Beruflich Dokumente
Kultur Dokumente
Structure of Lenticels On The Pneumatophores of Avicennia Marina: As Aerating Device Deliver Oxygen in Mangrove's Root
Hochgeladen von
Hery PurnobasukiOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Structure of Lenticels On The Pneumatophores of Avicennia Marina: As Aerating Device Deliver Oxygen in Mangrove's Root
Hochgeladen von
Hery PurnobasukiCopyright:
Verfügbare Formate
Jurnal BIOTA XVI (2): 309315.
(2011) ISSN: 0853-8670
Structure of Lenticels on the Pneumatophores of Avicennia marina: as Aerating Device Deliver Oxygen in Mangroves root
Struktur Lentisel pada Pneumatofor Avicennia marina: sebagai Alat Pengantar Oksigen Pada Akar Mangrove Hery Purnobasuki
Dept. of Biology, Faculty of Sciences and Technology, Airlangga University Jln. Mulyorejo (Kampus C Unair), Surabaya-60115 E-mail: herypurba@unair.ac.id or herypurba@yahoo.com
Abstract
Lenticels on the pneumatophores of Avicennia marina were studied by Scanning Electron Microscopy (SEM) in order to relate their development and structure of their function as aerating deliver oxygen in mangroves root. The results reveals that lenticels varied in size and range in morphology from classical crater-like with a mass of fluffy tissue in the centre. The lenticels are composed of complementary (filling) tissue, which consists of thin walled spheroidal cells by intercellular spaces. The cells may be suberized by positive stained of safranin. As it grows, the complementary tissue ruptures the periderm and the lenticels become functional as part of air conduit systems. Key words: lenticels, pneumatophores, root conduit systems
Abstrak
Telah dilakukan penelitian terhadap lentisel pada pneumatofor Avicennia marina dengan menggunakan mikroskop cahaya dan mikroskop pemindai electron untuk mengetahui keterkaitan perkembangan dan struktur dari fungsi lentisel sebagai alat pengantar oksigen pada akar mangrove. Hasil penelitian menunjukkan bahwa struktur lentisel bervariasi dalam hal ukuran dan morfologi, mulai dari bentuk seperti kawah dengan jaringan lunak di bagian tengahnya. Lentisel tersusun dari jaringan pelengkap yang tersusun atas sel-sel berdinding tipis dan membentu ruang-ruang interseluler. Sel-sel tersebut mengandung lignin yang terdeteksi positif dengan pewarnaan sfranin. Dalam pertumbuhannya, sel-sel pelengkap merusak lapisan peridermis dan selanjutnya lentisel berfungsi sebagai bagian dari sistem saluran penghantaran udara. Kata kunci: lentisel, pneumatofor, sistem saluran akar
Introduction
In well oxygenated soil, there is little difficulty in obtaining the oxygen needed for respiration. This is not so in waterlogged soils, and special aerating devices are required. Avicennia marina is a common mangrove species on tropical and subtropical sea shores, swamps and stream banks (Chapman, 1976; Tomlinson, 1986). In mature tree, the root system of A. 1
marina is complicated and it has four root types, i.e. cable roots, pneumatophores, feeding root, and anchor roots (Purnobasuki dan Suzuki, 2004, 2005a, 2005b). Pneumatophores grow vertically upward and expose their tip in air. The pneumatophores are covered by an impermeable periderm. At low tide, lenticels on the surface of the pneumatophores allow gas exchange between the atmosphere and the internal structure of root. Therefore, the pneumatophores has important function as a highly specialized ventilation mechanism, enabling the plant to survive in anaerobic soil and extreme conditions in the shore. Despite the importance of lenticels for survival and gas transport of mangrove roots, very little is known about the development and organization of lenticels tissue in mangrove root system. The structure of pneumatophores of Avicennia marina has been studied previously by Gordon and Dubinsky (1992), however there is still no clear how this air conducting network develop, especially the structure of lenticels in this species. The high flooding tolerance of certain species of forest trees such as mangrove has been attributed to one or more adaptive mechanism, one of this is ventilation structures of lenticels on their specialized root. Stems and roots that increase in thickness by secondary growth are generally bordered on the outside by a periderm, a protective tissue of secondary origin. Lenticels are circumscribed parts of the periderm in which the phellogen is more active and periodically produces a tissue with numerous intercellular spaces. Because of this relatively open arrangement of cells and the continuity of the intercellular spaces with those in the interior of the stems, the stem lenticels are supposed to provide pathways for transpiration and gas exchange (Rosner and Kartusch, 2003). The present study examines the structures and organization of lenticels of the pneumatophores of A. marina and to assess the relationships between tissue structure and habitat adaptation.
