Beruflich Dokumente
Kultur Dokumente
Article
Hochgeladen von
Sajeev RamCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Article
Hochgeladen von
Sajeev RamCopyright:
Verfügbare Formate
Advertiser profiles
BD Medical– Ben Venue Laboratories BENEO-Palatinit GmbH
Pharmaceutical PO Box 46568 Gottlieb-Daimler-Strasse 12
Systems Bedford, OH 44146 68165 Mannheim, Germany
tel. 440.232.3320 tel. +49 621 421 150
1 Becton Dr. fax +49 621 421 160
fax 440.439.6398
Franklin Lakes, NJ 07417 galeniq@beneo-palatinit.com
peter.hansbury@
tel. 800.225.3310 www.galeniq.com
boehringer-ingelheim.com
fax 201.847.4847 www.benvenue.com
BDPS_marketing@bd.com
www.bd.com/pharmaceuticals
Description Description
Ben Venue Laboratories develops BENEO-Palatinit is a 100% sub-
Description sidiary of Südzucker AG. As a
and manufactures sterile liquid and
BD Medical–Pharmaceutical member of IPEC, BENEO-Palatinit
lyophilized products under contract.
Systems is dedicated to devel- is implementing under IPEC–GMP
The company has expertise in han-
oping prefillable drug-delivery conditions for bulk pharmaceuti-
dling complex formulations and has
systems designed to fit the needs cal excipients. BENEO-Palatinit is
contributed to the creation of many
of the pharmaceutical industry. showing its multifunctional sugar-
significant pharmaceutical products
BD offers a range of products, free excipient galenIQ. Based on
for nearly 70 years. Products from
including glass and plastic pre- hydrogenated isomaltulose and
Ben Venue Laboratories are distrib-
fillable syringes, a nasal-spray its refined modifications, galenIQ
uted globally. Ben Venue Laborato-
system, and various self-injection is offered in different grades for
ries is a wholly owned subsidiary of
devices. Each of these differenti- solid dosage forms.
Boehringer Ingelheim Corporation.
ates pharmaceutical products
and contributes to the optimi- Products and services
Products and services
zation of drug therapy. All the galenIQ from BENEO-Palatinit is a
• Dedicated cytotoxic and geno-
company’s prefillable devices range of bulk excipients, including
toxic manufacturing facility
are designed to meet health- tailor-made grades for oral solid
opening Q2 2009
care professionals’ demands for dosage forms. Excellent alterna-
• Phase I to commercial on-site
safety and convenience and to tives for direct compression, cap-
production in batch sizes from
fulfill patients’ needs for comfort. sule-filling, or powder-mixture ap-
1 L to 2000 L
BD’s worldwide presence, market plications present galenIQ grades
• Liquid or lyophilized sterile
awareness, and pharmaceuti- 720 and 721. The galenIQ 800
products
cal packaging know-how allow series additionally offers special
• Centrally located 500,000-ft2
the company to propose suitable powder grades for wet granula-
manufacturing facility
solutions for all regional markets tion, compaction, and other ag-
• Largest contract sterile-
and parenteral drug-delivery glomeration processes.
injectable lyophilization capacity
needs. in the world
AAPS Booth 504
• Expertise in manufacturing
AAPS Booth 1443 complex formulations—micro-
spheres, liposomes, emulsions,
and nanotechnology
• Global product distribution.
