Beruflich Dokumente
Kultur Dokumente
9.effect of Activation - Full
Hochgeladen von
TJPRC PublicationsOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
9.effect of Activation - Full
Hochgeladen von
TJPRC PublicationsCopyright:
Verfügbare Formate
International Journal Metallurgical & Materials Science and Engineering (IJMMSE) ISSN 2278-2516 Vol.
3, Issue 1, Mar 2013, 75-84 TJPRC Pvt Ltd.
EFFECT OF ACTIVATION PARAMETERS ON THE SURFACE AND MECHANICAL PROPERTIES OF ACTIVATED CARBON SPHERES
JAGPAL SINGH MAHUR1, SAVITA DIXIT2, RAKESH SHRIVASTAVA1 & C. S. GOSWAMI3
1
R&D Centre, HEG Limited, Mandideep, Near Bhopal, Madhya Pradesh, India
2 3
MANIT, Bhopal, Madhya Pradesh, India,
K R G College, Gwalior, Madhya Pradesh, India
ABSTRACT
Spherical activated carbon with micro porous structure has been prepared from phenolic resin spheres by carbonizing them at 850C under the flow of nitrogen followed by activation in CO2 atmosphere at different temperature for fixed duration and for different duration at fixed temperature. The effect of activation parameters such as temperature, residence time and CO2 flow rate on the yield and surface properties of activated carbon spheres was studied. The effect of activation parameters on the mechanical properties of activated carbon spheres was also studied and correlated with surface properties of activated carbon spheres (ACS). The porosity of all activated samples was measured by physical adsorption of nitrogen at 77 K. The surface area was determined using multipoint BET equation and D-R method was used to determine the micro pore volume of activated carbon spheres. It was revealed from the experimental results that BET surface area and total pore volume increases with increasing residence time at constant temperature whereas mechanical strength of ACS decreases continuously on increasing residence time and activation temperature. BET surface area increases on increasing the activation temperature up to 950C due to the creation of new micro pores and then decreases due to the widening of pores under the same experimental conditions.
KEYWORDS: Activated Carbon Sphere, Adsorption, Micro Pore Analysis & BET Surface Area INTRODUCTION
Activated carbons have been commonly prepared from variety of carbonaceous materials such as wood, coconut shells, petroleum pitch, bituminous coal and polymers (Shrinivasakannan 2004; Wei 2003 & 2006; Lu 1992; SilvestreAlbero 2010 and Vilaplana-Ortego 2009) by carbonizing the carbonaceous material between 450C to 850C followed by physical activation/chemical process. Activated carbon exists in many forms i.e. powered activated carbon, granular activated carbon, palletized activated carbon, spherical activated carbon etc. Among all forms of activated carbons, spherical form i.e. activated carbon spheres (ACS) have many advantages as compared to the irregular shaped activated carbon such as the large surface area and high mechanical strength. Due to its spherical shape and rolling property, ACS can easily be filled uniformly and can be coated on the surface of any substrate to provide maximum exposure. Babel 2006; Junbing 1999; Tang 2000; Qiong 2004; Qiao 2001 and Singh et. al. have focused their work on the synthesis of activated carbon sphere from synthetic polymers. Micro porous structure of activated carbon spheres is mainly responsible for adsorption capability of ACS against toxic gases. The micro pore structure of ACS can be controlled by controlling the activation parameters (Bing 2002; Marsh 1971 and Takshi 2003). Apart from the activation parameters, porosity of activated carbon can also be controlled by catalytic gasification. Rodriguez-Reinoso et. al. 1991 pointed out one of the best approach to increase the meso and macro pore volume of activated carbon spheres.
76
Jagpal Singh Mahur, Savita Dixit, Rakesh Shrivastava & C. S. Goswami
In the recent years, the practice of treating the exhaust gas from combustion furnace with activated carbon has become a popular measure for preventing the public nuisance otherwise caused when exhaust gas containing oxides of sulphur and oxides of nitrogen is released into atmosphere. These particles (ACS) are useful in many applications such as ion selective resins. The carbonized microspheres of phenolic resin may be used as electrodes in Li-ion batteries. Activated carbon spheres are new generation material having a wide range of application in NBC (Neuclear, Biological and Chemical) suits, NBC Filters, NBC gloves and masks against toxic gases. Activated carbon spheres also find applications in the field of purification of chemicals and drugs, pollution control, treatment of potable water, solvent recovery and respirators meant for removal of toxic chemicals.
