Beruflich Dokumente
Kultur Dokumente
Beer's Law Simulator
Hochgeladen von
partho143Originalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Beer's Law Simulator
Hochgeladen von
partho143Copyright:
Verfügbare Formate
The Beers Law Simulator
In the laboratory, you explored a variety of aspects of visible spectrophotometry in More Lights, Color, Absorption. You should have derived Beers Law from experimental data. This simulation, using an interactive Excel spreadsheet, will enhance your understanding of Beers Law and how a number of variables influence the absorbance being measured. The multi-sheet interactive Excel spreadsheet is on the CHM 103 webpage (http://academic.pgcc.edu/~ssinex/chm103.html ). The tab, lower left, on the Excel workbook is used to move from sheet to sheet and is marked for each section below. The Mathematics of Beers Law Concentration tab Beers law is a linear relationship between absorbance (A) and three independent variables: the molar absorptivity (a), the pathlength (b), and the concentration (c). The law takes the form of y = mx + b, where m is the slope and b is the y-intercept. So for Beers law or A = abc, what is the value of the y-intercept? For a plot of the absorbance as a function of concentration, what is the slope in terms of the independent variables?
In any Beers law investigation, a certain wavelength is used and is held constant. What does the wavelength influence, since it does not appear in the mathematical statement of Beers law?
Investigating the Calibration Curve (A vs. c) Concentration tab The linear calibration curve is one of the most powerful analytical tools for scientists. How does changing the pathlength, b, influence the calibration curve? Sketch a graph.
How does changing the molar aborptivity, a, influence the calibration curve?
Why do the pathlength and the molar aborptivity have the effect you saw above?
The Effect of Pathlength (A vs. b) Pathlength tab The absorbance as a function of pathlength is another linear relationship. What is the slope, in terms of any of the three independent variables, for this graph?
CHM 103/Sinex
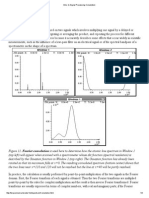
Investigating the Absorption Spectrum (A vs. ) - Wavelength tab The absorption spectrum is a plot of absorbance as a function of the wavelength. This is the first thing determined in any analysis, so that the wavelength of maximum absorbance, max, can be located on the plot. The remainder of the analysis is performed at this wavelength. Looking at the absorption spectrum, where is max? If the concentration changes, does max change? If the pathlength changes, does max change? How does the absorption spectrum change when the pathlength is varied? when the concentration is varied? Sketch a graph.
What variable does the wavelength influence? (You might want to go back to the concentration tab)
How does this variation plot? Sketch it.
How does this relate to the absorption spectrum?
The Influence of Errors on the Calibration Curve Concentration tab The spectrophotometer was set-up and calibrated using a distilled water blank (A = 0). For the analysis, the blank used for the analysis contained other reagents and gave an absorbance of 0.15. How would this influence the calibration curve? If you did not notice the blanks actual absorbance, how would the graph point it out?
A student ran a calibration curve and then knocked the wavelength dial on the spectrophotometer, so that the wavelength increased by 20 nm. How are the subsequent results of sample analysis influenced? Explain.
CHM 103/Sinex
Transmittance as a function of concentration (T vs. c) - Transmittance tab Transmittance is simply the ratio of the outgoing light intensity divided by the initial light intensity or T= I/Io . What type of relationship is found for transmittance as a function of concentration?
If T drops in half (from 0.80 to 0.40), how does c change?
Absorbance (A = -log T) was originally used to transform the data to a linear relation for ease of manual graphing. Today with graphing calculators and spreadsheets curve fitting is easy. Which relationship is easier to deal with A vs. c or T vs. c? Why?
Extrapolating your calibration curve (A vs. c) Bad Cal tab What happens to calibration curves at high concentrations?
The bending downward at high concentrations is typical. The non-linear position of the calibration does not adhere to Beers Law. Why?
How does this influence your final concentration if you were to extrapolate and use the linear calibration curve?
