Beruflich Dokumente
Kultur Dokumente
Speed of Reactions PDF
Hochgeladen von
afoo12340 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
74 Ansichten8 SeitenThere are four main factors that affect the rate of a chemical reaction: concentration, particle size, temperature, and catalysts. A higher concentration, smaller particle size, higher temperature, or presence of a catalyst will all increase the rate of reaction by increasing the frequency of successful collisions between reactant particles. The rate of a reaction can be measured by testing the volume of gas produced or mass change over time. A steeper slope on a graph of these measurements over time indicates a faster reaction rate.
Originalbeschreibung:
Speed of Reactions.pdf
Originaltitel
Speed of Reactions.pdf
Copyright
© Attribution Non-Commercial (BY-NC)
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenThere are four main factors that affect the rate of a chemical reaction: concentration, particle size, temperature, and catalysts. A higher concentration, smaller particle size, higher temperature, or presence of a catalyst will all increase the rate of reaction by increasing the frequency of successful collisions between reactant particles. The rate of a reaction can be measured by testing the volume of gas produced or mass change over time. A steeper slope on a graph of these measurements over time indicates a faster reaction rate.
Copyright:
Attribution Non-Commercial (BY-NC)
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
74 Ansichten8 SeitenSpeed of Reactions PDF
Hochgeladen von
afoo1234There are four main factors that affect the rate of a chemical reaction: concentration, particle size, temperature, and catalysts. A higher concentration, smaller particle size, higher temperature, or presence of a catalyst will all increase the rate of reaction by increasing the frequency of successful collisions between reactant particles. The rate of a reaction can be measured by testing the volume of gas produced or mass change over time. A steeper slope on a graph of these measurements over time indicates a faster reaction rate.
Copyright:
Attribution Non-Commercial (BY-NC)
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 8
Speed of Reactions
A chemical reaction can be represented by a
Chemical Equation. The starting chemicals are called Reactants and the new ones made the Products. The reactants go on the left-hand side of the arrow and the products on the righthand side. ie. Reactants Products
eg. Mg +HCl Mg(Cl)2 + H2
Factors affecting the speed of reactions(Rate of reactions)
There are four factors that affect the Rate of
Reactions. They are: Concentration Particle Size Temperature Catalyst.
Let us consider each in turn
For a reaction to occur, reacting molecules must
collide and stick to together. For a successful collision they must be moving fast enough ie. They must have sufficient Kinetic or movement energy. The minimum energy they must possess is called the Activation Energy.
Concentration: The Higher the concentration
the greater the rate of reaction. This is because higher concentrations have greater numbers of reacting molecules and therefore a greater chance of successful collision
Factors affecting Reaction Rates contd. contd
Particle Size: The smaller the particle size the
quicker the rate of reaction. This is a result of smaller particles allowing a greater surface area of reacting molecules to be in contact and therefore a greater chance of a successful collision. Temperature: The higher the temperature the greater the rate of reaction. When we heat a substance we give the particles more energy. As a result they move faster thus increasing the chance of a successful collision(it can be said that more particles now have more than the Activation Energy).
Factors affecting Reaction Rates(contd)
Catalysts: A catalyst speeds up the rate of reaction.
It does so by providing a surface on which reacting molecules can meet and collide. A catalyst will remain unchanged before and after a reaction. Catalysts in industrial reactions often have to be changed as they become Poisoned. Catalysts are said to be specific ie. A catalyst that works for one reaction does not work for any other. Enzymes: These are Catalysts in living things(Biological Catalysts). Enzymes only work when the reaction is at the correct temperature and pH.
Measuring The Speed of Reactions
Marble chips are added to Hydrochloric Acid and the volume of gas given off was noted every 10secs.
Marble chips are added to Acid and the mass of the flask noted ever 30secs as the CO2 gas is given off.