EXPERIMENTAL
Materials Phenolic Resin Spheres (PRS) prepared from resole type phenolic resin by suspension polymerization technique were used as starting material for preparation of activated carbon spheres. Phenolic resin spheres were screened to a particle size of 0.43 to 0.85 mm and selected for the study. Methods Phenolic resin sphere of size 0.43 to 0.85 mm were subjected to carbonization at 800C for 2 hrs soaking time in nitrogen atmosphere. The heating rate during carbonization was maintained at 1C/min and the flow rate of nitrogen was maintained at 200 ml/min. The carbonized spheres were then activated at 925C, 950C, 975C and 1000C for different length of time. After optimization of activation temperature, carbonized sphere were activated at 950C for 2 hrs using CO2 as activating agent, with different gas flow rates. Carbonization and activation process were carried out using a rotary furnace having constant temperature zone of 100 mm diameter and 100 mm length. Characterization The pyrolysis behavior of phenolic resin spheres was determined by Thermo Gravimetric Analysis (TGA) using TG/DTA apparatus from M/s Perkin Elmer instruments. The heating rate was set at 5C/min and flow rate of nitrogen was maintained at 200ml/min. Jeol make (Model-6380LA) Analytical Scanning Electron Microscope (SEM) was employed to study morphology of as such PRS, after carbonization and physical activation also. Quantachrome make (Autosorb-1) was used to determine the porosity of all activated samples by physical adsoption of nitrogen on activated samples at 77K. Surface area and micro pore volumes of the samples were determined using BET and Dubnin-Radushevich (D-R) equations, respectively. Mechanical strength: Mechanical strength is a most important and critical parameter for activated carbon spheres which indicate the capability to withstand the wear and tear of the adsorbent during end use applications. An apparatus for the testing of mechanical strength (crushing strength) of activated carbon spheres was fabricated as per ASTM C-695 standard. The ACS samples of average diameter 0.50 mm were selected randomly from a given batch and tested for their load resistance capacity.
Effect of Activation Parameters on the Surface and Mechanical Properties of Activated Carbon Spheres
77
RESULTS AND DISCUSSIONS
Thermal Degradation Behavior Figure 1 shows the thermo gravimetric curve of phenolic resin spheres. The pyrolysis starts slowly at 100C indicating the decomposition of phenolic resin spheres. A rapid weight loss occurs between 350C to 550C. Weight loss after 800C is negligible indicating the end of carbonization process. The final pyrolysis yield of phenolic resin spheres up to 800C obtained from TGA is found to be 66.7%.
110 100 Weight (%) 90 80 70 60 50 40 0 200 400 600 800 1000
Temperature ( C)
Figure 1: TGA Curve of Phenolic Resin Spheres Morphology Figure 2(a), 2(b) & 2(c) show the SEM micrograph of as such PRS, carbonized spheres and activated carbon spheres. It is evident from SEM micrographs that size of spheres taken for the study are varying from 0.43 to 0.85 mm, besides that particles are of spherical shape with smooth surface.
Figure 2(a)
Figure 2(b)
Figure 2(c)
Figure 2(a), 2(b) & 2(c): SEM Micrograph of as such PRS, Carbonized and Activated Spheres It is also clear from the SEM micrograph of phenolic resin sphere that no pore is visible after carbonization {Figure 2(b)} of phenolic resin spheres at 800C for 2 hrs. soaking time. Probably, it is due to the tarry material produced during carbonization was deposited on the pores developed due to devolatilization, which were again reopened during activation process {(Figure 2(c)}. Carbonization and Activation Behavior During carbonization, most of the non-carbon elements, hydrogen and oxygen are first removed in gaseous form by pyrolytic decomposition of PRS and the free atoms of elementary carbon are grouped into organized crystallographic
78
Jagpal Singh Mahur, Savita Dixit, Rakesh Shrivastava & C. S. Goswami
