Beruflich Dokumente
Kultur Dokumente
Drug Study
Hochgeladen von
John AlcantaraCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Drug Study
Hochgeladen von
John AlcantaraCopyright:
Verfügbare Formate
DRUG STUDY BRAND NAME: Garamycin, Garamycin Ophthalmic, Genoptic Classifications: antiinfective; aminoglycoside antibiotic Action: Broad-spectrum aminoglycoside
antibiotic derived from Micromonospora purpurea. Action is usually bacteriocidal. Indication: Parenteral use restricted to treatment of serious infections of GI, respiratory, and urinary tracts, CNS, bone, skin, and soft tissue (including burns) when other less toxic antimicrobial agents are ineffective or are contraindicated. Has been used in combination with other antibiotics. Also used topically for primary and secondary skin infections and for superficial infections of external eye and its adnexa. Dosage and Route: Moderate to Severe Infection Adult: IV/IM 1.52 mg/kg loading dose followed by 35 mg/kg/d in 23 divided doses Intrathecal 48 mg preservative free q.d. Topical 12 drops of solution in eye q4h up to 2 drops q1h or small amount of ointment b.i.d. or t.i.d. Child: IV/IM 67.5 mg/kg/d in 34 divided doses Intrathecal >3 mo, 12 mg preservative free q.d. Neonate: IV/IM 2.5 mg/kg q1224h Acute Pelvic Inflammatory Disease Adult: IV/IM 2 mg/kg followed by 1.5 mg/kg q8h Prophylaxis of Bacterial Endocarditis Adult: IV/IM 1.5 mg/kg 30 min before procedure, may repeat in 8 h Child: IV/IM < 27 kg, 2 mg/kg 30 min before procedure, may repeat in 8 h Adverse Effects: Special Senses: Ototoxicity (vestibular disturbances, impaired hearing), optic neuritis. CNS: neuromuscular blockade: skeletal muscle weakness, apnea, respiratory paralysis (high doses); arachnoiditis (intrathecal use). CV: hypotension or hypertension. GI: Nausea, vomiting, transient increase in AST, ALT, and serum LDH and bilirubin; hepatomegaly, splenomegaly. Hematologic: Increased or decreased reticulocyte counts; granulocytopenia, thrombocytopenia (fever, bleeding tendency), thrombocytopenic purpura, anemia. Body as a Whole: Hypersensitivity (rash, pruritus, urticaria, exfoliative dermatitis, eosinophilia, burning sensation of skin, drug fever, joint pains, laryngeal edema, anaphylaxis). Urogenital: Nephrotoxicity: proteinuria, tubular necrosis, cells or casts in urine, hematuria, rising BUN, nonprotein nitrogen, serum creatinine; decreased creatinine clearance. Other: Local irritation and pain following IM use; thrombophlebitis, abscess, superinfections, syndrome of hypocalcemia (tetany, weakness, hypokalemia, hypomagnesemia).
Nursing Responsibility: Assessment & Drug Effects Lab tests: Perform C&S and renal function prior to first dose and periodically during therapy; therapy may begin pending test results. Determine creatinine clearance and serum drug concentrations at frequent intervals, particularly for patients with impaired renal function, infants (renal immaturity), older adults, patients receiving high doses or therapy beyond 10 d, patients with fever or extensive burns, edema, obesity. Repeat C&S if improvement does not occur in 35 d; reevaluate therapy. Note: Dosages are generally adjusted to maintain peak serum gentamicin concentrations of 4 10 g/mL, and trough concentrations of 12 g/mL. Peak concentrations above 12 g/mL and trough concentrations above 2 g/mL are associated with toxicity. Draw blood specimens for peak serum gentamicin concentration 30 min1h after IM administration, and 30 min after completion of a 3060 min IV infusion. Draw blood specimens for trough levels just before the next IM or IV dose. Use nonheparinized tubes to collect blood.
Brand Name: Erceflora Classification: Antidiarrheals Suggested Dose: Adults 2-3 vials of 2 billion/5 mL susp Children 2-11 years 1-2 vials of 2 billion/5 mL susp Infants >1 month 1-2 vials of 2 billion/5 mL susp. Mode of Action: Contributes to the recovery of the intestinal microbial flora altered during the course of microbial disorders of diverse origin. It produces various vitamins, particularly group B vitamins thus contributing to correction of vitamin disorders caused by antibiotics & chemotherapeutic agents. Promotes normalization of intestinal flora. Indication: Acute diarrhea with duration of 14 days due to infection, drugs or poisons. Chronic or persistent diarrhea with duration of >14 days. Contraindication: Not for use in immunocompromised patients (cancer patients on chemotherapy, patients taking immunosuppressant meds) Drug Interaction: No known drug interactions. Side Effects/Adverse Reactions: No known side effects.
