Beruflich Dokumente
Kultur Dokumente
Medical Physics, Lecture-5.Ppt (Compatibility Mode)
Hochgeladen von
fadhaliOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Medical Physics, Lecture-5.Ppt (Compatibility Mode)
Hochgeladen von
fadhaliCopyright:
Verfügbare Formate
12/22/2011
Medical Physics
Thermal Physics
Dr. Mohamed Al-Fadhali
12/22/2011
Properties of materials change with temperature
Length Volume Resistance
He is hot
I feel hot
2 Dr. Mohamed Al- Fadhali
12/22/2011
Temperature Scales
Thermometers can be calibrated by placing them in thermal contact with an environment that remains at constant temperature Environment could be mixture of ice and water in thermal equilibrium Also commonly used is water and steam in thermal equilibrium
Celsius Scale
Temperature of an ice-water mixture is defined as 0 C This is the freezing point of water Temperature of a water-steam mixture is defined as 100 C This is the boiling point of water Distance between these points is divided into 100 segments
Kelvin Scale
When the pressure of a gas goes to zero, its temperature is 273.15 C This temperature is called absolute zero This is the zero point of the Kelvin scale (273.15 C = 0 K)
To convert: TC = TK 273.15
Fahrenheit Scales
Most common scale used in the US Temperature of the freezing point is 32 Temperature of the boiling point is 212 180 divisions between the points
12/22/2011
Comparing Temperature Scales
TC = TK - 273.15 9 TF = TC + 32 5 9 DTF = DTC 5
Type of Thermometer
v Change in electrical resistance (convenient
but not very linear) v Change in length of a bar (bimetallic strip) v Change in volume of a liquid v Change in volume of gas (very accurate but slow and bulky)
12/22/2011
Thermal Expansion
The thermal expansion of an object is a consequence of the
change in the average separation between its constituent atoms or molecules At ordinary temperatures, molecules vibrate with a small amplitude As temperature increases, the amplitude increases This causes the overall object as a whole to expand Rails expand and may buckle on a hot summer day
In most liquids or solids, when temperature rises molecules have more kinetic energy they are moving faster, on the average consequently, things tend to expand (works for a gas) amount of expansion DL depends on change in temperature DT L0 original length L0 coefficient of thermal expansion L0 + DL = L0 + a L0 DT DL = a L0 DT (linear expansion) V DV = b V0 DT (volume expansion) b @ 3a
DL
V + DV
12/22/2011
Linear (area, volume) Expansion
v For small changes in temperature
v The coefficient of linear expansion,
material
DL = a Lo Dt
a depends on the
v Similar in two dimensions (area expansion)
DA = g Ao Dt , g = 2a
v and in three dimensions (volume expansion)
DV = b Vo Dt for solids, b = 3a
Thermal Expansion and Teeth
Crazing:
Thermal expansion matching is important in choosing materials for fillings.
12/22/2011
Thermal Expansion and Teeth
Coefficients of linear expansion: Enamel: 11.4 x 10-6 C Dentin: 8.3 x 10-6 C
If you quickly switch from eating/drinking something hot to something cold, the brittle enamel will contract more than the dentin, and develop small cracks called crazes.
Example
A copper telephone wire has essentially no sag between poles 35.0 m apart on a winter day when the temperature is 20.0C. How much longer is the wire on a summer day when TC = 35.0C? Assume that the thermal coefficient of copper is constant throughout this range at its room temperature value.