formations. The yield after carbonization of PRS at 800C for 2 hrs soaking time was found to be 58.5%. Thus obtained carbonized samples were activated at 925C, 950C, 975C and 1000C for different length of time. Table1 shows the results of all activated carbon samples obtained using different set of activation conditions. Table 1: Properties of Phenolic Resin based Activated Carbon Spheres (ACS) Activation Conditions Temp., C 925 950 Time, hrs. 3 5 2 2 2 3 7 9 12 3 4 2 3 CO2 Low, ml/min 200 200 100 300 500 200 200 200 200 200 200 200 200 Burn Off, % 20.5 23.2 15.7 20.2 22.7 29.1 41.6 49.9 72.8 32.8 36.7 23.1 36.8 Yield, % 46.5 44.9 49.3 46.7 45.2 41.5 34.2 29.3 15.9 39.6 37 45 37.4 BET Surface Area, 2/g 858 953 447 629 738 960 1234 1520 2092 946 1015 650 944 Properties Total Pore Volume, cc/g 0.3498 0.3961 0.1925 0.2827 0.3273 0.4093 0.4824 0.7576 1.159 0.4198 0.4518 0.2924 0.436 Micro Pore Volume, cc/g 0.3331 0.3752 0.1753 0.2433 0.2878 0.3662 0.4541 0.5533 0.7221 0.3575 0.3822 0.2478 0.3524 DR-Micro Pore Fraction, % 95.2 94.7 91.1 86.1 87.9 89.5 83.8 73 62.3 85.2 84.6 84.7 80.8 Crushing Strength, kg/sphere 1.992 1.855 >2.0 >2.0 >2.0 1.841 1.765 1.396 0.953 1.895 1.804 >2.0 1.897
975 1000
Burn off of all activated carbon samples has been calculated on the basis of carbonized sample and yield was calculated on the basis of phenolic resin spheres. Yield of all activated carbon samples decreases as burn off increases indicating the gasification reaction of element carbon with CO2 to form carbon mono oxide. Nitrogen Adsorption Isotherm The adsorption isotherms of activated samples produced by activation at 950C for different length of time are shown in Figure 3. In all the cases, maximum adsorption takes place in the low pressure region indicating that samples are predominantly micro porous in nature. Nitrogen adsorption in the higher-pressure region indicates the presence of mesopores.
Nitrogen Adsorption Isotherm of samples activated at 950 C
800 700 600 500 400 300 200 100 0 0.00E+00 2.00E-01 4.00E-01 6.00E-01 8.00E-01 1.00E+00 1.20E+00
Relative Pre ssure (P/P0)
Volume Adsorbed (cc/g)
3 hrs. 7 hrs. 9 hrs 12 hrs
Figure 3: Adsorption Isotherm of Activated Carbon Spheres at 77K Effect of Temperature To study the effect of temperature on the properties of activated carbon spheres, CO2 gas flow rate and the activation time was kept constant. The burn off increases and yield of ACS decreases on increasing temperature from
Effect of Activation Parameters on the Surface and Mechanical Properties of Activated Carbon Spheres
79
925C to 1000C from a value of 20.5% to 36.8% and 46.5% to 37.4%, respectively. Total pore volume of activated samples increases from a value of 0.3498 cc/g to 0.4360 cc/g on increasing the temperature from 925C to 1000C. It is due to the rate of reaction of element carbon with CO2 shifts towards forward direction on increasing temperature. Surface area and micro pore volume of activated samples (Fig. 4a & Fig. 4b) increase rapidly up to 950C from 858 m2/g to 960 m2/g and 0.3331 cc/g to 0.3662 cc/g, respectively and then decreases slowly to a value of 941 m2/g and 0.3524 cc/g at 1000C, respectively. Increase in surface area and micro pore volume up to 950C may be due to the creation of new micro pores and decrease in surface area and micro pore volume after 950C may be due to the widening of pores. Fig. 4a & Fig.4b shows the graphical representation of effect of temperature on the properties of ACS.
980 960 S urface area (m 2/g) 940 920 900 880 Surf ace area 860 840 900 Yield 950 1000
50
Total pore volum e (cc/g)
0.5 0.4 0.3
0.37
0.36
30 20 10 0 1050
0.35 0.2 0.1 0 900 Total pore volume Micropore volume 950 1000
0.34
0.33 1050
Temperature ( C)
Temperature ( C)
Figure 4(a)
Figure 4(b)
Figure 4(a) & (4b): Effect of Temperature on the Properties of Activated Carbon Spheres
Effect of Soaking Time To study the effect of soaking time on the surface properties of activated carbon spheres, the gas flow rate and activation temperature during activation process were kept constant. It has been observed that the Yield of ACS decreases and the total pore volume increases from 41.5% to 15.9% and 0.4093 cc/g to 1.159 cc/g on increasing the activation time from 3 hrs. to 12 hrs., respectively. This is due to the continuous increase in porosity with respect to time. The surface area and micro pore volume also increases continuously from a value of 960 m2/g to 2092 m2/g and 0.3662 cc/g to 0.7221 cc/g, respectively on increasing soaking time due to the creation of new micro pores (Figure 5(a) & Figure 5(b).