Adverse Effects: No known adverse effects. Nursing Responsibility: 1.) Shake drug well before administration. Allows equal distribution of the drug in the fluid it is in. 2.) Monitor patient for any unusual effects from drug. Monitoring allows detection of possible side effects of the drug since there has been no known side effect of the drug. 3.) Administer drug within 30 minutes after opening container. To avoid contamination of the drug. 4.) Dilute drug with sweetened milk, orange juice or tea. To allow easy administration of the drug. 5.) Administer drug orally. Proper administration allows better effects of the drug and prevent possible complications Bacillus clausii is Gram-positive, motile, spore-forming and like most of the Bacillus bacteria, it is rod-shaped. Colonies of B. clausii form filamentous margins that appear cream-white in color. B. clausii is alkaliphilic and produces a class of subtilisins known as high-alkaline proteases. The protease from Bacillus clausii strain 221, the H-221 protease, was the first enzyme to be identified in an alkaliphilic Bacillus. The alkaliphilic nature of the organism has also proved it to be useful in preventing and treating various gastrointestinal disorders as an oral bacteriotherapy. This organism can be found in many alkaline environments, including soil and marine habitat.
Unasyn
Das könnte Ihnen auch gefallen
- Naplex Complete Study Outline A Topic-Wise Approach DiabetesVon EverandNaplex Complete Study Outline A Topic-Wise Approach DiabetesBewertung: 4 von 5 Sternen4/5 (2)
- Drug Study Gentamicin Sulfate and SalbutamolDokument7 SeitenDrug Study Gentamicin Sulfate and SalbutamolEduardNoch keine Bewertungen
- Urinary Germicides PharmaDokument11 SeitenUrinary Germicides PharmaMaria Pina Barbado PonceNoch keine Bewertungen
- MEDICATION: Garamycin, Garamycin Ophthalmic, Genoptic Classifications: Antiinfective Aminoglycoside Antibiotic ActionDokument4 SeitenMEDICATION: Garamycin, Garamycin Ophthalmic, Genoptic Classifications: Antiinfective Aminoglycoside Antibiotic ActionLea Mae HandayanNoch keine Bewertungen
- GentamicinDokument1 SeiteGentamicinCharm HopeNoch keine Bewertungen
- Drug StudyDokument7 SeitenDrug StudyPeetah PanNoch keine Bewertungen
- Amikacin SulfateDokument4 SeitenAmikacin SulfateCay SevillaNoch keine Bewertungen
- Drug StudyDokument10 SeitenDrug StudybaniniycsebNoch keine Bewertungen
- Rceflora: Admin Drug StudyDokument2 SeitenRceflora: Admin Drug StudyJudy Abella SazonNoch keine Bewertungen
- Antibiotic Penicillin: Ampicillin Ampicillin Sodium AmpicillinDokument3 SeitenAntibiotic Penicillin: Ampicillin Ampicillin Sodium AmpicillinMaria Delia SalvadoNoch keine Bewertungen
- Name of The Patient: Age: DiagnosisDokument5 SeitenName of The Patient: Age: DiagnosisMa Tesalonica GedaNoch keine Bewertungen
- Drug Study (DR)Dokument19 SeitenDrug Study (DR)09159054476Noch keine Bewertungen
- Nursing Considerations Assessment: History: Infections Kidney Disease Liver Disease, Hypothyroidism UlcerativeDokument5 SeitenNursing Considerations Assessment: History: Infections Kidney Disease Liver Disease, Hypothyroidism UlcerativeSophia limNoch keine Bewertungen
- A Drug Study On: Monaliza J. Lee, RN, MNDokument6 SeitenA Drug Study On: Monaliza J. Lee, RN, MNJeah Bearl AbellarNoch keine Bewertungen
- GaramycinDokument2 SeitenGaramycinjames_delicaNoch keine Bewertungen