12/22/2011
Applications of Thermal Expansion
1. Thermostats
Use a bimetallic strip Two metals expand differently
2. Pyrex Glass
Thermal stresses are smaller than for ordinary glass
3. Train rails
Keeping space between joints for possible expansion and contraction
Ideal Gas
Properties of gases o A gas does not have a fixed volume or pressure o In a container, the gas expands to fill the container Ideal gas: o Collection of atoms or molecules that move randomly o Molecules exert no long-range force on one another o Molecules occupy a negligible fraction of the volume of their container Most gases at room temperature and pressure behave approximately as an ideal gas
Moles
v Its convenient to express the amount of gas in a given volume in terms of the
number of moles, n
n=
mass molar mass
v One mole is the amount of the substance that contains as many particles as
there are atoms in 12 g of carbon-12
12/22/2011
Avogadros Hypothesis
Equal volumes of gas at the same temperature and pressure
contain the same numbers of molecules Consequences: At standard temperature and pressure, one mole quantities of all gases contain the same number of molecules This number is called Avogadros Number (NA =6.02 x 1023 particles / mole) Can also look at the total number of particles: N = n NA
The mass of an individual atom can be calculated as follows:
matom =
molar mass NA
The Ideal Gas Law
pV = nRT
What is the volume of 1 mol of gas at STP ? T = 0 C = 273 K 5 p = 1 atm = 1.01 x 10 Pa
V RT = n P 8 . 31 J / (mol K ) 273 K = 1 . 01 x 10 5 Pa = 0 . 0224 m 3 = 22 . 4 l
12/22/2011
Equation of State for an Ideal Gas
v Boyles Law
At a constant temperature, pressure is inversely proportional to the volume v Charles Law At a constant pressure, the temperature is directly proportional to the volume v Gay-Lussacs Law At a constant volume, the pressure is directly proportional to the temperature
Ideal Gas Law
v Summarizes Boyles Law, Charles Law, and Guy-
Lussacs Law PV = n R T
R is the Universal Gas Constant R = 8.31 J / mole K R = 0.0821 L atm / mole K
P V = N kB T
kB is Boltzmanns Constant kB = R / NA = 1.38 x 10-23 J/ K
12/22/2011
Questions
1. An ideal gas is confined to a container with constant volume. The number of moles is constant. By what factor will the pressure change if the absolute temperature triples? a. 1/9 b. 1/3 c. 3.0 d. 9.0 2. An ideal gas is confined to a container with adjustable volume. The number of moles and temperature are constant. By what factor will the volume change if pressure triples? a. 1/9 b. 1/3 c. 3.0 d. 9.0
Volume and Pressure of a Gas
In the kelvin scale, the lowest possible temperature is 0 K. (zero volume and zero pressure) Any two temperatures defined by the ratio
p1 T2 = p2 T1 or V1 T2 = V2 T1
The zero point is fixed Absolution Zero (-273.15C)
Example A bottle of hair spray is filled to a pressure of 1atm at 20C What is the canister pressure if it is placed into boiling water? p1 T2 = p2 T1 1 x 373 = p2 x 293
p2 = 373/293 p2 = 1.27 atm
10
12/22/2011
Pressure of an Ideal Gas
The pressure is proportional
to the number of molecules per unit volume and to the average translational kinetic energy of a molecule
P=
3 N 1 2 mv 2 V 2
Molecular Interpretation of Temperature
Temperature is proportional to the average kinetic energy of the
molecules
1 mv 2
KE
=
=
3 k BT 2
3 nRT 2
The total kinetic energy is proportional to the absolute temperature
total
In a monatomic gas, the KE is the only type of energy the molecules
can have
U =
3 nRT 2
U is the internal energy of the gas In a polyatomic gas, additional possibilities for contributions to the
internal energy are rotational and vibrational energy in the molecules
11
12/22/2011
Speed of the Molecules
Expressed as the root-mean-square (rms) speed
vrms =
3 kB T 3RT = m M
At a given temperature, lighter molecules move
faster, on average, than heavier ones
Lighter molecules can more easily reach escape speed from the earth
Internal Energy vs. Heat
v Internal Energy, U, is the energy associated with the
microscopic components of the system
Includes kinetic and potential energy associated with the random translational, rotational and vibrational motion of the atoms or molecules Also includes the intermolecular potential energy
v Heat is energy transferred between a system and its
environment because of a temperature difference between them
The system Q is used to represent the amount of energy transferred by heat between a system and its environment
12
12/22/2011
Units of Heat
v Calorie
Units SI CGS Joule (J) Calorie (cal)
An historical unit, before the connection between thermodynamics and mechanics was recognized A calorie is the amount of energy necessary to raise the temperature of 1 g of water from 14.5 C to 15.5 C .
A Calorie (food calorie) is 1000 cal
1 cal = 4.186 J
This is called the Mechanical Equivalent of Heat
v BTU (US Customary Unit)
BTU stands for British Thermal Unit A BTU is the amount of energy necessary to raise the temperature of 1 lb of water from 63 F to 64 F
Specific Heat
v Every substance requires a unique amount of energy per
unit mass to change the temperature of that substance by 1 C directly proportional to mass (thus, per unit mass) v The specific heat, c, of a substance is a measure of this amount
c=
Q m DT
13
12/22/2011
Example1:
How much heat is needed to raise temperature of aluminum by 5C?
Given: Mass: m=0.5 kg Temp. DT= 5 Specific heat: cAl =900 J/kgC
Heat is related to mass and temperature by
Q = mc Al D T
= (0.5kg ) 900 J kg o C + 5o C = +2250 Joules
)(
Thus, energy is flowing into the system! Find:
Q=?
