1 Seite6 4IonizEnergyShehbaz YaseenNoch keine Bewertungen
- PeriodicityDokument6 SeitenPeriodicityHadi AlnaherNoch keine Bewertungen
- Introduction To Molecular Orbital TheoryDokument17 SeitenIntroduction To Molecular Orbital TheoryGeoorge VouyiouklakisNoch keine Bewertungen
- Amorphous MetalDokument5 SeitenAmorphous MetalTeka KamNoch keine Bewertungen
- 4.0 Solid-State Nucleation and Growth PDFDokument17 Seiten4.0 Solid-State Nucleation and Growth PDFLEONARD NYIRONGONoch keine Bewertungen
- D Block ElementsDokument22 SeitenD Block Elementsketan kambleNoch keine Bewertungen
- Chemistry Project 2016Dokument20 SeitenChemistry Project 2016Divya KumawatNoch keine Bewertungen
- Crystal DefectsDokument3 SeitenCrystal DefectsZa GurlzNoch keine Bewertungen
- HalogensDokument3 SeitenHalogensselvabala_Noch keine Bewertungen
- Coordination NumberDokument11 SeitenCoordination NumberSyed Qasim ShahNoch keine Bewertungen
- Hydrothermal Process PPT 1Dokument22 SeitenHydrothermal Process PPT 1Awais AhmadNoch keine Bewertungen
- F321 Group 7Dokument5 SeitenF321 Group 7Doc_CrocNoch keine Bewertungen
- Writing Half Equations Worksheet ClassDokument1 SeiteWriting Half Equations Worksheet ClassJasmine YenNoch keine Bewertungen
- Molecular Orbital TheoryDokument6 SeitenMolecular Orbital TheoryleejhNoch keine Bewertungen
- ElectrolysisDokument31 SeitenElectrolysisteddaboyNoch keine Bewertungen
- 10 Science Notes 03 Metals and Non Metals 1Dokument10 Seiten10 Science Notes 03 Metals and Non Metals 1Rishu KaulNoch keine Bewertungen
- Properties of Metals and AlloysDokument19 SeitenProperties of Metals and AlloysIlhamsidiqNoch keine Bewertungen
- Ionisation Energy: AS ChemistryDokument15 SeitenIonisation Energy: AS Chemistry吴蔓华Noch keine Bewertungen
- Energy Band Theory ExplainedDokument4 SeitenEnergy Band Theory ExplainedDerbew Gahaw100% (1)
- Metals and Non-MetalsDokument11 SeitenMetals and Non-MetalsRoty005100% (4)
- Chapter Polymerisation MethodDokument56 SeitenChapter Polymerisation MethodwaniNoch keine Bewertungen
- Describe The Differences in Volatility, Solubility and Electrical Conductivity Between Ionic and Covalent CompoundsDokument6 SeitenDescribe The Differences in Volatility, Solubility and Electrical Conductivity Between Ionic and Covalent Compoundsmadhuri pawarNoch keine Bewertungen
- Solid State 6 JulyDokument18 SeitenSolid State 6 JulyQwertyNoch keine Bewertungen
- Ch3 - Chemical Bonding (IGCSE Study Notes)Dokument11 SeitenCh3 - Chemical Bonding (IGCSE Study Notes)Amal HassanNoch keine Bewertungen
- Formulae and Oxidation NumbersDokument14 SeitenFormulae and Oxidation NumbersDoc_CrocNoch keine Bewertungen
- Organic BasicsDokument10 SeitenOrganic BasicsDoc_CrocNoch keine Bewertungen
- HaloalkanesDokument6 SeitenHaloalkanesDoc_CrocNoch keine Bewertungen
- Organic BasicsDokument10 SeitenOrganic BasicsDoc_CrocNoch keine Bewertungen
- Formulae and Oxidation NumbersDokument14 SeitenFormulae and Oxidation NumbersDoc_CrocNoch keine Bewertungen
- AlcoholsDokument8 SeitenAlcoholsDoc_CrocNoch keine Bewertungen
- Rates of ReactionDokument7 SeitenRates of ReactionDoc_CrocNoch keine Bewertungen
- Qualitative AnalysisDokument3 SeitenQualitative AnalysisDoc_CrocNoch keine Bewertungen
- Acid Bases Salts and Reacting QuantitiesDokument11 SeitenAcid Bases Salts and Reacting QuantitiesDoc_CrocNoch keine Bewertungen
- AlkenesDokument12 SeitenAlkenesDoc_CrocNoch keine Bewertungen
- Instrumental AnalysisDokument9 SeitenInstrumental AnalysisDoc_Croc100% (2)
- The HalogensDokument5 SeitenThe HalogensDoc_Croc100% (1)
- Synthesis and SustainabilityDokument6 SeitenSynthesis and SustainabilityDoc_CrocNoch keine Bewertungen
- HaloalkanesDokument6 SeitenHaloalkanesDoc_CrocNoch keine Bewertungen
- AlkanesDokument5 SeitenAlkanesDoc_CrocNoch keine Bewertungen
- Organic BasicsDokument10 SeitenOrganic BasicsDoc_CrocNoch keine Bewertungen
- Chemical EquilibriumDokument7 SeitenChemical EquilibriumDoc_CrocNoch keine Bewertungen
- Group 2Dokument3 SeitenGroup 2Doc_CrocNoch keine Bewertungen
- Formulae and Oxidation NumbersDokument14 SeitenFormulae and Oxidation NumbersDoc_CrocNoch keine Bewertungen
- Rates of ReactionDokument6 SeitenRates of ReactionDoc_CrocNoch keine Bewertungen