2500 Surface area (m2/g) 2000 1500 1000 500 0 0 5 10 15 Time (hrs.) Yield Surface area 0 50
Total pore volume (cc/g) 1.5 1.2 0.9 0.4 0.6 0.3 0 0 5 10 15 Time (hrs.) 0.2 Total pore volume Micropore volume 0.8 Micropore volume (cc/g)
40 30 20 10 Yield (%)
0.6
Figure 5(a)
Figure 5(b)
Figure 5(a) & 5(b): Effect of Soaking Time on the Properties of Activated Carbon Spheres
M icropore volum e
40 Y ield (% )
80
Jagpal Singh Mahur, Savita Dixit, Rakesh Shrivastava & C. S. Goswami
Effect of Gas Flow Rate To study the effect of CO2 gas flow rate during activation process on the micro porosity of activated carbon sphere, the activation time and temperature were kept constant. Fig. 6a & Fig. 6b shows the graphical representation of samples activated at 950C for 2 hrs soaking time at different gas flow rate. It has been observed that the yield of ACS decreases and total pore volume increases slowly from 49.3% to 45.2% and 0.1925 cc/g to 0.3273 cc/g on increasing the gas flow rate from 100 ml/min to 500 ml/min, respectively. BET Surface area and micro pore volume also increase very slowly from a value of 447 m2/g to 738 m2/g and 0.1753 cc/g to 0.2878 cc/g, respectively on increasing the flow rate from 100 ml/min to 500 ml/min. The slow change in surface properties may be due to the excess amount of CO2 remains unreacted during activation process.
800 Surface area (m2/g) 50 49 600 Yield (%) 48 400 Surface area Yield 47 46 45 44 600 Total pore volume (cc/g) 0.35 0.3 0.25 0.2 0.15 0.1 0 200 400 CO2 f low rate (ml/min.) Total pore volume Micropore volume 0.35 Micropore volume (cc/g) 0.3 0.25 0.2 0.15 0.1 0.05 0 600
200
0 0 200 400 CO2 f low rate (ml/min.)
Figure 6(a)
Figure 6(b)
Figure 6(a) & 6(b): Effect of Flow Rate on the Properties of Activated Carbon Spheres Effect of Surface Properties on Mechanical Strength Crushing strength of activated carbon spheres is strongly dependent on the surface properties of. An adverse effect was observed on the crushing strength of activated carbon spheres on increasing activation temperature, activation time and the flow of activating gas. The crushing strength of activated carbon spheres decreases sharply as surface area, burn off, total pore volume and micropore volume increases. A graphical representation of crushing strength of ACS with respect to surface properties has been shown in Figure 7(a) & Figure 7(b).
2500 Surface area (m2/g) 2000 1500 1000 500 0 0.600 Surface area Burn off
80 Total pore volume (cc/g) 65 50 35 20 5 2.100 Burn off (%)
1.4 1.2 1 0.8 0.6 0.4 0.2 0.500 Total pore volume Micropor volume 1.000 1.500
0.8 Micropore volume (cc/g) 0.7 0.6 0.5 0.4 0.3 0.2 0.1 2.000
1.100
1.600
Crushing strength (kg/sphere)
Crushing strength (kg/g)
Figure 7(a)
Figure 7(b)
Figure 7(a) & 7(b): Effect of Surface Properties on the Crushing Strength of Activated Carbon Spheres
Effect of Activation Parameters on the Surface and Mechanical Properties of Activated Carbon Spheres
81
DR Micropore Analysis The microporosity of activated carbon spheres activated at 950C for different length of time have been determined by DR equation given below: log W = log W0 m[log(KP0/P)]2 where, W represents the weight adsorbed at relative pressure P/P0, W0 is the total weight adsorbed in micropores, m is the slop of straight line and K = (Pc/P0)(T/Tc)2, where, critical pressure of adsorbate (mm Hg), P0 is the saturated vapor pressure of adsorbate (mm Hg), T is the adsorption temperature (K) and Tc is the critical temperature of adsorbate (K).
3.50E-02 Weight adsorbed (g)
2.50E-02
3 hrs. 7 hrs. 9 hrs.
1.50E-02
12 hrs.
5.00E-03 2.00E+00
6.00E+00 log^2 (P0/P)
1.00E+01
1.40E+01
Figure 8: DR plot of Carbon Spheres Activated for different Length of Time Linearity of the DR plot between log W versus log2(P/P0) over a wide range of relative pressure P/P0 shown in Fig.7 indicates narrow micropores are presents in all the samples of activated carbon spheres.
