Beruflich Dokumente
Kultur Dokumente
Biology I Notes
Hochgeladen von
Andrea Ebelechukwu EkeyOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Biology I Notes
Hochgeladen von
Andrea Ebelechukwu EkeyCopyright:
Verfügbare Formate
10/12/11 enzymes are catalysts - allow reactions to occur faster in biological systems enzyme is a type of protein substrate binds
nds to enzyme enzyme-substrate complex formed reaction occurs and product is formed catalysts lower activation energy - less energy needs to be put in to allow reaction to occur
catalysts do not affect net change in free energy (G) - G for catalysed and uncatalysed reactions are the same enzymes position reactants so that they can react to form specific products enzymes used to synthesise/break down substances enzymes can add/remove charges (+) and (-) oxidation - loss of electrons (oxidised molecule loses the energy contained by electrons) reduction - gain of electrons (reduced molecules gain energy contained by electrons) enzyme conformation changes when substrate binds cofactors, coenzymes, prosthetic groups help enzymes function cofactors - metal ions coenzymes - many vitamins act as coenzymes, loosely bound to enzyme prosthetic groups - covalently bonded to enzyme (permanently integrated into enzyme) reaction rate sped up by presence of enzymes reaction rate sped up by higher substrate levels to a limit - limited by amount of enzyme available, natural systems have a limited amount of enzyme enzyme reactions do not occur in isolation - different metabolic systems present within living systems all living systems metabolism is catalysed by enzymes reactions is biological systems controlled by amount of enzyme, inhibition - genes control enzyme production, enzyme can be directly modified irreversible inhibition - a molecule binds to the active site and blocks the substrate, renders enzyme permanently unusable eg. DIPF inhibits trypsin enzyme, carbon monoxide poisoning competitive inhibition - competitive inhibitor binds to active site and blocks substrate, reversible noncompetitive inhibition - noncompetitive inhibitor binds to allosteric site and changes shape of enzyme and active site to block substrate allosteric site - non-active site area of enzyme
10/14/11
to overcome competitive inhibitor, increase concentration of substrate some enzymes are regulated by binding of inhibitor/activator molecules to allosteric site inactive without allosteric activator bound to enzyme feedback inhibition - the end product of a pathway is used as allosteric inhibitor for an enzyme in the pathway
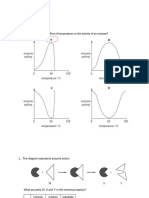
optimal pH - enzymes operate most efficiently at certain pH levels eg. pepsin (in stomach) has very acidic optimal pH optimal temperature - reaction rate increases as temperature increases until optimal temperature is reached, any temperature above optimal causes protein to begin denaturation temperature vs reaction rate graph is left-skewed distribution Chapter 9 - Cellular Pathways that Harness Chemical Energy glucose metabolised so cells can trap free energy in the form of ATP sun photosynthesis glucose glycolysis pyruvate cellular respiration (aerobic respiration) or fermentation (anaerobic respiration) aerobic (requiring oxygen) respiration is more efficient in trapping energy for use larger, higher order organisms (eg. humans) use this process
anaerobic (no requiring oxygen) respiration produces less ATP to use for work smaller, simpler organisms tend to use this process oxidation and reduction are coupled - redox reactions NAD+ is an oxidising agent (gains and carries electrons), NADH is a reducing agent (loses and passes on electrons) - cyclic process 10/17/11 reduction: NAD+ + 2 H NADH + H+ oxidation: NADH + H+ NAD+ + 2 H respiration in eukaryotes - glycolysis and fermentation occur in cytoplasm, citric acid cycle and pyruvate oxidation in mitochondrial matrix respiration in prokaryotes - glycolysis, fermentation, citric acid cycle occur in cytoplasm, pyruvate oxidation and respiratory chain associated with inner face of plasma membrane glycolysis and cellular respiration: glycolysis (glucose pyruvate) pyruvate oxidation citric acid cycle respiratory chain glycolysis and fermentation: glycolysis (glucose pyruvate) fermentation lactate or alcohol facultative anaerobes (eg. yeast) can use fermentation and cellular respiration overall cellular respiration reaction: C6H12O6(glucose) + 6 O2 6 CO2 + 6 H2O
energy-investing reactions in cellular respiration: Glycolysis enzymes and energy from 2 ATP used to break down glucose into G3P (glyceraldehyde 3-phosphate aka PGAL) G3P oxidised by NAD+ (NADH produced carries electrons to electron transport chain) to form BPG, BPG used to from 2 ATP and PGA (3-Phosphoglycerate aka PGA) PGA converted to PEP and 2 water molecules produced 2 ATP produced in conversion of PEP to pyruvate note: kinase enzyme produces ATP outside of mitochondria - substrate-level phosphorylation Pyruvate oxidation pyruvate oxidised by NAD+ to form Acetyl CoA (acetyl-conenzyme A) Citric acid cycle acetyl CoA used in citric acid cycle to produce NADH and FADH2 (electron carriers), H+, CO2 and 2 ATP - cycle occurs twice to oxidise 1 glucose 10/19/11