- Drug StudyDokument7 SeitenDrug StudysarahtotNoch keine Bewertungen
- Drug StudyDokument5 SeitenDrug StudyDick Morgan FerrerNoch keine Bewertungen
- CLARITHROMYCINDokument3 SeitenCLARITHROMYCINCay SevillaNoch keine Bewertungen
- Name of Drug Classification and Mechanism of Action Indication and Dosage Contraindications Side Effects/ Adverse Reactions Nursing ResponsibilitiesDokument4 SeitenName of Drug Classification and Mechanism of Action Indication and Dosage Contraindications Side Effects/ Adverse Reactions Nursing ResponsibilitiesRizza 이 동해 OcampoNoch keine Bewertungen
- Drugs Acting On The GitDokument29 SeitenDrugs Acting On The GitIsheanesu MugwisiNoch keine Bewertungen
- Drug Study NRMFDokument11 SeitenDrug Study NRMFKristine ReyesNoch keine Bewertungen
- Drug Study Clindamycin, Ipatropium BromideDokument8 SeitenDrug Study Clindamycin, Ipatropium Bromidepaupaulala100% (2)
- Drug StudyDokument7 SeitenDrug StudyGladys NacionNoch keine Bewertungen
- Drug USE Side Effects/ Adverse Effects Nursing ConsiderationsDokument4 SeitenDrug USE Side Effects/ Adverse Effects Nursing ConsiderationsNicole MapiliNoch keine Bewertungen
- TbactDokument7 SeitenTbactVinay KumarNoch keine Bewertungen
- PharmacologyDokument7 SeitenPharmacologyANNIE SHINE MAGSACAYNoch keine Bewertungen
- Drug Study QiDokument7 SeitenDrug Study QiJeremiah Mauricio100% (1)
- Quinocil: Ophthalmic Use: Treatment of Conjunctivitis Caused by Susceptible Strains of Aerobic GramDokument3 SeitenQuinocil: Ophthalmic Use: Treatment of Conjunctivitis Caused by Susceptible Strains of Aerobic GramTallal KhanNoch keine Bewertungen
- 5 Drug StudyDokument14 Seiten5 Drug StudyJennyLapitan100% (2)
- Nursing Care PlanDokument8 SeitenNursing Care PlanVincent QuitorianoNoch keine Bewertungen
- Osmolax: IndicationsDokument23 SeitenOsmolax: IndicationsyeyakNoch keine Bewertungen
- Drug StudyDokument10 SeitenDrug StudyBandana RajpootNoch keine Bewertungen
- Drug StudyDokument6 SeitenDrug StudyNajmah Saaban100% (1)
- Drug Monograph: Generic Name: Trade Name: Drug Class: IndicationsDokument8 SeitenDrug Monograph: Generic Name: Trade Name: Drug Class: IndicationsRawan AlmutairiNoch keine Bewertungen
- Gentamycin: Classification of Antihypertensive DrugsDokument3 SeitenGentamycin: Classification of Antihypertensive DrugsMargo Milad Fahim SaadNoch keine Bewertungen
- Additional Pharma CardsDokument21 SeitenAdditional Pharma CardsBrilie Karl Viray100% (1)
- Topic 2.1: Antibiotics: Unit 2: Anti-Infective MedicationsDokument50 SeitenTopic 2.1: Antibiotics: Unit 2: Anti-Infective MedicationsNirali ParmarNoch keine Bewertungen
- Antifungal AgentsDokument42 SeitenAntifungal AgentsOluwatobi AyomideNoch keine Bewertungen
- DactinomycinDokument4 SeitenDactinomycinKeith MadarangNoch keine Bewertungen
- Drug Study Paracetamol Ambroxol Ascorbic Acid CefuroximeDokument6 SeitenDrug Study Paracetamol Ambroxol Ascorbic Acid CefuroximeJaymark LambinoNoch keine Bewertungen
- Drug Study 1Dokument4 SeitenDrug Study 1bibet_martijaNoch keine Bewertungen
- Generic NameDokument1 SeiteGeneric NameBraezelle YuNoch keine Bewertungen
- Metronidazole: (Me Troe Ni' Da Zole)Dokument4 SeitenMetronidazole: (Me Troe Ni' Da Zole)Wino SantosNoch keine Bewertungen
- Finals PharmaDokument33 SeitenFinals PharmaKristine AnaenNoch keine Bewertungen
- Antifungaldrugs 150519204813 Lva1 App6892Dokument54 SeitenAntifungaldrugs 150519204813 Lva1 App6892Jennifer S ZiegenNoch keine Bewertungen