Materials and Method
Pneumatophore samples were taken from adult trees (1015 cm in trunk diameter at base, 0.51 m tall) of Avicennia marina (Forsk.) Vierh., which grow naturally in Urauchi (24 o23N, 123o46E) and Komi estuary (24o1919N, 123o54E), Iriomote Island, okinawa Prefecture, Japan. The sampled trees are sparsely distributed in seaward outer fringes of mangrove forest where plants are flooded by all high tides and easily influenced by strong winds and tidal forces. 2
Around the sampled trees, we collected carefully root of the trees during low tide and collected pneumatophore. SEM observation Pneumatophores were cut into cubic of 58 mm length pieces using razor blade for SEM preparation using Resin Casting Method (Mauseth and Fujii, 1994). The samples were observed and photographed on SEM (Hitachi S-4100). The tissue was fixed in FAA and dehydrated in a graded ethanol-t butyl alcohol and freeze dried at -10oC (Hitachi ES-2030 Freeze Dryer). The dried samples were embedded in styrene monomer-polyester resin with 1% benzoyl peroxide in the gelatin capsule and vacuum for several minutes. The samples were put at the oven 60oC overnight for polymerization. After removing gelatin, samples were trimmed using a chisel or sand paper. The trimmed sample was immeresed in a mixture of hydrogen peroxide-acetic acid (1:1) to remove lignin for one day or more at the oven 60 oC, after that is was washed in water and put on the sulfuric acid 64% to remove cell wall polysaccharides for one day. The resin cast was rinsed with water and cleaned by agitation in water using mini supersonic cleaner. The sample was dehydrated again in a graded ethanol-t butyl alcohol and freeze dried. The dried sample was glued to the specimen stub that coated with conductive carbon tape. The sample was coated with platinum palladium in vacuum evaporator (Hitachi E-1030 ion sputter), observed and photographed on Scanning Electron Microscope (Hutachi S-4100).
Results and Discussion
The surface of pneumatophores of A. marina had many small spots or mere pimples of lenticels. Lenticels spread in the surfaces of pneumatophores and only clearly showed at the region of aerial part of these roots. These varied in size and range in morphology from classical crater-like (Fig. 1) with a mass of fluffy tissue in the centre to unopened pustules. The clorenchyma of the pneumatophores cortex beneath seemed to be responsible for overall green color in the surface of aerial part of pneumatophores. In A. marina, there were two kinds of pneumatophores, the smooth and the rough types. The rough type, which was found at the region of pneumatophores above the surface of water, had numerous lenticels, which project markedly from the surface, whereas the smooth type (young pneumatophores) had fewer or no lenticels, submerged underground (Fig. 1B)
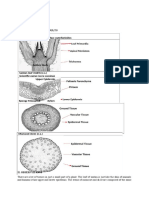
Figure 1. Morphological structure of pneumatophores as gas exchange in A. marina. The lenticels mere pimples or spot on the surface of pneumatophores (AB), Bar= 2 cm. The chlorenchyma of the pneumatophore cortex beneath may be responsible for overall green color and the dot line reveals the ground level. C. Root tip of pneumatophores with many lenticels, appearance as crater-like. Bar= 8 mm. D. More distance for root tip with young lateral root (feeding root) growth. Bar= 4 mm. fe. feeding roots; lc. Lenticels.
The lenticels of this species is composed of complementary (filling) tissue, which consists of thin walled spheroidal cells by intercellular spaces (Fig 2 and 3). They concave disc, stacked with no intercellular matrix (Fig 3C and D). The cells may be suberized by positive stained of safranin. Lenticels appear up to 0.5 cm below the pneumatophore tip and the uppermost lenticels are mere pimples on the surface of the pneumatophores in A. marina. As it grows, the complementary tissue ruptures the periderm and the lenticel become functional (Fig. 2D). After that, it supposed that a suberized layer is formed by the meristem beneath the complementary tissue of the lenticel. Eventually the meristem begins to form complementary tissue inside this suberized layer eventually ruptures and is cast off along with all of the old complementary tissue. A scar remains at the edges of the lenticels (Fig. 2D) shows a short section of the suberized layer and some complementary tissues. From the presence of several sets of scar tissue in some lenticels it is obvious that this may be happen many times in the life of one lenticel.