How do we measure the Rates of Reaction
The following is obtained by adding a metal to an acid and measuring the volume of H2 gas given off every 10secs This line represents the final volume of gas produced.This will be the same if the mass of Mg and no. of moles of Acid remain constant
Volume of gas(cm3)
The steeper the slope the quicker the Time(seconds) reaction ------- This line represents a smaller Particle Size ------- This line represents a higher Temperature
This line represents Mg metal + 2M HCl acid added together
------- This line represents a greater Concentration
Measuring Reactions rates.
We can measure the rate of reaction by following
the change in mass throughout the course of the reaction. Let us consider chalk and excess acid added together and the mass of the flask taken every 30secs The steeper the slope the
Mass (g)
CaCO3 + HCl CaCl2 + CO2 + H2O
The Mass of the flask gets lighter as the CO2 is released
faster the reaction.The line levels off when all the Chalk has been used up When the line levels off the reaction has Time (s) finished
Das könnte Ihnen auch gefallen
- Rates of ReactionDokument11 SeitenRates of Reaction류성의Noch keine Bewertungen
- IGCSE Chemistry - Rates and EquilibriumDokument22 SeitenIGCSE Chemistry - Rates and EquilibriumChemistryKlipz100% (7)
- Chap 7 IGCSE Chemistry NotesDokument10 SeitenChap 7 IGCSE Chemistry NotesMisbah Kamran0% (1)
- Rates of Reaction ALDokument57 SeitenRates of Reaction ALJana Abo elfotohNoch keine Bewertungen
- 2.2.4 Rate of Chemical ReactionDokument12 Seiten2.2.4 Rate of Chemical Reactiondansochristiana574Noch keine Bewertungen
- Unit - The Periodic TableDokument20 SeitenUnit - The Periodic TableMaurya AdeshraNoch keine Bewertungen
- Collision TheoryDokument38 SeitenCollision TheorySaadiah MohammadNoch keine Bewertungen
- 10.3 Kinetic Factors Affecting-2Dokument54 Seiten10.3 Kinetic Factors Affecting-2Hafizszulfeyzul FeyzulNoch keine Bewertungen
- Rate of ReactionDokument48 SeitenRate of ReactionHAFSAH AHMADNoch keine Bewertungen
- 1a Rates of Reaction Slides PDFDokument26 Seiten1a Rates of Reaction Slides PDFArshad KhanNoch keine Bewertungen
- Rate of ReactionDokument18 SeitenRate of ReactionExeteurNoch keine Bewertungen
- SPM Chemistry Form 4 Chapter 1Dokument37 SeitenSPM Chemistry Form 4 Chapter 1kslpeter87Noch keine Bewertungen
- Rates of ReactionsDokument55 SeitenRates of ReactionsJOSHUA NYANGENANoch keine Bewertungen
- Definition N FactorsDokument37 SeitenDefinition N FactorsJedidah JongNoch keine Bewertungen
- New Rate of Reaction Year 9Dokument19 SeitenNew Rate of Reaction Year 9nwandu.elliot.ebenezerNoch keine Bewertungen
- Speed of ReactionDokument3 SeitenSpeed of ReactionM.zuhair asifNoch keine Bewertungen
- Chemical KineticsDokument10 SeitenChemical KineticsitsshaunboteNoch keine Bewertungen
- Chapter 8 Chemical ReactionsDokument15 SeitenChapter 8 Chemical ReactionsAmmar RizwanNoch keine Bewertungen
- Introduction To Chemical KineticsDokument19 SeitenIntroduction To Chemical KineticsGodwin EdekheNoch keine Bewertungen