NADH formed by hydride ion reduction coenzyme A detached from molecules when it has served its purpose GTP is produced and converted to ATP for use Electron transport chain proteins embedded in inner mitochondrial membrane accept electrons carried by NADH and FADH2 (which become NAD+, FAD) and pass them along to a terminal electron acceptor oxygen is terminal electron acceptor - O2 reduced by electron transport chain (ETC) to form water, ETC cannot function without oxygen oxygen can accept electrons from ETC because of cytochrome c oxidase protons stripped from NADH and FADH2 - protons (H+) pumped out of matrix into intermembrane space, H2O produced within matrix ATP synthesised via chemiosmosis - higher concentration of H+ in intermembrane space (due to ETC proton pumps) causes H+ to diffuse across inner mitochondrial membrane H+ diffuses through ATP synthase embedded in inner membrane, chemiosmosis powers ATP production
experiment in textbook showed that proton (H+) gradient drives ATP synthesis another experiment showed that ATP synthase is necessary for ATP synthesis Anaerobic respiration (fermentation) glycolysis occurs to for 2 pyruvate molecules from 1 glucose molecule fermentation produces lactic acid or ethanol (alcohol) constant supply of NAD+ needed to sustain fermentation CO2 produced in alcohol fermentation Aerobic vs Anaerobic efficiency - 32 ATP produced in cellular respiration, 2 ATP produced in fermentation
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5784)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (72)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Characterization of Carbohydrates (Formal Report)Dokument11 SeitenCharacterization of Carbohydrates (Formal Report)Man Dejelo100% (6)
- Milton Noguiera Da Silva Junior, Master Thesis in Applied Mathematics at Leiden UniversityDokument275 SeitenMilton Noguiera Da Silva Junior, Master Thesis in Applied Mathematics at Leiden UniversityMilton Nogueira da Silva JuniorNoch keine Bewertungen
- LadmerDokument58 SeitenLadmerMeah Pacheco100% (1)
- WFCF Ingredient FormDokument4 SeitenWFCF Ingredient Formmeli meliNoch keine Bewertungen
- Flow Chart Anatomy and PhysiologyDokument1 SeiteFlow Chart Anatomy and PhysiologyAvinashNoch keine Bewertungen
- Approved Curriculam BS-Dental Technology Feb.-2017. 1Dokument57 SeitenApproved Curriculam BS-Dental Technology Feb.-2017. 1Aman WazirNoch keine Bewertungen
- Biochemistry Concepts and Connections 1st Edition Appling Solutions ManualDokument7 SeitenBiochemistry Concepts and Connections 1st Edition Appling Solutions Manualscarletwilliamnfz100% (30)
- Transgenic Cattle: Methods of Production and ApplicationsDokument3 SeitenTransgenic Cattle: Methods of Production and ApplicationsSunitha MachirajuNoch keine Bewertungen
- Casarotto Et Al. 2021Dokument35 SeitenCasarotto Et Al. 2021Valeria PNoch keine Bewertungen
- Macromolecules labster resultsDokument2 SeitenMacromolecules labster resultsAnita AmaoNoch keine Bewertungen
- Protein - Britannica Online Encyclopedia PDFDokument46 SeitenProtein - Britannica Online Encyclopedia PDFanant mishraNoch keine Bewertungen
- CisgenesisDokument61 SeitenCisgenesissaurabh784100% (1)
- Enzyme Lab - Effect of PHDokument2 SeitenEnzyme Lab - Effect of PHWalwin HareNoch keine Bewertungen
- IB Biology SL - Working With DataDokument68 SeitenIB Biology SL - Working With DataShelley Lima100% (1)
- Pharmacology I Lecture 2 (Antibiotics) : Protein Synthesis InhibitorsDokument40 SeitenPharmacology I Lecture 2 (Antibiotics) : Protein Synthesis Inhibitorsعلي الفواديNoch keine Bewertungen
- What Is Resting Membrane Potential?: Mechanism of An Action PotentialDokument5 SeitenWhat Is Resting Membrane Potential?: Mechanism of An Action PotentialAli InamNoch keine Bewertungen
- 9700 Scheme of Work (For Examination From 2022) - 3Dokument1 Seite9700 Scheme of Work (For Examination From 2022) - 3DuckyNoch keine Bewertungen
- Bubble Lab ExerciseDokument9 SeitenBubble Lab ExerciseRic Anthony LayasanNoch keine Bewertungen
- Bisgop EnxymeDokument28 SeitenBisgop EnxymefjkgldjfNoch keine Bewertungen
- Chapter 8Dokument39 SeitenChapter 8Jennifer GillNoch keine Bewertungen
- Bulletin 7475Dokument516 SeitenBulletin 7475sylvi293Noch keine Bewertungen
- A1.2 2025 Topic Test v3Dokument5 SeitenA1.2 2025 Topic Test v3valentinaNoch keine Bewertungen
- LAB2FINALDokument7 SeitenLAB2FINALAldren BeliberNoch keine Bewertungen
- Enzymes Classified Past Paper 2 Solved GCSE O Levels Biology 0610Dokument40 SeitenEnzymes Classified Past Paper 2 Solved GCSE O Levels Biology 0610IGCSE Physics & Chemistry100% (1)
- Lecture III-2 DNA and Chromosome StructuresDokument21 SeitenLecture III-2 DNA and Chromosome StructuresJÜnn BatacNoch keine Bewertungen
- Lipid Profile L - DeterminatinDokument33 SeitenLipid Profile L - DeterminatinaliNoch keine Bewertungen
- Curcumin As A Promising Neuroprotective Agent For The Treatment of Spinal Cord Injury: A Review of The LiteratureDokument13 SeitenCurcumin As A Promising Neuroprotective Agent For The Treatment of Spinal Cord Injury: A Review of The LiteratureEna BagarićNoch keine Bewertungen
- Mitochondria in Health and DiseasesDokument436 SeitenMitochondria in Health and DiseasesRoberta MorganaNoch keine Bewertungen
- Glycogen Metabolism.Dokument49 SeitenGlycogen Metabolism.Santino MajokNoch keine Bewertungen
- Plasma Lipoproteins - METABOLISM PDFDokument4 SeitenPlasma Lipoproteins - METABOLISM PDFRishabhNoch keine Bewertungen