- Cancer Cell-Specific AgentsDokument5 SeitenCancer Cell-Specific AgentsRomwella May AlgoNoch keine Bewertungen
- Antifungal DrugsDokument43 SeitenAntifungal DrugsMohammed WasimNoch keine Bewertungen
- PHARMACOLOGYDokument37 SeitenPHARMACOLOGYEcquin Capzy100% (1)
- Drug StudyDokument6 SeitenDrug StudyMarielle Denise Tagtag BugtongNoch keine Bewertungen
- Cefazolin AncefDokument4 SeitenCefazolin AncefAmanda La SalaNoch keine Bewertungen
- Drug StudyDokument23 SeitenDrug StudyEdward Baes33% (3)
- General Principles of Clinical ToxicologyDokument34 SeitenGeneral Principles of Clinical ToxicologySigita KazūneNoch keine Bewertungen
- Erce FloraDokument2 SeitenErce FloraRye Anch0% (1)
- Drug Study OrthoDokument17 SeitenDrug Study OrthoMc Crister SilangNoch keine Bewertungen
- Bacillus Clausii (Erceflora)Dokument1 SeiteBacillus Clausii (Erceflora)Rye Anch88% (8)
- T Bact OintmentDokument8 SeitenT Bact OintmentAditya RajNoch keine Bewertungen
- AzithromycinDokument4 SeitenAzithromycinBrittany ClontzNoch keine Bewertungen
- Alert Medical Series: Emergency Medicine Alert I, II, IIIVon EverandAlert Medical Series: Emergency Medicine Alert I, II, IIINoch keine Bewertungen
- OmepronDokument3 SeitenOmepronJohn AlcantaraNoch keine Bewertungen
- Apn2 Case Study DkaDokument25 SeitenApn2 Case Study DkaJohn AlcantaraNoch keine Bewertungen
- NovomixDokument5 SeitenNovomixJohn AlcantaraNoch keine Bewertungen
- NovomixDokument5 SeitenNovomixJohn AlcantaraNoch keine Bewertungen
- Diabetic Ketoacidosis Case StudyDokument6 SeitenDiabetic Ketoacidosis Case StudyJohn AlcantaraNoch keine Bewertungen
- Pathophysiology: Diet Sedentary Lifestyle Familial History of Hypertension Age - Common in 40 and AboveDokument4 SeitenPathophysiology: Diet Sedentary Lifestyle Familial History of Hypertension Age - Common in 40 and AboveJohn AlcantaraNoch keine Bewertungen
- Activity Intolerance Related To Decreased Cardiac Output, Due To EndocarditisDokument4 SeitenActivity Intolerance Related To Decreased Cardiac Output, Due To EndocarditisJohn AlcantaraNoch keine Bewertungen
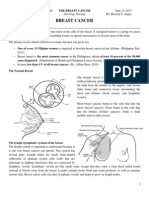
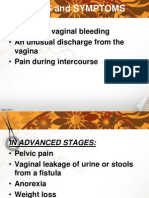
- BREAST CANCER ReportDokument9 SeitenBREAST CANCER ReportJohn AlcantaraNoch keine Bewertungen
- The Mechanical VentilatorDokument28 SeitenThe Mechanical VentilatorJohn AlcantaraNoch keine Bewertungen
- Breast CancerDokument18 SeitenBreast CancerJohn AlcantaraNoch keine Bewertungen
- Ovarian CancerDokument27 SeitenOvarian CancerJohn Alcantara100% (1)
- Presented By: Jan Patrick C. DiezDokument25 SeitenPresented By: Jan Patrick C. DiezJohn AlcantaraNoch keine Bewertungen
- NCP Risk For Fluid Volume DeficitDokument2 SeitenNCP Risk For Fluid Volume DeficitJeanineReyes44% (9)
- Cervical Cancer Powerpoint New EditDokument24 SeitenCervical Cancer Powerpoint New EditJohn AlcantaraNoch keine Bewertungen
- BREAST CANCER ReportDokument9 SeitenBREAST CANCER ReportJohn AlcantaraNoch keine Bewertungen
- Buerger's DiseaseDokument23 SeitenBuerger's DiseaseJohn Alcantara100% (1)
- Drug StudyDokument3 SeitenDrug StudyJohn AlcantaraNoch keine Bewertungen
- Procedure Performed Procedure Performed: Adamson University College of NursingDokument1 SeiteProcedure Performed Procedure Performed: Adamson University College of NursingJohn AlcantaraNoch keine Bewertungen
- Buerger's DiseaseDokument7 SeitenBuerger's DiseaseJohn AlcantaraNoch keine Bewertungen