- Qualitative AnalysisDokument3 SeitenQualitative AnalysisDoc_CrocNoch keine Bewertungen
- Acid Bases Salts and Reacting QuantitiesDokument11 SeitenAcid Bases Salts and Reacting QuantitiesDoc_CrocNoch keine Bewertungen
- Enthalpy ChangesDokument17 SeitenEnthalpy ChangesDoc_Croc100% (1)
- Periodic Trends and PatternsDokument5 SeitenPeriodic Trends and PatternsDoc_CrocNoch keine Bewertungen
- Structure and ShapesDokument10 SeitenStructure and ShapesDoc_CrocNoch keine Bewertungen
- Forces Between Atoms and MoleculesDokument13 SeitenForces Between Atoms and MoleculesDoc_CrocNoch keine Bewertungen
- Electronic Structure and IonisationDokument9 SeitenElectronic Structure and IonisationDoc_CrocNoch keine Bewertungen
- Atomic Structure Isotopes and MolesDokument11 SeitenAtomic Structure Isotopes and MolesDoc_CrocNoch keine Bewertungen
- Atomic Structure Isotopes and MolesDokument11 SeitenAtomic Structure Isotopes and MolesDoc_CrocNoch keine Bewertungen
- 165 Gmaw Zug Asme (Imam Mustofa 3g) WPQDokument4 Seiten165 Gmaw Zug Asme (Imam Mustofa 3g) WPQMuhammad Fitransyah Syamsuar PutraNoch keine Bewertungen
- Measurement of Conductance and Kohlrauch's LawDokument23 SeitenMeasurement of Conductance and Kohlrauch's Lawknowledge of sciencesNoch keine Bewertungen
- Presentation On White RustDokument12 SeitenPresentation On White RustapparaomsNoch keine Bewertungen
- THG Hooks Forged Hooks Catalogue SheetDokument5 SeitenTHG Hooks Forged Hooks Catalogue SheetjhonNoch keine Bewertungen
- Gypsum in CementDokument2 SeitenGypsum in CementManish Kumar100% (1)
- Tenax 88s HR (E 50 6 Mn1ni B 32 h5)Dokument1 SeiteTenax 88s HR (E 50 6 Mn1ni B 32 h5)brunizzaNoch keine Bewertungen
- Vapour Pressure LabDokument8 SeitenVapour Pressure LabTuqeer MuhammadNoch keine Bewertungen
- A New Technique of Processing For Waste-Expanded Polystyrene Foams As AggregatesDokument7 SeitenA New Technique of Processing For Waste-Expanded Polystyrene Foams As Aggregatestimtoihochoi1Noch keine Bewertungen
- Naming CompoundsDokument84 SeitenNaming CompoundsangelaNoch keine Bewertungen
- Stellite 6 Datasheet PDFDokument2 SeitenStellite 6 Datasheet PDFEswaran100% (1)
- Feedwater System 2Dokument45 SeitenFeedwater System 2ika yuliyani murtiharjonoNoch keine Bewertungen
- Eam of Analysis EditedDokument140 SeitenEam of Analysis EditedAvishek KumarNoch keine Bewertungen
- Lubricantes JCBDokument24 SeitenLubricantes JCBNoe GarciaNoch keine Bewertungen
- Benedicto, Capstone Mod 12-13Dokument19 SeitenBenedicto, Capstone Mod 12-13Roy Benedicto Jr.Noch keine Bewertungen
- KS4 Reversible ReactionsDokument25 SeitenKS4 Reversible ReactionsAisha KhanNoch keine Bewertungen
- OringmatDokument39 SeitenOringmatRoby MastreNoch keine Bewertungen
- Module 1Dokument14 SeitenModule 1Harsha MayankNoch keine Bewertungen
- SMAW Welding Guide for 4130 SteelDokument39 SeitenSMAW Welding Guide for 4130 SteelSwapnil ModakNoch keine Bewertungen
- Importance of nucleic acidsDokument2 SeitenImportance of nucleic acidsMyla Angelica AndresNoch keine Bewertungen
- Chem 31 PROCEDURES (Practicals)Dokument9 SeitenChem 31 PROCEDURES (Practicals)FMDCNoch keine Bewertungen
- Chemical Equilibrium and Le Chatelier's Principle: Chemistry 1Dokument17 SeitenChemical Equilibrium and Le Chatelier's Principle: Chemistry 1azamatNoch keine Bewertungen
- CIE IGCSE Resistant Materials GuideDokument23 SeitenCIE IGCSE Resistant Materials GuidekevinNoch keine Bewertungen
- Choosing the Right Fire Extinguisher for Your NeedsDokument2 SeitenChoosing the Right Fire Extinguisher for Your Needsfaisalhotline9500Noch keine Bewertungen
- Experiment 6 - Comparative Investigation of Organic CompoundsDokument6 SeitenExperiment 6 - Comparative Investigation of Organic CompoundsIson Dy100% (2)
- Thermal EOR Methods GuideDokument42 SeitenThermal EOR Methods GuidePetroleum PetroleumNoch keine Bewertungen
- The Role of Light and Carbon Dioxide in PhotosynthesisDokument10 SeitenThe Role of Light and Carbon Dioxide in PhotosynthesisEPHRAIM JOASH ABEJO GAGANTINGNoch keine Bewertungen
- Chem 229 Problem Set 6Dokument2 SeitenChem 229 Problem Set 6Su KelesogluNoch keine Bewertungen
- Litesse IDokument1 SeiteLitesse IAnthonyPonceParedesNoch keine Bewertungen
- BOD Full ReportDokument11 SeitenBOD Full ReportAhmad Farid75% (4)