The educational value of this Excel simulation only comes from playing with it and trying to figure out what is going to happen. Then change the variable and see what happens. Learn and think about the situations posed NOT memorize! An answer key is available for this set of questions at: http://academic.pgcc.edu/~ssinex/BLS_key.pdf
CHM 103/Sinex
Das könnte Ihnen auch gefallen
- Daniel C. Harris: Quantitative Chemical Analysis, 9th Ed.: Books and Software in ReviewDokument3 SeitenDaniel C. Harris: Quantitative Chemical Analysis, 9th Ed.: Books and Software in ReviewOryza SativaNoch keine Bewertungen
- Statistical Analysis With Software Application: Polytechnic University of The PhilippinesDokument15 SeitenStatistical Analysis With Software Application: Polytechnic University of The PhilippinesDianne Mae Saballa Cruz0% (1)
- Multiple Integrals and Its Application in Telecomm EngineeringDokument38 SeitenMultiple Integrals and Its Application in Telecomm EngineeringNaveed Ramzan71% (7)
- HMIWebDisplayBuildingGuide EngDokument181 SeitenHMIWebDisplayBuildingGuide Engpartho143100% (2)
- Asme B46.1 2002Dokument5 SeitenAsme B46.1 2002Suryanarayanan Venkataramanan14% (7)
- UT 4 Lectura Recomendada 20061001 Structured Analysis of Competing Hypotheses - Wheaton ChidoDokument4 SeitenUT 4 Lectura Recomendada 20061001 Structured Analysis of Competing Hypotheses - Wheaton ChidoRodrigo Spaudo CarrascoNoch keine Bewertungen
- Class Research Agenda: Inquiries, Investigation and Immersion Quarter 1 - Module 1Dokument22 SeitenClass Research Agenda: Inquiries, Investigation and Immersion Quarter 1 - Module 1john ray rivasNoch keine Bewertungen
- Twyman Lothian PpaerDokument8 SeitenTwyman Lothian PpaerchristianNoch keine Bewertungen
- Lesson 4 - Scientific InvestigationsDokument14 SeitenLesson 4 - Scientific InvestigationsELise SHafriNoch keine Bewertungen
- Lesson 4 - Scientific InvestigationsDokument14 SeitenLesson 4 - Scientific InvestigationsRais RahimiNoch keine Bewertungen
- ConvolutionDokument3 SeitenConvolutionSiddharth KumarNoch keine Bewertungen
- Beers Law Handouts 1213Dokument4 SeitenBeers Law Handouts 1213Anusia ThevendaranNoch keine Bewertungen
- 1.5 Scientific InvestigationsDokument24 Seiten1.5 Scientific InvestigationsTeoh MilayNoch keine Bewertungen
- Multipath Cross-Correlation Flowmeters: V. Skwarek and V. HansDokument6 SeitenMultipath Cross-Correlation Flowmeters: V. Skwarek and V. HansriannataNoch keine Bewertungen
- IbchintroDokument17 SeitenIbchintroapi-293306937Noch keine Bewertungen
- International Journal of Pharma Professional's ResearchDokument4 SeitenInternational Journal of Pharma Professional's ResearchChichi FauziyahNoch keine Bewertungen
- BeerDokument18 SeitenBeerdipok12Noch keine Bewertungen
- AbsorbansiDokument11 SeitenAbsorbansiJonathan CookNoch keine Bewertungen
- Maths in Focus - Margaret Grove - ch8Dokument54 SeitenMaths in Focus - Margaret Grove - ch8Sam Scheding100% (1)
- Introduction To Spectrochemical Methods 1Dokument50 SeitenIntroduction To Spectrochemical Methods 1sanelisofuturemoyoNoch keine Bewertungen
- The Power of Slope SpectrosDokument2 SeitenThe Power of Slope SpectrosJohn SiricoNoch keine Bewertungen
- Pathint PDFDokument13 SeitenPathint PDFLucas CasagrandeNoch keine Bewertungen
- Application of Transfer Matrix Method in AcousticsDokument7 SeitenApplication of Transfer Matrix Method in AcousticsaruatscribdNoch keine Bewertungen
- The Power of Slope SpectrosDokument3 SeitenThe Power of Slope SpectroseshihNoch keine Bewertungen
- Chapter 1 - Spectroscopy MethodsDokument77 SeitenChapter 1 - Spectroscopy MethodsHariss JailudinNoch keine Bewertungen