CONCLUSIONS
This study has demonstrated that activated carbon spheres with high surface area and high pore volume can be prepared from commercially available phenolic resin by controlling the activation conditions. BET surface area and micro pore volume of activated samples increase rapidly up to 950C from 858 m2/g to 960 m2/g and 0.3331 cc/g to 0.3662 cc/g, respectively and then decreases slowly to a value of 941 m2/g and 0.3524 cc/g at 1000C, respectively. Therefore, from this study, it was found that the optimum temperature for activation of phenolic resin spheres was 950C. Activation of phenolic resin spheres at 950C for 12 hrs soaking time found to have a surface area 2092 m2/g and total pore volume 1.159 cc/g. Total pore volume of activated samples increases from a value of 0.3498 cc/g to 0.436 cc/g on increasing the temperature from 925C to 1000C. Percent micro pore fraction of ACS increases when activation of PRS was carried out at relatively lower than the optimum temperature whereas it decreases when activation was carried out at relatively higher than the optimum temperature. The BET surface area, micro pore volume and total pore volume increase from a value of 960 m2/g to 2092 m2/g, 0.3662 cc/g to 0.7221 cc/g and 0.4093 cc/g to 1.159 cc/g respectively on increasing soaking time from 3 hrs. to 12 hrs. Small variation in the surface properties of activated carbon spheres was observed with changing the gas flow rate during
82
Jagpal Singh Mahur, Savita Dixit, Rakesh Shrivastava & C. S. Goswami
activation process. Surface area and micro pore volume increase very slowly from a value of 300 m2/g to 392 m2/g and 0.1198 cc/g to 0.1583 cc/g respectively on increasing the gas flow rate from 80 ml/min to 800 ml/min. The crushing strength of activated carbon spheres decreases sharply as surface area, burn off, total pore volume and micropore volume increases. The crushing was found to be greater than 2kg/sphere at the surface area below 738 m2/g. The crushing was found to be 0.953 kg/sphere at the highest surface area of 2092 m2/g.
ACKNOWLEDGEMENTS
Authors are thankful to M/s HEG Limited, Madideep, near Bhopal, (M.P.) for providing all necessary support to carry out the research work.
REFERENCES
1. Babe, K. Izabella Kruciska, Romualda Ciso, Magorzata Koszewska Comparative Analysis of the Properties of Active Carbon Fibres Obtained from Different Precursors FIBRES & TEXTILES in Eastern Europe October / December 2006, Vol. 14, No. 4 (58), P. 79-86. 2. Junbing, Yang. Ling Licheng, Liu Lang. Study on the activation characteristics of phenolic resin based spherical carbon. New carbon material (in Chinese) 1999; 14 (4): 11-16. 3. Junbing, Yang. Ling Licheng, Liu Lang. Preparation and properties of phenolic resin based spherical activated carbon. Extended Abstract, 24th biennial Conference on Carbon, Charleston (South Carolina, USA) P-676 677. 4. Lu, G.Q., Do, D.D., A kinetic study of coal reject-derived char activation with CO2, H2O, and air, Carbon, 30, 2129(1992). 5. Marsh H, Rand B. Process of Activation of Carbons by Gasification with carbon dioxide. I. Gasification of pure poly (furfuryl alcohol). Carbon 1971: 9 (1): 47-61. 6. Qiao W.M., Korai Y, Mochida I, Hori Y, Maeda T. Preparation of an activated carbon artifact: factors
influencing strength when using a thermoplastic polymer as binder. Carbon 2001; 39:2355-68. 7. Qiong Cai, Zheng-Hong Huang, Feiyu Kang, Jun Bing Yang. Preparation of activated carbon microspheres from phenolic resin by supercritical water activation. Carbon 42 (2004) P775-783. 8. Rodriguez-Reinoso F, Almela-Alarcon M, Linares-Solano A, Salinas-Martinez deLecea C, Sepulved-Escribano A. In: Extended abstracts, 17th biennal conference on carbon, 1985:273. 9. Rodriguez-Reinoso F, In: Lahaye J, Ehrburgor P, editors. Fundamental issues in control of carbon gasification reactivity. Dordrecht: Kluwer, 1991:533. 10. Silvestre-Albero, A. J., M. Ramos-Fernandez, M. Martinez-Escandell, A. Sepulveda-Escribano, J. SilvestreAlbero and F. Rodriguez-Reinoso High saturation capacity of activated carbons prepared from mesophase pitch in the removal of volatile organic compounds Carbon, 48, 548556 (2010). 11. Singh, Arjun., Neeta Verma, R K Yadav, Darshan Lal and V.S.Tripathi. Prepration of Activated Carbon from Phenolic beeds : Effect of Formaldehyde-to-Phenol Ratio. Carbon and Carbon Material Recent Trends (2003) P 169 176.