- Introduction To Chemical KineticsDokument19 SeitenIntroduction To Chemical KineticsGodwin EdekheNoch keine Bewertungen
- Rates of Chemical Reaction - PowerpointDokument16 SeitenRates of Chemical Reaction - Powerpointmerezemenike272Noch keine Bewertungen
- Rates of ReactionDokument3 SeitenRates of ReactionTofunmi OyeboluNoch keine Bewertungen
- Module Form 5 .Rate of ReactionDokument8 SeitenModule Form 5 .Rate of ReactionChew Gee Lan100% (1)
- 2958 - Rate of ReactionDokument40 Seiten2958 - Rate of ReactioncorporolrexNoch keine Bewertungen
- Rate of ReactionDokument5 SeitenRate of ReactionlettyNoch keine Bewertungen
- Activation Energy: Key ConceptsDokument2 SeitenActivation Energy: Key ConceptsChristopher BanolNoch keine Bewertungen
- Rate of Reaction NotesDokument27 SeitenRate of Reaction NotesYong SiewkuanNoch keine Bewertungen
- Chemical ReactionDokument4 SeitenChemical ReactionaleezaNoch keine Bewertungen
- Topic.6 Chemical ReactionsDokument22 SeitenTopic.6 Chemical ReactionsJoyce AmirNoch keine Bewertungen
- Ass 2013Dokument6 SeitenAss 2013api-252561013Noch keine Bewertungen
- Document 61Dokument2 SeitenDocument 61Keandra JordanNoch keine Bewertungen
- Rate of ReactionDokument22 SeitenRate of ReactionlettyNoch keine Bewertungen
- Chapter 1 Form 5 Chemistry 2015Dokument21 SeitenChapter 1 Form 5 Chemistry 2015Alvieno Situl MintowNoch keine Bewertungen
- Chemical ReactionsDokument100 SeitenChemical Reactionsmohidb642Noch keine Bewertungen
- Rates of ReactionDokument7 SeitenRates of Reactionapi-25909541Noch keine Bewertungen
- Chemical KineticsDokument6 SeitenChemical KineticsWiktoria KaczmarzykNoch keine Bewertungen
- This Is A Redox Equation. The Ion Permanganate (Purple) Is Reduced To Ion Manganese (II) Which Is ColourlessDokument3 SeitenThis Is A Redox Equation. The Ion Permanganate (Purple) Is Reduced To Ion Manganese (II) Which Is ColourlessMelissa Ann VannanNoch keine Bewertungen
- 6 Chemical ReactionsDokument19 Seiten6 Chemical ReactionsAhmed AyazNoch keine Bewertungen
- Secondary Chemistry Rate of ReactionDokument17 SeitenSecondary Chemistry Rate of ReactiondreamydamselNoch keine Bewertungen
- Lajur FixDokument18 SeitenLajur FixMoniqsa Purbo SyahraniNoch keine Bewertungen
- 377chemistry Unit 4 Notes CompleteDokument65 Seiten377chemistry Unit 4 Notes Completemuddasser91100% (3)
- KINETICSDokument47 SeitenKINETICSMarilia BonorinoNoch keine Bewertungen
- Measures The Speed at Which Reactants Are Converted Into Products in A Chemical ReactionDokument2 SeitenMeasures The Speed at Which Reactants Are Converted Into Products in A Chemical ReactionwhitneyNoch keine Bewertungen
- Chemical KineticsDokument25 SeitenChemical KineticsAngelo PunzalanNoch keine Bewertungen
- Collision TheoryDokument61 SeitenCollision TheoryIda Yuni Sukadi50% (2)
- Speed of Reaction SummaryDokument3 SeitenSpeed of Reaction Summarychong5660% (5)
- Factors That Affect The Rate of A Chemical ReactionDokument18 SeitenFactors That Affect The Rate of A Chemical ReactionWonder Bee NzamaNoch keine Bewertungen
- Saturday X-Tra X-Sheet: 16 Rates of Reaction: Key ConceptsDokument6 SeitenSaturday X-Tra X-Sheet: 16 Rates of Reaction: Key ConceptsKingford MwesoNoch keine Bewertungen