AAPS Booth 1949
130 Pharmaceutical Technology NOVEMBER 2008 pharmtech.com
Das könnte Ihnen auch gefallen
- Advances in Liquid Fill Capsule TechnologyDokument4 SeitenAdvances in Liquid Fill Capsule TechnologyAlireza GhaffariNoch keine Bewertungen
- Biological e LTD 1507890378Dokument18 SeitenBiological e LTD 1507890378nrcr2588Noch keine Bewertungen
- Providing WFI and Pure Steam in Best QualityDokument11 SeitenProviding WFI and Pure Steam in Best QualityBulent InanNoch keine Bewertungen
- 2021 - Seedtreatment Agropages 44Dokument1 Seite2021 - Seedtreatment Agropages 44Catherine TangNoch keine Bewertungen
- Ready Pack Information SheetDokument2 SeitenReady Pack Information SheetSatish HiremathNoch keine Bewertungen
- Pharmaceutical Steam Generators and Water Distillation SystemsDokument12 SeitenPharmaceutical Steam Generators and Water Distillation Systemssoajanii100% (2)
- Apex VA 13 Pages.Dokument13 SeitenApex VA 13 Pages.Gustavo RojasNoch keine Bewertungen
- Act 1 Manuf LecDokument5 SeitenAct 1 Manuf LecXz GuzmanNoch keine Bewertungen
- Schott-NEXT 12022 MobileDokument17 SeitenSchott-NEXT 12022 Mobiled3392104002Noch keine Bewertungen
- TDS DR Bio 7211 Compostable Filler FilmsDokument3 SeitenTDS DR Bio 7211 Compostable Filler FilmsMukul SareenNoch keine Bewertungen
- Tds DR Bio 7212 Pla FillerDokument3 SeitenTds DR Bio 7212 Pla FillerMukul SareenNoch keine Bewertungen
- 4.sterile Technology PDFDokument68 Seiten4.sterile Technology PDFOula HatahetNoch keine Bewertungen
- Bogash 1963Dokument5 SeitenBogash 1963wahyu santikaNoch keine Bewertungen
- Catalogo Parenteral BormioliDokument19 SeitenCatalogo Parenteral BormioliIgorAntonelliNoch keine Bewertungen
- Coa Fb-50 Plus Textile AntifoamDokument4 SeitenCoa Fb-50 Plus Textile AntifoamShandy Yudha Nugraha100% (1)
- Stelis Biosource CorporateDokument18 SeitenStelis Biosource CorporatePiyush JainNoch keine Bewertungen
- Ceapro Corp Presentation March 2017Dokument32 SeitenCeapro Corp Presentation March 2017kaiselkNoch keine Bewertungen
- 1 (6) - 部分28Dokument1 Seite1 (6) - 部分28Ricky ChiuNoch keine Bewertungen
- Pharmaceutical Repackaging Facility Project Report Executive SummaryDokument21 SeitenPharmaceutical Repackaging Facility Project Report Executive SummaryHitesh Patel100% (1)
- Vaccine Formulation PlantDokument10 SeitenVaccine Formulation PlantHarmeet SekhonNoch keine Bewertungen
- Designing Effective Drug Formulations - PA - Q416 - 34Dokument4 SeitenDesigning Effective Drug Formulations - PA - Q416 - 34Sateesh KumarNoch keine Bewertungen
- Narmada Gelatines Ltd. - A Long Term Investment OpportunityDokument14 SeitenNarmada Gelatines Ltd. - A Long Term Investment OpportunityShrikant BagrodiaNoch keine Bewertungen
- Oppanol - Brochure - 2020 - DS - SCREEN (1) 3Dokument8 SeitenOppanol - Brochure - 2020 - DS - SCREEN (1) 3Ala Eddine MarzouguiNoch keine Bewertungen
- HBL 1 Company ProfileDokument53 SeitenHBL 1 Company ProfileAakashSharmaNoch keine Bewertungen
- Poly (Ethylene-Co-Vinyl Acetate) : Technical BriefDokument2 SeitenPoly (Ethylene-Co-Vinyl Acetate) : Technical BriefprashantNoch keine Bewertungen
- RETAIL PRESCRIPTION AUDIT OF CIPLA PRODUCTS IN NOIDA (PharmaDokument81 SeitenRETAIL PRESCRIPTION AUDIT OF CIPLA PRODUCTS IN NOIDA (PharmaNeeraj Yadav100% (2)
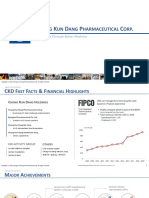