Effect of Activation Parameters on the Surface and Mechanical Properties of Activated Carbon Spheres
83
12. Singh, Arjun., Neeta Verma, R K Yadav, Darshan Lal and V.S.Tripathi. Prepration of Activated Carbon from Phenolic beeds : Effect of Activation Time. Carbon and Carbon Material Recent Trends (2003) P 169 176. 13. Srinivasakannan C. and Mohamad Zailani Abu Bakar, Production of activated carbon from rubber wood sawdust, Volume 27, Issue 1, July 2004, P. 89-96. 14. Takashi K. Control of pore structure in carbon, Carbon 2003; 38:269-86. 15. Teng H., Wang S.C. Influence of oxidation on the preparation of porous carbon from Phenol-Formaldehyde Resin with KOH activation. Ind. Eng. Chem. Res. 2000; 39, 673. 16. Vilaplana-Ortego, E. M.A. Lillo-Rodenas, J. Alcan iz-Monge, D. Cazorla-Amoros, A. Linares-Solano Isotropic petroleum pitch as a carbon precursor for the preparation of activated carbons by KOH activation Carbon, 4 7, (2009) 2112 2143. 17. Wei, SU ZHOU Li. and ZHOU Yaping Preparation of Microporous Activated Carbon from Raw Coconut Shell by Two-step Procedure Chinese J. Chem. Eng., 14(2) 266269 (2006). 18. Yang, Jun-Bing., Li-Cheng Ling, Lang Liu, Fei-Yu Kang, Zheng-Hong Huang, Hui Wu. Preparation and
properties of phenolic resin based activated carbon spheres with controlled pore size distribution. Carbon 40 (2002) P911-916. 19. Zhou, Su. W, L., Zhou, Y.P., Preparation of microporous activated carbon from coconut shells without activating agents, Carbon, 41, 861- 863 (2003).
Das könnte Ihnen auch gefallen
- Flame Retardant Textiles For Electric Arc Flash Hazards: A ReviewDokument18 SeitenFlame Retardant Textiles For Electric Arc Flash Hazards: A ReviewTJPRC PublicationsNoch keine Bewertungen
- Comparative Study of Original Paithani & Duplicate Paithani: Shubha MahajanDokument8 SeitenComparative Study of Original Paithani & Duplicate Paithani: Shubha MahajanTJPRC PublicationsNoch keine Bewertungen
- Baluchari As The Cultural Icon of West Bengal: Reminding The Glorious Heritage of IndiaDokument14 SeitenBaluchari As The Cultural Icon of West Bengal: Reminding The Glorious Heritage of IndiaTJPRC PublicationsNoch keine Bewertungen
- 2 52 1642055366 1ijpslirjun20221Dokument4 Seiten2 52 1642055366 1ijpslirjun20221TJPRC PublicationsNoch keine Bewertungen
- Using Nanoclay To Manufacture Engineered Wood Products-A ReviewDokument14 SeitenUsing Nanoclay To Manufacture Engineered Wood Products-A ReviewTJPRC PublicationsNoch keine Bewertungen
- 2 29 1645708157 2ijtftjun20222Dokument8 Seiten2 29 1645708157 2ijtftjun20222TJPRC PublicationsNoch keine Bewertungen
- Development and Assessment of Appropriate Safety Playground Apparel For School Age Children in Rivers StateDokument10 SeitenDevelopment and Assessment of Appropriate Safety Playground Apparel For School Age Children in Rivers StateTJPRC PublicationsNoch keine Bewertungen
- 2 52 1649841354 2ijpslirjun20222Dokument12 Seiten2 52 1649841354 2ijpslirjun20222TJPRC PublicationsNoch keine Bewertungen
- 2 33 1641272961 1ijsmmrdjun20221Dokument16 Seiten2 33 1641272961 1ijsmmrdjun20221TJPRC PublicationsNoch keine Bewertungen
- 2 4 1644229496 Ijrrdjun20221Dokument10 Seiten2 4 1644229496 Ijrrdjun20221TJPRC PublicationsNoch keine Bewertungen
- The Conundrum of India-China Relationship During Modi - Xi Jinping EraDokument8 SeitenThe Conundrum of India-China Relationship During Modi - Xi Jinping EraTJPRC PublicationsNoch keine Bewertungen
- 2 31 1648794068 1ijpptjun20221Dokument8 Seiten2 31 1648794068 1ijpptjun20221TJPRC PublicationsNoch keine Bewertungen