- Force Spectroscopy With The Atomic Force Microscope: Application NoteDokument8 SeitenForce Spectroscopy With The Atomic Force Microscope: Application Notekishorkumarn8212Noch keine Bewertungen
- Speeding Up Cosmological Boltzmann CodesDokument7 SeitenSpeeding Up Cosmological Boltzmann CodesWilliam AlgonerNoch keine Bewertungen
- Determination of Mixtures by UV Absorption Spectroscopy: Chem 155 Lab Dr. TerrillDokument8 SeitenDetermination of Mixtures by UV Absorption Spectroscopy: Chem 155 Lab Dr. Terrillprakush_prakushNoch keine Bewertungen
- Semblance Merupakan Representasi Dari Energi Gelombang SeismikDokument4 SeitenSemblance Merupakan Representasi Dari Energi Gelombang SeismikMega Dwi AstutiNoch keine Bewertungen
- Unit Iv Spectro PhotometersDokument30 SeitenUnit Iv Spectro PhotometersMathavaraja JeyaramanNoch keine Bewertungen
- Physics 3.0: Why Computer Science Will Lead The Next Physics RevolutionDokument16 SeitenPhysics 3.0: Why Computer Science Will Lead The Next Physics RevolutionjazzyjhaLNoch keine Bewertungen
- Calculating SpectraDokument16 SeitenCalculating Spectraziggie_lenzNoch keine Bewertungen
- Jiles-Atherton Magnetic Hysteresis Parameters IdentificationDokument6 SeitenJiles-Atherton Magnetic Hysteresis Parameters IdentificationAhmed Magdy BeshrNoch keine Bewertungen
- SpectrophotometerDokument4 SeitenSpectrophotometerSana AbbasiNoch keine Bewertungen
- EIS Nota AC-1 EG&GDokument13 SeitenEIS Nota AC-1 EG&GHarry MarshallNoch keine Bewertungen
- A Novel, Cost-Effective Method For Loudspeakers Parameters MeasurementDokument7 SeitenA Novel, Cost-Effective Method For Loudspeakers Parameters MeasurementdzymytchNoch keine Bewertungen
- Internationa Research Training Group: Diffusion in Porous Materials Workshop Leipzig 9th-12th May 2006Dokument51 SeitenInternationa Research Training Group: Diffusion in Porous Materials Workshop Leipzig 9th-12th May 2006Clarence AG YueNoch keine Bewertungen
- Time Series Via Fractals GeometryDokument35 SeitenTime Series Via Fractals GeometryhemaNoch keine Bewertungen
- Spectrophotometry FundamentalsDokument15 SeitenSpectrophotometry FundamentalschipulinoNoch keine Bewertungen
- Paper Paper Paper Paper Paper: Overhead Contact Line A Overhead Contact Line BDokument6 SeitenPaper Paper Paper Paper Paper: Overhead Contact Line A Overhead Contact Line BResearch1 amtdcNoch keine Bewertungen
- MHS Physics Problems Chapter 1Dokument27 SeitenMHS Physics Problems Chapter 1Dina Ashraf0% (1)
- Knotes Chapter-2 Amplitude ModulationDokument66 SeitenKnotes Chapter-2 Amplitude ModulationGp Bhupinder100% (1)
- Curve FittingDokument4 SeitenCurve Fittingsardinha2Noch keine Bewertungen
- H2 Measurement 2012Dokument21 SeitenH2 Measurement 2012Ronnie QuekNoch keine Bewertungen
- Aspirin Lab 012714Dokument22 SeitenAspirin Lab 012714David SepulvedaNoch keine Bewertungen
- A Novel Path-Loss Model For UWB Off-Body PropagationDokument5 SeitenA Novel Path-Loss Model For UWB Off-Body PropagationHadi RadNoch keine Bewertungen
- MF2030 Final Exam Solution HT10Dokument12 SeitenMF2030 Final Exam Solution HT10Athul_V_Dev_1688Noch keine Bewertungen
- Beers Law NotesDokument5 SeitenBeers Law NotesJerome GironNoch keine Bewertungen
- Power Engineering Letters: Carlos Pérez-RojasDokument3 SeitenPower Engineering Letters: Carlos Pérez-RojasMUNIECOFANTASMANoch keine Bewertungen
- Transient Dynamic AnalysisDokument14 SeitenTransient Dynamic Analysissunil481100% (1)
- 5-INSTRUMENTATION OF UV - VISIBLE SPECTROSCOPY (Reg)Dokument13 Seiten5-INSTRUMENTATION OF UV - VISIBLE SPECTROSCOPY (Reg)YolandaNoch keine Bewertungen
- Simulating Org A No GenesisDokument5 SeitenSimulating Org A No GenesisDenis MenshikovNoch keine Bewertungen
- Transient Movement of Fluid Spheres Using Lattice Boltzmann MethodDokument15 SeitenTransient Movement of Fluid Spheres Using Lattice Boltzmann MethodCheyenneNoch keine Bewertungen