Calorimeter
A technique for determining the specific heat of a
substance is called calorimetry A calorimeter is a vessel that is a good insulator that allows a thermal equilibrium to be achieved between substances without any energy loss to the environment
Calorimetry
Analysis performed using a calorimeter Conservation of energy applies to the isolated system The energy that leaves the warmer substance equals the energy that enters the water Qcold = -Qhot Negative sign keeps consistency in the sign convention of T
14
12/22/2011
Example: A 0.010-kg piece of unknown metal heated to 100C and dropped into the bucket containing 0.5 kg of water at 20C. Determine specific heat of metal if the final temperature of the system is 50C
Given: m1=0.010 kg m2=0.5 kg Specific heat (water): cW =4186 J/kgC Temperatures: T1=100 C T2=20 C Tf=50 C Find: Specific heat =? Mass: Conservation of energy: heat lost by metal is the same as heat acquired by water:
Qwater + Qmetal = 0
Solve this equation:
Qwater + Qmetal = 0 = mmetalcmetalDTmetal + mH2OcH 2ODTH2O = (- 0.5)cmetal + 62790J = 0
5
= (0.01kg)cmetal 50o C - 100o C + (0.5kg) 4186J kgoC 50o C - 20o C
)(
cmetal = 1.25 10 J kg C
o
iron
Latent Heat
During a phase change, the amount of heat is given as
Q=mL L is the latent heat of the substance
Latent means hidden or concealed
Choose a positive sign if you are adding energy to the
system and a negative sign if energy is being removed from the system Latent heat of fusion is used for melting or freezing Latent heat of vaporization is used for boiling or condensing
15
12/22/2011
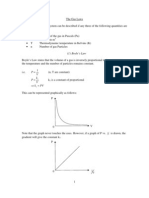
Graph of Ice to Steam
Methods of Heat Transfer
v Methods include Conduction Convection Radiation
1. Conduction
The transfer can be viewed on an atomic scale It is an exchange of energy between microscopic particles by collisions Less energetic particles gain energy during collisions from more energetic particles Rate of conduction depends upon the characteristics of the substance
16
12/22/2011
Conduction example
The molecules vibrate about their equilibrium positions Particles near the flame vibrate with larger amplitudes These collide with adjacent molecules and transfer some energy Eventually, the energy travels entirely through the rod
Conduction can occur only if there is a difference in temperature between two parts of the conducting medium
Conduction
The slab allows energy to
transfer from the region of higher temperature to the region of lower temperature P= Q T -T = kA h c t L
Thermal conductivity
Heat flow
17
12/22/2011
P=
Q T -T = kA h c t L
A is the cross-sectional area L = x is the thickness of the slab or the
length of a rod P is in Watts when Q is in Joules and t is in seconds k is the thermal conductivity of the material
Good conductors have high k values and good insulators have low k values
2. Convection
v Energy transferred by the
movement of a substance
When the movement results from differences in density, it is called natural conduction When the movement is forced by a fan or a pump, it is called forced convection
18
12/22/2011
Convection example
Air directly above the
flame is warmed and expands The density of the air decreases, and it rises The mass of air warms the hand as it moves by Applications:
Radiators Cooling automobile engines
3. Radiation
v Radiation does not require physical contact v All objects radiate energy continuously in
the form of electromagnetic waves due to thermal vibrations of the molecules v Rate of radiation is given by Stefans Law
19
12/22/2011
Radiation example
The electromagnetic waves carry the energy from
the fire to the hands No physical contact is necessary
Radiation equation
P = AeT4
P is the rate of energy transfer, in Watts = 5.6696 x 10-8 W/m2 K4 A is the surface area of the object e is a constant called the emissivity
e varies from 0 to 1
T is the temperature in Kelvins
20
12/22/2011
Energy Absorption and Emission by Radiation
With its surroundings, the rate at which the
object at temperature T with surroundings at To radiates is
Pnet = Ae(T4 T4o) When an object is in equilibrium with its surroundings, it radiates and absorbs at the same rate
Its temperature will not change
Example: Determine solar energy over the area of 1 m . Temperature of
2
Suns surface is 6000 K and temperature of surroundings is 300 K.
Given: Area: A= 1 m2 Temperatures: T1=6000 K T2=300 K Use Stefans law:
Power = sAe (T 4 - T04 )
Temperature of Suns surface
Temperature on the Earth
Find: Power =?