- 2 51 1647598330 5ijmpsjun202205Dokument10 Seiten2 51 1647598330 5ijmpsjun202205TJPRC PublicationsNoch keine Bewertungen
- 2 51 1651909513 9ijmpsjun202209Dokument8 Seiten2 51 1651909513 9ijmpsjun202209TJPRC PublicationsNoch keine Bewertungen
- 2 44 1653632649 1ijprjun20221Dokument20 Seiten2 44 1653632649 1ijprjun20221TJPRC PublicationsNoch keine Bewertungen
- 2 67 1653022679 1ijmperdjun202201Dokument12 Seiten2 67 1653022679 1ijmperdjun202201TJPRC PublicationsNoch keine Bewertungen
- 2 51 1656420123 1ijmpsdec20221Dokument4 Seiten2 51 1656420123 1ijmpsdec20221TJPRC PublicationsNoch keine Bewertungen
- Covid-19: The Indian Healthcare Perspective: Meghna Mishra, Dr. Mamta Bansal & Mandeep NarangDokument8 SeitenCovid-19: The Indian Healthcare Perspective: Meghna Mishra, Dr. Mamta Bansal & Mandeep NarangTJPRC PublicationsNoch keine Bewertungen
- Effect of Degassing Pressure Casting On Hardness, Density and Tear Strength of Silicone Rubber RTV 497 and RTV 00A With 30% Talc ReinforcementDokument8 SeitenEffect of Degassing Pressure Casting On Hardness, Density and Tear Strength of Silicone Rubber RTV 497 and RTV 00A With 30% Talc ReinforcementTJPRC PublicationsNoch keine Bewertungen
- An Observational Study On-Management of Anemia in CKD Using Erythropoietin AlphaDokument10 SeitenAn Observational Study On-Management of Anemia in CKD Using Erythropoietin AlphaTJPRC PublicationsNoch keine Bewertungen
- Self-Medication Prevalence and Related Factors Among Baccalaureate Nursing StudentsDokument8 SeitenSelf-Medication Prevalence and Related Factors Among Baccalaureate Nursing StudentsTJPRC PublicationsNoch keine Bewertungen
- Dr. Gollavilli Sirisha, Dr. M. Rajani Cartor & Dr. V. Venkata RamaiahDokument12 SeitenDr. Gollavilli Sirisha, Dr. M. Rajani Cartor & Dr. V. Venkata RamaiahTJPRC PublicationsNoch keine Bewertungen
- Effectiveness of Reflexology On Post-Operative Outcomes Among Patients Undergoing Cardiac Surgery: A Systematic ReviewDokument14 SeitenEffectiveness of Reflexology On Post-Operative Outcomes Among Patients Undergoing Cardiac Surgery: A Systematic ReviewTJPRC PublicationsNoch keine Bewertungen
- Analysis of Bolted-Flange Joint Using Finite Element MethodDokument12 SeitenAnalysis of Bolted-Flange Joint Using Finite Element MethodTJPRC PublicationsNoch keine Bewertungen
- A Review of "Swarna Tantram"-A Textbook On Alchemy (Lohavedha)Dokument8 SeitenA Review of "Swarna Tantram"-A Textbook On Alchemy (Lohavedha)TJPRC PublicationsNoch keine Bewertungen
- 2 67 1645871199 9ijmperdfeb202209Dokument8 Seiten2 67 1645871199 9ijmperdfeb202209TJPRC PublicationsNoch keine Bewertungen
- Vitamin D & Osteocalcin Levels in Children With Type 1 DM in Thi - Qar Province South of Iraq 2019Dokument16 SeitenVitamin D & Osteocalcin Levels in Children With Type 1 DM in Thi - Qar Province South of Iraq 2019TJPRC PublicationsNoch keine Bewertungen
- 2 67 1645017386 8ijmperdfeb202208Dokument6 Seiten2 67 1645017386 8ijmperdfeb202208TJPRC PublicationsNoch keine Bewertungen
- 2 67 1648211383 1ijmperdapr202201Dokument8 Seiten2 67 1648211383 1ijmperdapr202201TJPRC PublicationsNoch keine Bewertungen
- Numerical Analysis of Intricate Aluminium Tube Al6061T4 Thickness Variation at Different Friction Coefficient and Internal Pressures During BendingDokument18 SeitenNumerical Analysis of Intricate Aluminium Tube Al6061T4 Thickness Variation at Different Friction Coefficient and Internal Pressures During BendingTJPRC PublicationsNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- ICMT Valve With ICAD Motor Control Using A Pressure Input SignalDokument2 SeitenICMT Valve With ICAD Motor Control Using A Pressure Input SignalvickersNoch keine Bewertungen