- CKD Corporate Presentation - 20200631Dokument14 SeitenCKD Corporate Presentation - 20200631AhmedNoch keine Bewertungen
- Trans Tech HVAC TTHVAC Company Profile 08 2018.01.18Dokument20 SeitenTrans Tech HVAC TTHVAC Company Profile 08 2018.01.18GAURAV KUMBHARNoch keine Bewertungen
- TraducidoDokument23 SeitenTraducidoLuis Fabricio PlazaNoch keine Bewertungen
- Conda ProductList 2018Dokument26 SeitenConda ProductList 2018KATHENoch keine Bewertungen
- Functional Products Inc.: Additives For Biobased ProductsDokument7 SeitenFunctional Products Inc.: Additives For Biobased ProductsMenoddin shaikhNoch keine Bewertungen
- Tds - Proxel GXL - en - 08-04-2020Dokument2 SeitenTds - Proxel GXL - en - 08-04-2020Maximiliano MackeviciusNoch keine Bewertungen
- Advantages of Ester Over Mineral OilDokument8 SeitenAdvantages of Ester Over Mineral OilMenoddin shaikhNoch keine Bewertungen
- Nipanox BHT - Version 2010Dokument2 SeitenNipanox BHT - Version 2010anh.tranNoch keine Bewertungen
- BASF Glyoxal BrochureDokument8 SeitenBASF Glyoxal BrochureMukund KsNoch keine Bewertungen
- Sample Data & FieldsDokument4 SeitenSample Data & FieldsKadirul IslamNoch keine Bewertungen
- Rheovis PU 1215: Formulation AdditivesDokument3 SeitenRheovis PU 1215: Formulation AdditivesAyhan CansuNoch keine Bewertungen
- 〈1〉 INJECTIONS AND IMPLANTED DRUG PRODUCTS (PARENTERALS) -PRODUCT QUALITY TESTSDokument6 Seiten〈1〉 INJECTIONS AND IMPLANTED DRUG PRODUCTS (PARENTERALS) -PRODUCT QUALITY TESTSthanh nguyễnNoch keine Bewertungen
- 01 enDokument12 Seiten01 enZoro DNoch keine Bewertungen
- LG Chem Life Sciences FactsheetDokument14 SeitenLG Chem Life Sciences FactsheetmaxNoch keine Bewertungen
- Elementis-Bentone De-TdsDokument2 SeitenElementis-Bentone De-Tdsmgamal1080Noch keine Bewertungen
- Eco-Friendly Compostable Resin Reduces Plastic WasteDokument3 SeitenEco-Friendly Compostable Resin Reduces Plastic WasteMukul SareenNoch keine Bewertungen
- Biocon Launching The New Cancer DrugDokument14 SeitenBiocon Launching The New Cancer DrugAkshat Gupta100% (1)
- Oral Films 1 PDFDokument11 SeitenOral Films 1 PDFCristian Camilo Villa100% (1)
- PT JAPFA COMFEED INDONESIA Tbk FY2021 Strengthening Synergies for a Sustainable FutureDokument25 SeitenPT JAPFA COMFEED INDONESIA Tbk FY2021 Strengthening Synergies for a Sustainable FutureJeffry Shandy LeeNoch keine Bewertungen
- BIO-Heat Trifold EUDokument2 SeitenBIO-Heat Trifold EUmadimadi11Noch keine Bewertungen
- SPINE EU ENG ITA REV 07 31 05 2022 - Compressed 1Dokument32 SeitenSPINE EU ENG ITA REV 07 31 05 2022 - Compressed 1Guo KangNoch keine Bewertungen
- Polifosfórico - TDS.20012 PPA 116 ENDokument1 SeitePolifosfórico - TDS.20012 PPA 116 ENKarolina J Pérez VelásquezNoch keine Bewertungen
- Okeke Louis Chemical Analyst in PharmDokument52 SeitenOkeke Louis Chemical Analyst in PharmOkorie VictorNoch keine Bewertungen
- VenAir ManguerasDokument56 SeitenVenAir ManguerasSadhala01Noch keine Bewertungen
- 10.1021@cen 09409 Buscon008Dokument1 Seite10.1021@cen 09409 Buscon008Amal ..Noch keine Bewertungen
- A A A A C C C C A A A A D D D D Eeee M M M M IIII C C C C S S S S C C C C IIII Eeee N N N N C C C C Eeee S S S SDokument3 SeitenA A A A C C C C A A A A D D D D Eeee M M M M IIII C C C C S S S S C C C C IIII Eeee N N N N C C C C Eeee S S S SnelisaNoch keine Bewertungen