- Collision Theory & CatalystDokument33 SeitenCollision Theory & CatalystSHEENA MAE DALGUNTASNoch keine Bewertungen
- Colision TheoryDokument85 SeitenColision Theoryactive learning educationNoch keine Bewertungen
- Chapter 8a Reaction Kinetics AS LEVEL NOTESDokument8 SeitenChapter 8a Reaction Kinetics AS LEVEL NOTESArslnNoch keine Bewertungen
- Kinetika ReaksiDokument77 SeitenKinetika ReaksiafrizalfaoniNoch keine Bewertungen
- Rate of Reactions 18 April 2024Dokument46 SeitenRate of Reactions 18 April 2024Amahle KudaNoch keine Bewertungen
- Chemical ReactionsDokument45 SeitenChemical ReactionsSafwan MahmudNoch keine Bewertungen
- Chemical Reactions - ROR ReversibleDokument44 SeitenChemical Reactions - ROR ReversibleAlia AdrianaNoch keine Bewertungen
- Rates of ReactionDokument31 SeitenRates of Reactionspamzz063Noch keine Bewertungen
- Rates of Reactions FactorsDokument7 SeitenRates of Reactions FactorsiamjebastinsamuelNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersVon EverandPractice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: Kinetics and EquilibriumVon EverandPractice Makes Perfect in Chemistry: Kinetics and EquilibriumNoch keine Bewertungen
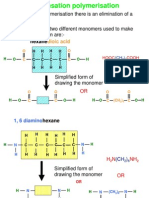
- Condensation PolymerisationDokument14 SeitenCondensation Polymerisationafoo1234100% (2)
- EstersDokument2 SeitenEstersafoo1234Noch keine Bewertungen
- CHEMISTRY 2005 For TeachersDokument63 SeitenCHEMISTRY 2005 For Teachersafoo1234Noch keine Bewertungen
- Hydrocarbons2 Cracking Isomers Topic6 120206Dokument14 SeitenHydrocarbons2 Cracking Isomers Topic6 120206afoo1234Noch keine Bewertungen
- Chemical SafetyDokument22 SeitenChemical Safetyafoo12340% (1)
- STMDokument5 SeitenSTMafoo1234Noch keine Bewertungen
- Isb 2011Dokument16 SeitenIsb 2011afoo12340% (2)
- BIO GR-8 MCQ - July, Aug 03Dokument6 SeitenBIO GR-8 MCQ - July, Aug 03afoo1234Noch keine Bewertungen
- Group 1 SlidesDokument5 SeitenGroup 1 Slidesafoo1234Noch keine Bewertungen
- Fraction Practice Test Additional MaterialsDokument2 SeitenFraction Practice Test Additional Materialsafoo1234Noch keine Bewertungen
- Madharasathul Ahmadhiyya: First Term ExaminationDokument9 SeitenMadharasathul Ahmadhiyya: First Term Examinationafoo1234Noch keine Bewertungen
- Grade 10 / 04: Madhrasathul Ahmadhiyya First Term Test - 2008Dokument9 SeitenGrade 10 / 04: Madhrasathul Ahmadhiyya First Term Test - 2008afoo1234Noch keine Bewertungen
- Madhrasathul Ahmadhiyya: First Term Test - 2008 Grade 10Dokument14 SeitenMadhrasathul Ahmadhiyya: First Term Test - 2008 Grade 10afoo1234Noch keine Bewertungen
- Madhrasathul Ahmadhiyya, Male' Scond Term Test 2003Dokument5 SeitenMadhrasathul Ahmadhiyya, Male' Scond Term Test 2003afoo1234Noch keine Bewertungen
- Madhrasathul Ahmadhiyya, Male' First Term Test 2008 Commerce Grade 10 P - 2 D: 2 HoursDokument2 SeitenMadhrasathul Ahmadhiyya, Male' First Term Test 2008 Commerce Grade 10 P - 2 D: 2 Hoursafoo1234Noch keine Bewertungen
- Madhrasathul Ahmadhiyya, Male' First Term Test 2002Dokument6 SeitenMadhrasathul Ahmadhiyya, Male' First Term Test 2002afoo1234Noch keine Bewertungen