- Sadi Mohammad Naved: Duties/ResponsibilitiesDokument3 SeitenSadi Mohammad Naved: Duties/ResponsibilitiesNick SanchezNoch keine Bewertungen
- DesuperheatersDokument8 SeitenDesuperheatersmuhdrijasmNoch keine Bewertungen
- Cisco Catalyst Switching Portfolio (Important)Dokument1 SeiteCisco Catalyst Switching Portfolio (Important)RoyalMohammadkhaniNoch keine Bewertungen
- ParkerOriga PDFDokument338 SeitenParkerOriga PDFilyesNoch keine Bewertungen
- cGMP ChecklistDokument31 SeitencGMP ChecklistWerner Schrammel100% (1)
- IECDokument52 SeitenIECM.r. Munish100% (2)
- DNS Amplification Attacks ExplainedDokument13 SeitenDNS Amplification Attacks ExplainedhammNoch keine Bewertungen
- DTMF Proximity DetectorDokument1 SeiteDTMF Proximity DetectorAlagappan ArunachalamNoch keine Bewertungen
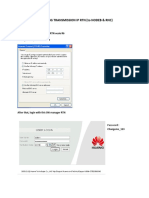
- How to ping NodeB and RNC using RTN transmission IPDokument14 SeitenHow to ping NodeB and RNC using RTN transmission IPPaulo DembiNoch keine Bewertungen
- Animal-Cube-Puzzle I e LTR PDFDokument3 SeitenAnimal-Cube-Puzzle I e LTR PDFJose Oswaldo Sierra MatheusNoch keine Bewertungen
- UDRPDokument10 SeitenUDRPDomainNameWire100% (1)
- BASH Shell Scripting SyllabusDokument4 SeitenBASH Shell Scripting SyllabusAdzmely Mansor100% (1)
- Maharashtra State Electricity Distribution Co - LTD., O & M Division, NANDURBARDokument3 SeitenMaharashtra State Electricity Distribution Co - LTD., O & M Division, NANDURBARPuru BornareNoch keine Bewertungen
- Experimental Design and Optimization of Free EnergDokument5 SeitenExperimental Design and Optimization of Free Energesubalew tadesseNoch keine Bewertungen
- Ahmad Ridzuan Ibrahim (CD 5103)Dokument24 SeitenAhmad Ridzuan Ibrahim (CD 5103)Kabil RajNoch keine Bewertungen
- Sda-02-Dd-02 - Pile & Pile Cap - Sheet-1 - R0Dokument1 SeiteSda-02-Dd-02 - Pile & Pile Cap - Sheet-1 - R0Himani PatelNoch keine Bewertungen
- Model 2000 Flow ComputerDokument8 SeitenModel 2000 Flow ComputerAdnan SalihbegovicNoch keine Bewertungen
- Plan for Inspection and Testing of LV Power CablesDokument1 SeitePlan for Inspection and Testing of LV Power CablesRami KsidaNoch keine Bewertungen
- Cyber Dynamic Line UsDokument8 SeitenCyber Dynamic Line UsMilan PitovicNoch keine Bewertungen
- West Bengal's Leather Industry OpportunitiesDokument5 SeitenWest Bengal's Leather Industry OpportunitiesDeepak AgarwalNoch keine Bewertungen
- NV 24 Globe ActuatorDokument12 SeitenNV 24 Globe ActuatorRodrigo AlvesNoch keine Bewertungen
- Iphone 6 Full schematic+IC BoardDokument86 SeitenIphone 6 Full schematic+IC BoardSIDNEY TABOADANoch keine Bewertungen
- ASME B31.3 2020 CambiosDokument10 SeitenASME B31.3 2020 CambiosJosé Juan Jiménez AlejandroNoch keine Bewertungen
- PGCB ReportDokument36 SeitenPGCB ReportNayemul Hasan NayemNoch keine Bewertungen
- Sap Abap Programming SyllabusDokument5 SeitenSap Abap Programming SyllabusSURAJ KUMAR SAHUNoch keine Bewertungen
- C++ Chapter 12 - ClassesDokument62 SeitenC++ Chapter 12 - Classesعلي العريبيNoch keine Bewertungen
- Call of Duty MG08/15 LMG Weapon WikiDokument1 SeiteCall of Duty MG08/15 LMG Weapon WikiSelin HNoch keine Bewertungen
- AeDokument12 SeitenAeRoberto SanchezNoch keine Bewertungen
- CIRCULAR WATER TANK DESIGN-Layout1 AkhilDokument1 SeiteCIRCULAR WATER TANK DESIGN-Layout1 AkhilVENKAT KALYANNoch keine Bewertungen