Power = sAe (6000 K 4 - 300 K 4 ) = 7.3 10 7 J s
= (5.67 10 - 8 )(1m 2 )(1)(1.3 1015 K 4 )
21
12/22/2011
Ideal Absorbers and Reflectors An ideal absorber is defined as an object that absorbs all of the energy incident on it e=1 This type of object is called a black body
An ideal absorber is also an ideal radiator of energy
An ideal reflector absorbs none of the energy incident on it e=0
Applications of Radiation
Clothing
Black fabric acts as a good absorber White fabric is a better reflector
Thermography
The amount of energy radiated by an object can be measured with a thermograph
Body temperature
Radiation thermometer measures the intensity of the infrared radiation from the eardrum
22
12/22/2011
Question
The use of fiberglass insulation in the outer walls of a building is intended to minimize heat transfer through what process? a. conduction b. radiation c. convection d. vaporization
Work in Thermodynamic Processes
v State of a system Description of the system in terms of state variables
Pressure Volume Temperature Internal Energy
A macroscopic state of an isolated system can be specified only if the system is in internal thermal equilibrium
23
12/22/2011
Work
Work is an important energy transfer
mechanism in thermodynamic systems Heat is another energy transfer mechanism Example: gas cylinder with piston
o The gas is contained in a cylinder with a moveable piston o The gas occupies a volume V and exerts pressure P on the walls of the cylinder and on the piston
A force is applied to
slowly compress the gas
The compression is slow enough for all the system to remain essentially in thermal equilibrium
W = - P V
This is the work done on the gas
24
12/22/2011
Work on a Gas Cylinder
W = - P V
When the gas is compressed V is negative The work done on the gas is positive When the gas is allowed to expand V is positive The work done on the gas is negative When the volume remains constant No work is done on the gas
Notes about the Work Equation
W = - P V If the pressure remains constant during the expansion or compression, the process is called an isobaric process If the pressure changes, the average pressure may be used to estimate the work done
W = - P V
Work=Area under the curve Work done on the gas
25
12/22/2011
Question
Find work done by the gas in this cycle.
P2
P1
Note: work is equal to the area:
W = ( p2 - p1 )(V2 - V1 )
V1 V2
Other Processes
Isovolumetric Volume stays constant Vertical line on the PV diagram Isothermal Temperature stays the same Adiabatic No heat is exchanged with the surroundings
26
12/22/2011
Example:
Calculate work done by expanding gas of 1 mole if initial pressure is 4000 Pa, initial volume is 0.2 m3, and initial temperature is 96.2 K. Assume a two processes: (1) isobaric expansion to 0.3 m3, Tf=144.3 K (2) isothermal expansion to 0.3 m3.
Given: n = 1 mole Ti = 96.2 K Tf = 144.3 K Vi = 0.2 m3 Vf = 0.3 m3 P = const Find: 1. Isobaric expansion:
W = PDV = P (V f - Vi ) = 4000 Pa (0.3m 3 - 0.2m3 ) = 400 J
Also:
Tf Ti Pf V f = PiVi
3 nR = V f = 0.3m = 1.5 Vi 0.2m3 nR
W=?
A 50% increase in temperature!
Example:
Calculate work done by expanding gas of 1 mole if initial pressure is 4000 Pa, initial volume is 0.2 m3, and initial temperature is 96.2 K. Assume a two processes: (1) isobaric expansion to 0.3 m3, Tf=144.3 K (2) isothermal expansion to 0.3 m3. Given: n = 1 mole Ti = 96.2 K Vi = 0.2 m3 Vf = 0.3 m3 T = const 2. Isothermal expansion:
V f W = nRT ln V i Vf = PiVi ln V i 3 0 .3 m = (4000 Pa ) 0.2 m 3 ln = 324 J 0 .2 m 3
Find:
Also:
Pf = Pi Vi 0.2m3 = 4000 Pa = 2667 Pa Vf 0.3m3
W=?
A ~67% decrease in pressure!
27
12/22/2011
Processes for Transferring Energy
v By doing work
Requires a macroscopic displacement of the point of application of a force
v By heat
Occurs by random molecular collisions
v Results of both
Change in internal energy of the system Generally accompanied by measurable macroscopic variables
Pressure Temperature Volume
First Law of Thermodynamics
v Consider energy conservation in thermal processes. Must include:
Q (Heat) Positive if energy is transferred to the system W (Work) Positive if done on the system U(Internal energy ) Positive if the temperature increases The relationship among U, W, and Q can be expressed as
U = Uf Ui = Q + W
This means that the change in internal energy of a system is equal to the sum of the energy transferred across the system boundary by heat and the energy transferred by work
28
12/22/2011
Example:
If 500 J of heat added to ideal gas that is expanding from 0.2 m3 to 0.3 m3 at a constant pressure of 4000 Pa, what is the change in its internal energy?
Given: n = 1 mole Vi = 0.2 m3 Vf = 0.3 m3 P = const Q=500 J 1. Isobaric expansion:
W = PDV = P (V f - Vi ) = 4000 Pa (0.3m 3 - 0.2m3 ) = 400 J
Use 1st law of thermodynamics: Find:
Q = DU + W DU = Q - W = 500 J - 400 J = 100 J
DU=?
What if volume is kept constant?