- Quality control analysis of finished goods damageDokument14 SeitenQuality control analysis of finished goods damageWindi Sari PattyNoch keine Bewertungen
- Michem™ Dispersion Urethane 4075: Technical Data SheetDokument2 SeitenMichem™ Dispersion Urethane 4075: Technical Data Sheetsriatul2006Noch keine Bewertungen
- Pharmaceuticals & Medical Supplies: Amarila Malik Professor, Faculty of Pharmacy - Universitas IndonesiaDokument22 SeitenPharmaceuticals & Medical Supplies: Amarila Malik Professor, Faculty of Pharmacy - Universitas IndonesiaAngga AnugrawanNoch keine Bewertungen
- Ibn SinaDokument9 SeitenIbn SinaMuntasir MahmoodNoch keine Bewertungen
- High Gravity BrewingDokument4 SeitenHigh Gravity BrewingMohd SuhailNoch keine Bewertungen
- K 2007 Additives ReviewDokument7 SeitenK 2007 Additives ReviewNguyen Xuan Giang0% (1)
- GMP in Pharmaceutical Industry: Global cGMP & Regulatory ExpectationsVon EverandGMP in Pharmaceutical Industry: Global cGMP & Regulatory ExpectationsBewertung: 5 von 5 Sternen5/5 (2)
- Adhoc NetworksDokument19 SeitenAdhoc NetworkssuriyakrishnanNoch keine Bewertungen
- SaamDokument1 SeiteSaamSajeev RamNoch keine Bewertungen
- 2 Marks MPCDokument26 Seiten2 Marks MPCSajeev RamNoch keine Bewertungen
- OsDokument3 SeitenOsSajeev RamNoch keine Bewertungen
- Background 3Dokument2 SeitenBackground 3Sajeev RamNoch keine Bewertungen
- ChennaiDokument41 SeitenChennaiRudolf WhitterNoch keine Bewertungen
- Form 29 - Notice of Transfer of Ownership of VehicleDokument1 SeiteForm 29 - Notice of Transfer of Ownership of Vehiclemanjinderjodhka8903Noch keine Bewertungen
- Form 30Dokument2 SeitenForm 30Sriraghuraman Gopal Rathnam0% (1)
- Low Price Samsung MobilesDokument3 SeitenLow Price Samsung MobilesSajeev RamNoch keine Bewertungen
- Plan BasicDokument8 SeitenPlan BasicSajeev RamNoch keine Bewertungen
- A Frequency Based ApprochDokument12 SeitenA Frequency Based ApprochSajeev RamNoch keine Bewertungen
- EMS Action PlanDokument7 SeitenEMS Action PlanSajeev RamNoch keine Bewertungen
- Background 3Dokument2 SeitenBackground 3Sajeev RamNoch keine Bewertungen
- Background 3Dokument2 SeitenBackground 3Sajeev RamNoch keine Bewertungen
- EMS Action PlanDokument7 SeitenEMS Action PlanSajeev RamNoch keine Bewertungen
- Ems 2Dokument20 SeitenEms 2Sajeev RamNoch keine Bewertungen
- I 1, 2, - . - , N) of Spanning Subgraphs of K Pages, Satisfying The Following Two PropertiesDokument6 SeitenI 1, 2, - . - , N) of Spanning Subgraphs of K Pages, Satisfying The Following Two PropertiesSajeev RamNoch keine Bewertungen
- Targeted Cationic Liposomes, Technologies, and Developments: Anas El-AneedDokument4 SeitenTargeted Cationic Liposomes, Technologies, and Developments: Anas El-AneedSajeev RamNoch keine Bewertungen
- Grignard Reactions: Preparation, Properties and ApplicationsDokument15 SeitenGrignard Reactions: Preparation, Properties and ApplicationsHamed IjazNoch keine Bewertungen
- The Effect of Different Alkalinity Levels On Litopenaeus Vannamei Reared With Bio Oc Technology (BFT)Dokument17 SeitenThe Effect of Different Alkalinity Levels On Litopenaeus Vannamei Reared With Bio Oc Technology (BFT)Manu MorpheusNoch keine Bewertungen
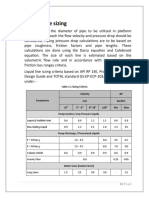
- Line Sizing CriteriaDokument5 SeitenLine Sizing CriteriaBILAL ILYASNoch keine Bewertungen