- 1043Dokument6 Seiten1043Eng-Sa'di Y. TamimiNoch keine Bewertungen
- Dimensional Analysis and SimilitudeDokument47 SeitenDimensional Analysis and SimilitudeFabrizio NEBESSENoch keine Bewertungen
- Monte Carlo Methods For Electron Transport in SemiconductorsDokument11 SeitenMonte Carlo Methods For Electron Transport in SemiconductorsPulkit AgrawalNoch keine Bewertungen
- Optical Properties of Thin Semiconductor Films: Grolik Benno, Kopp Joachim October, 31st 2003Dokument11 SeitenOptical Properties of Thin Semiconductor Films: Grolik Benno, Kopp Joachim October, 31st 2003Mnar AlalmNoch keine Bewertungen
- Adjustment of SST Turbulence Model For An Improved Prediction of Stalls On Wind Turbine BladesDokument7 SeitenAdjustment of SST Turbulence Model For An Improved Prediction of Stalls On Wind Turbine BladesMalik NaweratNoch keine Bewertungen
- Color I MeterDokument3 SeitenColor I MeterAnura BandaraNoch keine Bewertungen
- ADE 1D StabilityDokument6 SeitenADE 1D StabilityMartin AntoNoch keine Bewertungen
- Interactions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsVon EverandInteractions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsNoch keine Bewertungen
- Computational Fluid Dynamics: Principles and ApplicationsVon EverandComputational Fluid Dynamics: Principles and ApplicationsBewertung: 5 von 5 Sternen5/5 (1)
- Easy(er) Electrical Principles for Extra Class Ham License (2012-2016)Von EverandEasy(er) Electrical Principles for Extra Class Ham License (2012-2016)Noch keine Bewertungen
- Management of ChangeDokument2 SeitenManagement of Changepartho143Noch keine Bewertungen
- Form Object (Access)Dokument11 SeitenForm Object (Access)partho143Noch keine Bewertungen
- Space Motion of RocketsDokument14 SeitenSpace Motion of Rocketspartho143Noch keine Bewertungen
- TI-84CE Graphing CalculatorDokument4 SeitenTI-84CE Graphing Calculatorpartho143Noch keine Bewertungen
- Urinalysis and Body Fluids: Unit 2 Session 5Dokument4 SeitenUrinalysis and Body Fluids: Unit 2 Session 5partho143Noch keine Bewertungen
- 04 SET MagnetismDokument5 Seiten04 SET Magnetismpartho143Noch keine Bewertungen
- Class11 - Digestion and Absorption Assignment (2018-2019)Dokument1 SeiteClass11 - Digestion and Absorption Assignment (2018-2019)partho143Noch keine Bewertungen
- Job Application (Includes Both)Dokument3 SeitenJob Application (Includes Both)partho143Noch keine Bewertungen
- Mind Map RubricDokument1 SeiteMind Map Rubricpartho143Noch keine Bewertungen
- Add, Edit, Delete and Run Access Queries With VBADokument5 SeitenAdd, Edit, Delete and Run Access Queries With VBApartho143Noch keine Bewertungen
- Finding Roots of Polynomials Using Mathcad: PolyrootsDokument1 SeiteFinding Roots of Polynomials Using Mathcad: Polyrootspartho143Noch keine Bewertungen
- Beginners PianoDokument25 SeitenBeginners Pianopartho143100% (1)
- Guide To OPCDokument9 SeitenGuide To OPCpartho143Noch keine Bewertungen
- 023 BP RefiningDokument27 Seiten023 BP Refiningpartho143Noch keine Bewertungen
- RD WR - AspDokument2 SeitenRD WR - Asppartho143Noch keine Bewertungen
- Add or Remove A Macro From CodeDokument2 SeitenAdd or Remove A Macro From Codepartho143Noch keine Bewertungen
- Interactive Excel Spreadsheet: CHM 102/sinexDokument12 SeitenInteractive Excel Spreadsheet: CHM 102/sinexpartho143Noch keine Bewertungen
- Linear and Nonlinear Regression in Mathcad: Scalar CaseDokument3 SeitenLinear and Nonlinear Regression in Mathcad: Scalar Casepartho143Noch keine Bewertungen
- Using Excel For Handling, Graphing, and Analyzing Scientific DataDokument2 SeitenUsing Excel For Handling, Graphing, and Analyzing Scientific Datapartho143Noch keine Bewertungen