- Madhrasathul Ahmadhiyya Second Term Examinations - Grade 10 Fisheries Science - Paper 2 Duration: One and A Half Hours Paper 2Dokument2 SeitenMadhrasathul Ahmadhiyya Second Term Examinations - Grade 10 Fisheries Science - Paper 2 Duration: One and A Half Hours Paper 2afoo1234Noch keine Bewertungen
- Grade 10 ECONOMICS Paper 2 Duration: 2 Hours Information and InstructionsDokument2 SeitenGrade 10 ECONOMICS Paper 2 Duration: 2 Hours Information and Instructionsafoo1234Noch keine Bewertungen
- SN Unit 6 June 2008 MS PDFDokument21 SeitenSN Unit 6 June 2008 MS PDFafoo1234Noch keine Bewertungen
- Madhrasathul Ahmadhiyya, Male' First Term Test 2002Dokument1 SeiteMadhrasathul Ahmadhiyya, Male' First Term Test 2002afoo1234Noch keine Bewertungen
- Dharumavantha School Examinations: First Term 2009 Grade 10Dokument15 SeitenDharumavantha School Examinations: First Term 2009 Grade 10afoo1234Noch keine Bewertungen
- Dharumavantha School Examinations: First Term 2009 Grade 10Dokument11 SeitenDharumavantha School Examinations: First Term 2009 Grade 10afoo1234Noch keine Bewertungen
- SN Unit 6 June 2007 PDFDokument16 SeitenSN Unit 6 June 2007 PDFafoo1234Noch keine Bewertungen
- Madhrasathul Ahmadhiyya First Term Test 2002 Grade: 10 Principles of Accounting Paper One Page 1 of 5Dokument5 SeitenMadhrasathul Ahmadhiyya First Term Test 2002 Grade: 10 Principles of Accounting Paper One Page 1 of 5afoo1234Noch keine Bewertungen
- Madhrasathul Ahmadhiyya First Term Test - 2001 Chemistry-10 Paper 4 Page 1 of 6Dokument6 SeitenMadhrasathul Ahmadhiyya First Term Test - 2001 Chemistry-10 Paper 4 Page 1 of 6afoo1234Noch keine Bewertungen
- Dharumavantha School Examinations: First Term 2009 Grade 10Dokument12 SeitenDharumavantha School Examinations: First Term 2009 Grade 10afoo1234Noch keine Bewertungen
- SN Unit 6 Jan 2010 Article PDFDokument12 SeitenSN Unit 6 Jan 2010 Article PDFafoo1234Noch keine Bewertungen
- Preliminary Ordinary Level 2009: Dharumavantha School ExaminationsDokument20 SeitenPreliminary Ordinary Level 2009: Dharumavantha School Examinationsafoo1234Noch keine Bewertungen
- SN Unit 6 Jan 2010 PDFDokument20 SeitenSN Unit 6 Jan 2010 PDFafoo1234Noch keine Bewertungen
- Welcome From Governor Brian Schweitzer: Montana Means BusinessDokument2 SeitenWelcome From Governor Brian Schweitzer: Montana Means BusinessMark ReinhardtNoch keine Bewertungen
- Political Factors of Retail SectorDokument3 SeitenPolitical Factors of Retail SectorSachin Kumar Bassi100% (1)
- Complete The Sentences With There Is or There AreDokument5 SeitenComplete The Sentences With There Is or There AreS Leonardo CpdaNoch keine Bewertungen
- Company Profile (RIL)Dokument6 SeitenCompany Profile (RIL)Viju Hiremath50% (2)
- Biblioteca en PapelDokument18 SeitenBiblioteca en PapelCristina Garcia Aguilar0% (1)
- International Business: Case Analysis - Bharathi Airtel in Africa Group - 7 Section - ADokument4 SeitenInternational Business: Case Analysis - Bharathi Airtel in Africa Group - 7 Section - AVignesh nayakNoch keine Bewertungen