- Medication Safety ResourcesDokument30 SeitenMedication Safety ResourcesmariaNoch keine Bewertungen
- Grastric Gavage & LavageDokument17 SeitenGrastric Gavage & LavageJuliet Pua74% (19)
- CNUR 304 - Mental HealthDokument91 SeitenCNUR 304 - Mental HealthAbby PohlNoch keine Bewertungen
- CaseDokument4 SeitenCasedvd303Noch keine Bewertungen
- Uworld - PSYCHIATRYDokument24 SeitenUworld - PSYCHIATRYNikxyNoch keine Bewertungen
- Myasthenia Gravis Presentation and Treatment Variations: A Case Study ApproachDokument5 SeitenMyasthenia Gravis Presentation and Treatment Variations: A Case Study ApproachLiyasariNoch keine Bewertungen
- Review of LiteratureDokument29 SeitenReview of Literaturekarthik keyanNoch keine Bewertungen
- Epilepsy in Pregnancy (2) 633 - Hywell Dda Guideline 2022Dokument15 SeitenEpilepsy in Pregnancy (2) 633 - Hywell Dda Guideline 2022michaelwillsonNoch keine Bewertungen
- Glucocorticoid Effects On The Immune System - UpToDateDokument23 SeitenGlucocorticoid Effects On The Immune System - UpToDatedita novia maharani100% (1)
- IMC of Pharam IndustryDokument7 SeitenIMC of Pharam IndustryVAIBHAV SINGHNoch keine Bewertungen
- Malteser-Apotheke: Legal Notice No. 148Dokument22 SeitenMalteser-Apotheke: Legal Notice No. 148Pavalache ClaudioNoch keine Bewertungen
- Seminar Topics (Raju Reddy)Dokument7 SeitenSeminar Topics (Raju Reddy)Saggam RaviNoch keine Bewertungen
- Calcium Channel BlockersDokument2 SeitenCalcium Channel BlockersBrittany RamirezNoch keine Bewertungen
- Obat - Obatan Di Bedah SarafDokument16 SeitenObat - Obatan Di Bedah Sarafditya_madridistasNoch keine Bewertungen
- Jurnal AnestesiDokument5 SeitenJurnal AnestesiridwanNoch keine Bewertungen
- IssuancesDokument347 SeitenIssuancesReia RuecoNoch keine Bewertungen
- Lecture 16-17 - Opioids AnalgesicsDokument20 SeitenLecture 16-17 - Opioids AnalgesicsJedoNoch keine Bewertungen
- B.Pharmacy IY - R17 - Remedial MathsDokument2 SeitenB.Pharmacy IY - R17 - Remedial MathsrajeshNoch keine Bewertungen
- Daftar Obat Ethical FixDokument4 SeitenDaftar Obat Ethical FixPanjalu YudaprajaNoch keine Bewertungen
- Apr MicrobialidentificationDokument82 SeitenApr MicrobialidentificationAntónio FerreiraNoch keine Bewertungen
- Reading Guide MIC E-TestDokument13 SeitenReading Guide MIC E-TestTrọng TínNoch keine Bewertungen
- CPR PPT FinalDokument83 SeitenCPR PPT FinalSimran Josan100% (1)
- GerdDokument38 SeitenGerdZANoch keine Bewertungen
- Diabetes Mellitus With Peripheral Neuropathy: Presented By: Amol LavateDokument12 SeitenDiabetes Mellitus With Peripheral Neuropathy: Presented By: Amol Lavatelavate amol bhimraoNoch keine Bewertungen
- Drug LiteratureDokument5 SeitenDrug LiteraturekaqueridoNoch keine Bewertungen
- Data ResepDokument26 SeitenData ResepFACHRIZAL AMRIENoch keine Bewertungen
- Preventive and Social Medicine: Concept of Health and DiseaseDokument7 SeitenPreventive and Social Medicine: Concept of Health and DiseaseskNoch keine Bewertungen
- Prescription 2: Ingredient Physical Description Solubility Dose Comparison Given UsualDokument4 SeitenPrescription 2: Ingredient Physical Description Solubility Dose Comparison Given UsualRhonabelle DadulaNoch keine Bewertungen
- 3rd All Surgery Question FileDokument136 Seiten3rd All Surgery Question FileСымбат КулдасоваNoch keine Bewertungen
- Dci 230030Dokument14 SeitenDci 230030MARIA LEIVANoch keine Bewertungen