- Finding Roots of Scalar Functions Using in MathcadDokument2 SeitenFinding Roots of Scalar Functions Using in Mathcadpartho143Noch keine Bewertungen
- Generate A Model Tab: PsDokument6 SeitenGenerate A Model Tab: Pspartho143Noch keine Bewertungen
- Sample VariationDokument8 SeitenSample Variationpartho143Noch keine Bewertungen
- Click Here: Developer's Guide To Excelets/SinexDokument3 SeitenClick Here: Developer's Guide To Excelets/Sinexpartho143Noch keine Bewertungen
- Developer's Guide To Excelets/SinexDokument1 SeiteDeveloper's Guide To Excelets/Sinexpartho143Noch keine Bewertungen
- Efect Size e TRDokument3 SeitenEfect Size e TRThiagoNoch keine Bewertungen
- MBA-BS-Recap Session Model Questions-13-07-2023-SV1Dokument74 SeitenMBA-BS-Recap Session Model Questions-13-07-2023-SV1seyon sithamparanathanNoch keine Bewertungen
- Learning Through ExperimentsDokument23 SeitenLearning Through ExperimentsNoridan NohNoch keine Bewertungen
- 12.086 / 12.586 Modeling Environmental Complexity: Mit OpencoursewareDokument7 Seiten12.086 / 12.586 Modeling Environmental Complexity: Mit Opencoursewareivan.tolicNoch keine Bewertungen
- Multiple Choice Questions On Biostatistics (CSIR UGC NET - ICMR JRF Exam)Dokument4 SeitenMultiple Choice Questions On Biostatistics (CSIR UGC NET - ICMR JRF Exam)Binod KumarNoch keine Bewertungen
- Groys Medium ReligionDokument12 SeitenGroys Medium ReligionFabián MartínezNoch keine Bewertungen
- Practice Problems For Midterm 1Dokument83 SeitenPractice Problems For Midterm 1LingYNNoch keine Bewertungen
- Workflow of Statistical Data AnalysisDokument105 SeitenWorkflow of Statistical Data AnalysissharathdhamodaranNoch keine Bewertungen
- Data Analysis Text BookDokument292 SeitenData Analysis Text BookclaireNoch keine Bewertungen
- How To Calculate Confidence IntervalDokument3 SeitenHow To Calculate Confidence Intervalreddy iibfNoch keine Bewertungen
- UoSOutline MKTG1001 2011Dokument11 SeitenUoSOutline MKTG1001 2011michael_todd_44Noch keine Bewertungen
- From The Ground UpDokument18 SeitenFrom The Ground UpPH Ph GnsoNoch keine Bewertungen
- Parameter and StatisticDokument18 SeitenParameter and StatisticGemark D. Gebone67% (3)
- Poisson Distribution ExamplesDokument2 SeitenPoisson Distribution ExamplesEdward KahwaiNoch keine Bewertungen
- B. Georgeot and D. L. Shepelyansky - Integrability and Quantum Chaos in Spin Glass ShardsDokument4 SeitenB. Georgeot and D. L. Shepelyansky - Integrability and Quantum Chaos in Spin Glass ShardsTellusz4532Noch keine Bewertungen
- Managerial Statistics Exam #6Dokument1 SeiteManagerial Statistics Exam #6Mary GabriolaNoch keine Bewertungen
- Forecasting Techniques Qualitative TechniquesDokument3 SeitenForecasting Techniques Qualitative TechniqueskhalilNoch keine Bewertungen
- Math Unit 2 Grade 3 Lesson 29 31Dokument158 SeitenMath Unit 2 Grade 3 Lesson 29 31Arlene SonNoch keine Bewertungen
- Chapter 4: Random Variables and Probability Distributions: Solve The ProblemDokument4 SeitenChapter 4: Random Variables and Probability Distributions: Solve The ProblemEunice WongNoch keine Bewertungen
- Eapp q2 Module 7Dokument18 SeitenEapp q2 Module 7Mark Laurence RubioNoch keine Bewertungen
- Research Method Ch2sDokument30 SeitenResearch Method Ch2sCarms YasayNoch keine Bewertungen
- Planning and Decision MakingDokument16 SeitenPlanning and Decision MakingNgeno KipkiruiNoch keine Bewertungen
- Exp Method StepsDokument5 SeitenExp Method Stepskankshi ChopraNoch keine Bewertungen
- Chapter 14 (14.1 - 14.2)Dokument22 SeitenChapter 14 (14.1 - 14.2)JaydeNoch keine Bewertungen
- Rizaldi - Tugas W9 Anova 2 WayDokument14 SeitenRizaldi - Tugas W9 Anova 2 WayUH SHE UP OFFICIALNoch keine Bewertungen