The First Law and Human Metabolism
v The First Law can be applied to living organisms v The internal energy stored in humans goes into
other forms needed by the organs and into work and heat v The metabolic rate (U / T) is directly proportional to the rate of oxygen consumption by volume
Basal metabolic rate (to maintain and run organs, etc.) is about 80 W
29
12/22/2011
Various Metabolic Rates
Fig. T12.1, p. 369 Slide 11
30
Das könnte Ihnen auch gefallen
- 17 Thermal-Heat and Kinetics GasDokument34 Seiten17 Thermal-Heat and Kinetics Gaskirana wahyuniNoch keine Bewertungen
- P103 Chapter10 TAT wk3wk4Dokument62 SeitenP103 Chapter10 TAT wk3wk4Muhammad SaeedNoch keine Bewertungen
- Chapter11 PDFDokument43 SeitenChapter11 PDFsailas simukanzyaNoch keine Bewertungen
- Lec. 2Dokument32 SeitenLec. 2Ali. AboudNoch keine Bewertungen
- Phys 2 Lecture 01 Thermodynamics-1Dokument19 SeitenPhys 2 Lecture 01 Thermodynamics-1Maruja TheaNoch keine Bewertungen
- Laboratory: Submitted To: Mabel YaconDokument16 SeitenLaboratory: Submitted To: Mabel YaconMacky NoveraNoch keine Bewertungen
- Thermal Physics Key ConceptsDokument43 SeitenThermal Physics Key ConceptsFarhan Mukhtiar YousafzaiNoch keine Bewertungen
- Thermal Physics IB Physics Topic 3 & Option CDokument55 SeitenThermal Physics IB Physics Topic 3 & Option CMoiz Dhanerawala100% (1)
- Thermal Properties of MatterDokument49 SeitenThermal Properties of MatterAbhisheak DineshNoch keine Bewertungen
- Thermodynamics ReviewerDokument118 SeitenThermodynamics ReviewerAngelo Luigi Yasay100% (2)
- Thermal PhysicsDokument9 SeitenThermal Physicssrivastava_binduNoch keine Bewertungen
- Thermal Properties of MatterDokument31 SeitenThermal Properties of MatterShanmuga PriyaNoch keine Bewertungen
- Chapter Ten Thermodynamics: Temperature and The Zeroth Law of ThermodynamicsDokument7 SeitenChapter Ten Thermodynamics: Temperature and The Zeroth Law of ThermodynamicsTony AtefNoch keine Bewertungen
- Lecture1 Physics2Dokument13 SeitenLecture1 Physics2Kathryn IcuspitNoch keine Bewertungen
- Lec-1 Intro To ThermodynamicsDokument37 SeitenLec-1 Intro To ThermodynamicsMuhammad SarmadNoch keine Bewertungen
- Transformation by SteamDokument43 SeitenTransformation by SteamLlama jennerNoch keine Bewertungen
- TEMPERATURE AND THERMOMETRY: MEASURING HEATDokument8 SeitenTEMPERATURE AND THERMOMETRY: MEASURING HEATElizabeth AnyegaNoch keine Bewertungen
- Oleh: Dewi Ekawati Indah Dwiphayanti Laelatul Fitriani Juwita Chandra DewiDokument31 SeitenOleh: Dewi Ekawati Indah Dwiphayanti Laelatul Fitriani Juwita Chandra DewiNurulWardhani11Noch keine Bewertungen
- Chapter 5 Gas Laws and Kinetic Theory - 2Dokument43 SeitenChapter 5 Gas Laws and Kinetic Theory - 2Rahim RahimunNoch keine Bewertungen
- Thermodynamics NotesDokument14 SeitenThermodynamics NotesFairy QueenNoch keine Bewertungen
- Intro To ThermodynamicsDokument37 SeitenIntro To ThermodynamicsOmer IqbalNoch keine Bewertungen
- Chapter Ten Lecture Ten Thermodynamics: TemperatureDokument16 SeitenChapter Ten Lecture Ten Thermodynamics: TemperatureTony AtefNoch keine Bewertungen
- Thermal Physics: 1. Temperature and The Zeroth Law of ThermodynamicsDokument9 SeitenThermal Physics: 1. Temperature and The Zeroth Law of ThermodynamicsHanazonoSakuraNoch keine Bewertungen
- 1.4.6 To 1.4 Gases Notes and ReviewDokument16 Seiten1.4.6 To 1.4 Gases Notes and ReviewEmpress ZNoch keine Bewertungen
- Thermodynamics basics and heat transferDokument20 SeitenThermodynamics basics and heat transferarunyogNoch keine Bewertungen
- Thermal Physics TemperatureDokument8 SeitenThermal Physics TemperatureJosh CohenNoch keine Bewertungen