- DEP 39011012 - Inf - 2018-02 - A01Dokument67 SeitenDEP 39011012 - Inf - 2018-02 - A01g_325899365Noch keine Bewertungen
- Top Steel Companies in IndiaDokument3 SeitenTop Steel Companies in Indiazukmos67% (3)
- Spring 2013 Lecture 2 - 4Dokument15 SeitenSpring 2013 Lecture 2 - 4XiuQingNoch keine Bewertungen
- Quantitative Analysis of Carbohydrates I - Lab 4Dokument27 SeitenQuantitative Analysis of Carbohydrates I - Lab 4Noriko Medoruma0% (3)
- Laprak Distilasi UapDokument11 SeitenLaprak Distilasi UapRetnani Arum PertiwiNoch keine Bewertungen
- 4.7 Lab - Percentage of Water in PopcornDokument3 Seiten4.7 Lab - Percentage of Water in PopcornVansh PatelNoch keine Bewertungen
- Hegatec VacuumBeltDryerDokument2 SeitenHegatec VacuumBeltDryerpintaratNoch keine Bewertungen
- The Radio Chemistry of Mercury - Us AECDokument211 SeitenThe Radio Chemistry of Mercury - Us AEClondonbluetopazNoch keine Bewertungen
- AntimonyDokument72 SeitenAntimony沈益Noch keine Bewertungen
- 10.1016/j.foodres.2014.01.057: Food Research InternationalDokument90 Seiten10.1016/j.foodres.2014.01.057: Food Research Internationaledywiyono2013Noch keine Bewertungen
- Lesson Plan in DNA Grade 9 PracticumDokument2 SeitenLesson Plan in DNA Grade 9 PracticumHarry Chestered Gipulan Empistan100% (2)
- Afa Reviewer ADokument28 SeitenAfa Reviewer AJane CastroNoch keine Bewertungen
- 2do Taller de Química Inorgánica IIDokument3 Seiten2do Taller de Química Inorgánica IIKaritto EspitiaNoch keine Bewertungen
- Introduction To Storage TanksDokument49 SeitenIntroduction To Storage TanksMachineryeng100% (2)
- HAZOP Training290620Dokument93 SeitenHAZOP Training290620NasrulNoch keine Bewertungen
- Novabrite RGB Full Color High Power Led Application Note: R&D CenterDokument15 SeitenNovabrite RGB Full Color High Power Led Application Note: R&D CenterVinu KumarNoch keine Bewertungen
- READYMIX Safety Data SheetDokument9 SeitenREADYMIX Safety Data Sheetalbarajeel forwarding001Noch keine Bewertungen
- Optek TOP5 Brewery Applications EnglishDokument16 SeitenOptek TOP5 Brewery Applications EnglishoptekNoch keine Bewertungen
- Physical and Chemical Properties QuizDokument20 SeitenPhysical and Chemical Properties Quizgnanasekar0% (1)
- Clinical Chemistry - Theory, Analysis, CorrelationDokument344 SeitenClinical Chemistry - Theory, Analysis, Correlationaristides.quinteroNoch keine Bewertungen
- Will Silver Bromide PrecipitateDokument14 SeitenWill Silver Bromide PrecipitateLeonidasNoch keine Bewertungen
- College of Engineering and Computer TechnologyDokument2 SeitenCollege of Engineering and Computer TechnologyRoss Sonny CruzNoch keine Bewertungen
- Mass-Transfer Diffusion Coefficients in Binary Systems: AppendixDokument3 SeitenMass-Transfer Diffusion Coefficients in Binary Systems: AppendixAnisaNoch keine Bewertungen
- Project On Fuel Cells: A Concise Look at Fuel Cell TechnologyDokument12 SeitenProject On Fuel Cells: A Concise Look at Fuel Cell TechnologySagar RastogiNoch keine Bewertungen
- Enhanced Hybrid Science 6 Quarter1 Module 1 Week1Dokument10 SeitenEnhanced Hybrid Science 6 Quarter1 Module 1 Week1KATHLENE CORPUS100% (1)
- Properties and Uses: Report by Group 2Dokument56 SeitenProperties and Uses: Report by Group 2Carl Ashlee Perez AsiNoch keine Bewertungen
- Charles Law ExplainedDokument3 SeitenCharles Law ExplainedKaren May UrlandaNoch keine Bewertungen