- Genpact PlacementPapersDokument7 SeitenGenpact PlacementPapersChandrasekhar UchihaNoch keine Bewertungen
- Project Controls Manager ResumeDokument12 SeitenProject Controls Manager ResumeManor SafeieNoch keine Bewertungen
- Calorii LegumeDokument15 SeitenCalorii LegumeAdina Paula GăburoiNoch keine Bewertungen
- Internet ReceiptDokument2 SeitenInternet Receiptbrm1shubhaNoch keine Bewertungen
- Salary Tds Computation Sheet Sec 192bDokument1 SeiteSalary Tds Computation Sheet Sec 192bpradhan13Noch keine Bewertungen
- E.O. No. 518Dokument3 SeitenE.O. No. 518Rene ValentosNoch keine Bewertungen
- 1697 - 24 (E)Dokument2 Seiten1697 - 24 (E)Shabnam Macanmaker100% (1)
- Kandhari Beverages Private Limited: Particulars Amount RatingDokument3 SeitenKandhari Beverages Private Limited: Particulars Amount RatingRobin GargNoch keine Bewertungen
- SL - No State/UT Active Naef Total Number Foreign Companies RegisteredDokument4 SeitenSL - No State/UT Active Naef Total Number Foreign Companies RegisteredrickysainiNoch keine Bewertungen
- Phrasal Verbs 3: Michael Vince, Peter Sunderland, Advanced Language Practice, Oxford, Macmillan, 2003, P. 156-161Dokument10 SeitenPhrasal Verbs 3: Michael Vince, Peter Sunderland, Advanced Language Practice, Oxford, Macmillan, 2003, P. 156-161pusha26Noch keine Bewertungen
- Stock Market Crash in Bangladesh 2010-11 - A Case StudyDokument4 SeitenStock Market Crash in Bangladesh 2010-11 - A Case StudyMainul HossainNoch keine Bewertungen
- CV 10022018 PDFDokument2 SeitenCV 10022018 PDFZia UllahNoch keine Bewertungen
- SEBI Grade A Free Study Material Accountancy Accounting Standards RFDokument15 SeitenSEBI Grade A Free Study Material Accountancy Accounting Standards RFshiivam sharmaNoch keine Bewertungen
- AGENDA For Rural ConclaveDokument4 SeitenAGENDA For Rural ConclaveRimple SanchlaNoch keine Bewertungen
- Assessing Earnings Quality at NuwareDokument7 SeitenAssessing Earnings Quality at Nuwaresatherbd21100% (4)
- Battle Creek Storage Systems Budgeted The Following Factory OverDokument1 SeiteBattle Creek Storage Systems Budgeted The Following Factory OverAmit PandeyNoch keine Bewertungen
- Accounting in Business: Accounting or Accountancy Is The Measurement, Processing and Communication of FinancialDokument3 SeitenAccounting in Business: Accounting or Accountancy Is The Measurement, Processing and Communication of FinancialRaheelAfzaalNoch keine Bewertungen
- Module 1 Nature and Scope of The NGASDokument22 SeitenModule 1 Nature and Scope of The NGAScha11Noch keine Bewertungen
- Top100 Million TonnersDokument1 SeiteTop100 Million TonnersAlejandro LeoneNoch keine Bewertungen
- SSSXXDokument6 SeitenSSSXXNath OruNoch keine Bewertungen
- Diversification of Hindustan Unilever LimitedDokument21 SeitenDiversification of Hindustan Unilever LimitedAtul Nikam50% (2)
- Dahlan IskanDokument2 SeitenDahlan IskanIzza AkbarNoch keine Bewertungen
- Contract Costing LMS UEWDokument32 SeitenContract Costing LMS UEWPrince Nanaba EphsonNoch keine Bewertungen
- Budgeting WsDokument5 SeitenBudgeting Wsapi-290878974Noch keine Bewertungen