- Ch17 Young Freedman2Dokument14 SeitenCh17 Young Freedman2Andrew MerrillNoch keine Bewertungen
- Applied Physics: Lec - 1: Introduction To ThermodynamicsDokument37 SeitenApplied Physics: Lec - 1: Introduction To ThermodynamicsTayyaba TanveerNoch keine Bewertungen
- Chapter Four Heat and ThermodynamicsDokument44 SeitenChapter Four Heat and ThermodynamicsmesfinNoch keine Bewertungen
- Unit 1: Thermodynamics Grade 12Dokument25 SeitenUnit 1: Thermodynamics Grade 12mesfinNoch keine Bewertungen
- Thermodynamics ReviewerDokument118 SeitenThermodynamics ReviewerRenz Karl DeclaroNoch keine Bewertungen
- Physics Ch3 NotesDokument4 SeitenPhysics Ch3 NotesAli GorganiNoch keine Bewertungen
- State of Matter-Gas)Dokument48 SeitenState of Matter-Gas)bigsnailz100% (1)
- 11.Fisika1-Kalor KinetikDokument47 Seiten11.Fisika1-Kalor KinetikWahyu Septriandi SaktiNoch keine Bewertungen
- Heat and TempDokument74 SeitenHeat and TempPortia A. EgkenNoch keine Bewertungen
- PHY112 Lecture1Dokument131 SeitenPHY112 Lecture18wc9sncvpwNoch keine Bewertungen
- Topic+2 +1st+Law+Thermodynamics+1+Aug+2022Dokument40 SeitenTopic+2 +1st+Law+Thermodynamics+1+Aug+2022mpumelaqqNoch keine Bewertungen
- Chapter 19Dokument47 SeitenChapter 19maxim santos100% (1)
- Physics 06-Temperature, Heat, and Thermodynamics (2018)Dokument123 SeitenPhysics 06-Temperature, Heat, and Thermodynamics (2018)This is ScribeNoch keine Bewertungen
- Chem - Week - 3Dokument30 SeitenChem - Week - 3cadaxeshpatelNoch keine Bewertungen
- Thermal Energy Transfer and Equilibrium ExplainedDokument12 SeitenThermal Energy Transfer and Equilibrium ExplainedBoedisantosoNoch keine Bewertungen
- L 5 - Specific HeatDokument51 SeitenL 5 - Specific Heatمصطفى العباديNoch keine Bewertungen
- Course: B.Tech Mechanical Subject: Elements of Mechanical Engineering Unit-1Dokument84 SeitenCourse: B.Tech Mechanical Subject: Elements of Mechanical Engineering Unit-1Amogh VaishnavNoch keine Bewertungen
- Thermodynamics I: Temperature, Heat and Gas LawsDokument19 SeitenThermodynamics I: Temperature, Heat and Gas LawsAbdelkader Faklani DouNoch keine Bewertungen
- Detailed Notes - Section 06 Thermal Physics - AQA Physics A-LevelDokument9 SeitenDetailed Notes - Section 06 Thermal Physics - AQA Physics A-LevelDeepesh SureshNoch keine Bewertungen
- Heat Capacity and Thermal ExpansionDokument14 SeitenHeat Capacity and Thermal ExpansionSunday Glo M. CabuyaoNoch keine Bewertungen
- Temperature and HeatDokument48 SeitenTemperature and Heatalexzandrei.rara937Noch keine Bewertungen
- Thermal PhysicsDokument24 SeitenThermal PhysicsSuraj GopaulNoch keine Bewertungen
- 11 State of Matter Study NotesDokument15 Seiten11 State of Matter Study NotesVivek KumarNoch keine Bewertungen
- Temperature: Can Be Thought of AsDokument5 SeitenTemperature: Can Be Thought of AsTarek Mohamed Ahmed AhmedNoch keine Bewertungen
- L102 13bDokument33 SeitenL102 13belangovanvnrNoch keine Bewertungen
- Grade 12 Physics NoteDokument35 SeitenGrade 12 Physics NoteNebilahNoch keine Bewertungen
- Joule Appparatus ManualDokument4 SeitenJoule Appparatus ManualBalRam DhimanNoch keine Bewertungen
- SFG 3023 Chapter 1Dokument67 SeitenSFG 3023 Chapter 1Nik AshrafNoch keine Bewertungen
- Chapter-6 Temperature - HeatDokument8 SeitenChapter-6 Temperature - Heat2220678Noch keine Bewertungen
- GAS Ideal Gas Law - Build Your Own Temperature Scale Lab Manual (English)Dokument5 SeitenGAS Ideal Gas Law - Build Your Own Temperature Scale Lab Manual (English)Isiwjsbnwhshz Hshshzhbshs0% (1)
- Physics 2 1Dokument104 SeitenPhysics 2 1Kimberly GonzalesNoch keine Bewertungen
- Thermodynamics AllDokument141 SeitenThermodynamics AllSidharth AryaNoch keine Bewertungen
- Survey On Student Learning Outcomes Assessment - SummaryReportDokument4 SeitenSurvey On Student Learning Outcomes Assessment - SummaryReportfadhaliNoch keine Bewertungen
- Curriculum Mapping TemplatesDokument6 SeitenCurriculum Mapping TemplatesfadhaliNoch keine Bewertungen
- Curriclumtypes 110225065422 Phpapp01 130901064106 Phpapp01Dokument26 SeitenCurriclumtypes 110225065422 Phpapp01 130901064106 Phpapp01JONRY GUMINTAD HELAMONNoch keine Bewertungen
- Jazan University Physics Quiz 1Dokument1 SeiteJazan University Physics Quiz 1fadhaliNoch keine Bewertungen
- Physics Gradute AttributesDokument1 SeitePhysics Gradute AttributesfadhaliNoch keine Bewertungen
- Exam 112phys, 1st Mid TermDokument3 SeitenExam 112phys, 1st Mid TermfadhaliNoch keine Bewertungen
- Lab Experiment InterferenceDokument4 SeitenLab Experiment InterferencefadhaliNoch keine Bewertungen
- 2 5 04Dokument2 Seiten2 5 04Joel Martínez GonzálezNoch keine Bewertungen
- Newton Rings PDFDokument4 SeitenNewton Rings PDFfadhaliNoch keine Bewertungen
- Curriculum Assessment ChecklistDokument1 SeiteCurriculum Assessment ChecklistfadhaliNoch keine Bewertungen
- 112phys, 2nd Mid TermDokument4 Seiten112phys, 2nd Mid TermfadhaliNoch keine Bewertungen
- Template For Program Assessment PlanDokument6 SeitenTemplate For Program Assessment PlanfadhaliNoch keine Bewertungen
- 412phys (Laser) Course ReviewDokument11 Seiten412phys (Laser) Course ReviewfadhaliNoch keine Bewertungen
- 412phys (Laser) Course ReviewDokument11 Seiten412phys (Laser) Course ReviewfadhaliNoch keine Bewertungen
- 112PHYS Course Specification-Dr. M FadhaliDokument10 Seiten112PHYS Course Specification-Dr. M FadhalifadhaliNoch keine Bewertungen
- 412phys (Laser) Course ReviewDokument11 Seiten412phys (Laser) Course ReviewfadhaliNoch keine Bewertungen
- Ch08, Optical InterferenceDokument2 SeitenCh08, Optical InterferencefadhaliNoch keine Bewertungen
- 412PHYS - CS - Update April2016Dokument8 Seiten412PHYS - CS - Update April2016fadhaliNoch keine Bewertungen
- Modeling of Vibration Sensing With Variable Fabry-Perot SystemDokument6 SeitenModeling of Vibration Sensing With Variable Fabry-Perot SystemfadhaliNoch keine Bewertungen
- Medical Physics, Lecture-5.Ppt (Compatibility Mode)Dokument30 SeitenMedical Physics, Lecture-5.Ppt (Compatibility Mode)fadhaliNoch keine Bewertungen
- Dr. M Fadhali CV (Aug.2012)Dokument15 SeitenDr. M Fadhali CV (Aug.2012)fadhaliNoch keine Bewertungen
- Medical Phyysics-Lecture-2 (Dr. M Fadhali) .PPT (Compatibility Mode)Dokument9 SeitenMedical Phyysics-Lecture-2 (Dr. M Fadhali) .PPT (Compatibility Mode)fadhaliNoch keine Bewertungen
- Lecture-4 (Dr. M Fadhali) .PPT (Compatibility Mode) 2slidesDokument9 SeitenLecture-4 (Dr. M Fadhali) .PPT (Compatibility Mode) 2slidesMohamed FadhaliNoch keine Bewertungen
- Blackbody RadiationDokument5 SeitenBlackbody RadiationfadhaliNoch keine Bewertungen
- Lecture-3 (Dr. M Fadhali - General Physics)Dokument12 SeitenLecture-3 (Dr. M Fadhali - General Physics)fadhaliNoch keine Bewertungen
- Fadhali - Laser Micromachining 2Dokument22 SeitenFadhali - Laser Micromachining 2fadhaliNoch keine Bewertungen
- OPJ DR - FadhaliDokument5 SeitenOPJ DR - FadhalifadhaliNoch keine Bewertungen
- Nitrate Reduction in Sulfate Reducing BacteriaDokument10 SeitenNitrate Reduction in Sulfate Reducing BacteriaCatalinaManjarresNoch keine Bewertungen
- Levenbach Causal2017Dokument15 SeitenLevenbach Causal2017Jenna GrantNoch keine Bewertungen
- Animal Quiz: SuperlativesDokument2 SeitenAnimal Quiz: SuperlativesLuis LemusNoch keine Bewertungen
- ISE I Conversation Task - Rules and RegulationsDokument3 SeitenISE I Conversation Task - Rules and RegulationsElena B. HerreroNoch keine Bewertungen
- Henderson - Historical Documents of The Middle AgesDokument536 SeitenHenderson - Historical Documents of The Middle AgesVlad VieriuNoch keine Bewertungen
- GII-07 Training MaterialDokument191 SeitenGII-07 Training MaterialIris Amati MartinsNoch keine Bewertungen
- Literature ReviewDokument4 SeitenLiterature Reviewapi-549241187Noch keine Bewertungen
- Conic SectionDokument58 SeitenConic SectionNailah Sakinah100% (1)
- UNDERSTANDING-WPS OfficeDokument51 SeitenUNDERSTANDING-WPS OfficeCristina Lance100% (1)
- Viennas Cafe Louvre in The 1920s and 1930Dokument18 SeitenViennas Cafe Louvre in The 1920s and 1930Friso HoeneveldNoch keine Bewertungen
- Why Research Is Important in The BusinessDokument2 SeitenWhy Research Is Important in The BusinessBricx BalerosNoch keine Bewertungen
- Jolly Phonics Teaching Reading and WritingDokument6 SeitenJolly Phonics Teaching Reading and Writingmarcela33j5086100% (1)
- Test Unit 3Dokument2 SeitenTest Unit 3RAMONA SECUNoch keine Bewertungen
- HCF and LCMDokument3 SeitenHCF and LCMtamilanbaNoch keine Bewertungen
- Chapter3 Sampling Proportions PercentagesDokument10 SeitenChapter3 Sampling Proportions PercentagesHamzaZahidNoch keine Bewertungen
- Numl Lahore Campus Break Up of Fee (From 1St To 8Th Semester) Spring-Fall 2016Dokument1 SeiteNuml Lahore Campus Break Up of Fee (From 1St To 8Th Semester) Spring-Fall 2016sajeeNoch keine Bewertungen
- 2 - RUBRIC PHY110 (For Student)Dokument3 Seiten2 - RUBRIC PHY110 (For Student)Puteri AaliyyaNoch keine Bewertungen
- Life Convict Laxman Naskar Vs State of West Bengal & Anr On 1 September, 2000Dokument6 SeitenLife Convict Laxman Naskar Vs State of West Bengal & Anr On 1 September, 2000Kimberly HardyNoch keine Bewertungen
- Medicalization of Racial Features Asian American Women and Cosmetic SurgeryDokument17 SeitenMedicalization of Racial Features Asian American Women and Cosmetic SurgeryMadalina ElenaNoch keine Bewertungen
- CBCP Monitor Vol. 17 No. 9Dokument20 SeitenCBCP Monitor Vol. 17 No. 9Areopagus Communications, Inc.Noch keine Bewertungen
- Table Topics Contest Toastmaster ScriptDokument4 SeitenTable Topics Contest Toastmaster ScriptchloephuahNoch keine Bewertungen
- Answer Here:: FAMILY NAME - FIRST NAME - CLASSCODEDokument4 SeitenAnswer Here:: FAMILY NAME - FIRST NAME - CLASSCODEUchayyaNoch keine Bewertungen
- Ad844 PDFDokument20 SeitenAd844 PDFkavi_mishra92Noch keine Bewertungen
- DRF1301 1000V 15A 30MHz MOSFET Push-Pull Hybrid DriverDokument4 SeitenDRF1301 1000V 15A 30MHz MOSFET Push-Pull Hybrid DriverAddy JayaNoch keine Bewertungen
- Marwar Steel Tubes Pipes StudyDokument39 SeitenMarwar Steel Tubes Pipes Studydeepak kumarNoch keine Bewertungen
- Raman Spectroscopy: 1 Theoretical BasisDokument9 SeitenRaman Spectroscopy: 1 Theoretical BasisJèManziNoch keine Bewertungen
- The Meaning of Al FatihaDokument11 SeitenThe Meaning of Al Fatihammhoward20Noch keine Bewertungen
- Parashara'S Light 7.0.1 (C) Geovision Software, Inc., Licensed ToDokument5 SeitenParashara'S Light 7.0.1 (C) Geovision Software, Inc., Licensed TobrajwasiNoch keine Bewertungen
- Are Moral Principles Determined by SocietyDokument2 SeitenAre Moral Principles Determined by SocietyKeye HiterozaNoch keine Bewertungen
- Research Online Research OnlineDokument11 SeitenResearch Online Research OnlineMunib HussainNoch keine Bewertungen