The lenticels development of these roots briefly describe as follows. The parenchymatous cells adjoining the aerenchyma tubular structure in the cortex increase in size and divide. The resulting daughter-cells give rise to complementary tissue which fills up the air -chamber in regular form of files formation. A curved cell-layer, convex toward the inside, undergoes tangential divisions and becomes t he lenticellar meristem (Fig. 2A), which continually cuts off additional complementary cells on its outer side. The pressure of the steadily expanding regular files of complementary tissue causes the epidermis layer to bulge outwards and finally to burst and make the epidermis layer become rupture; the complementary tissue than protrudes in places, hence to rough surface of the lenticels. On older pneumatophores, which are already covered with periderm, the lenticels arise from the phellogen, which at certain points, produces complementary tissue with abundant intercellular spaces in place of the normal uninterrupted layers of cork (Fig. 2 and 3B) and thus becomes locally converted into lenticelullar meristem. The layer of cork above a developing lenticel suffers the same fate as the epidermis in the previous explanation, being distended and finally ruptures by the growing complementary tissue. As it growth, the complementary tissue ruptures the periderm and the lenticels become functional. After a while, a suberized layer was formed by the meristem beneath the complementary tissue (Fig. 2A) of the lenticel. Eventually the meristem begins to form complementary tissue inside this suberized layer which ruptures and its cast off along with all of the old complementary tissue (Fig. 2D). Observation of longitudinal sections of pneumatophores of A. marina gave similar results in cross section. However the observation showed that the mass of complementary cells make regularly alignment and that there were intercellular spaces or gaps between the adjoining files of complementary cells. These spaces or gaps may provide a connection between the aerenchyma tubular structures in the cortex area (Fig. 3A and B). It observed also there was no intercellular matrix on these spaces.
Discussion
Lenticels have an important role on the gas pathway of root of A. marina as first gate that related to atmosphere directly. The SEM observations on the present study revealed different stages of the lenticels (Fig. 2). I suppose that these are different stages in the development of the lenticels; the partially-opened one (Fig 2A), a partially-mature lenticels (Fig 2B) and the fully5
opened one (Fig. 2C and D). From the structural analyses I suggest that a developmental process taking places in the lenticels. The young immature lenticels are closed, with a relatively low number of complementary cells. Later, more complementary cells are formed, creating an increasing pressure inside the lenticels. When the pressure is high enough, the cork breaks open, forming the partially-opened state of the partially-mature lenticels. As the complementary cells continue to be formed, the additional pressures enlarge the opening, creating the fully-opened mature lenticels. Although gas exchange can occur in the partially-mature lenticels, the fullyopened mature lenticels is probably more effective in the aeration process, as its larger opening and the larger amount of complementary cells inside the lenticels, allow more air to diffuse into the lenticels and to more rapidly. Chapman (1939, 1940), using light microscopy, describes lenticels of Avicennia nitida with and without an opening, but associated them with different kinds of pneumatophores. Thus, the suggested development stages for lenticels of A. Marina may be similar in some or all the species in the genus. The presence of the complementary cells with no intercellular matrix will encourages free diffusion of air between the cells, whose concave shape also helps to fulfill this function. Since the reaction to Safranin was positive, it is possible that, because of their suberin and or cutin cover, the accumulation of complementary cells may act as a hydrophobic layer through which air may diffuse while water penetration is prevented.
Figure 2. Cross section of pneumatophore of A. marina at a lenticels which has undergone several growth cycles. A. closed lenticels (Bar= 100 m). B. some epiderm rupture and lenticels start to open (Bar= 150 m). CD. open lenticels (Bar= 150 m). ar. aerenchyma; cc. complementary tissue; m. meristem; pe. periderm; s. suberized tissue. Arrows indicate rupture suberized layers with old complementary tissues.
Figure 3. Longitudinal section of pneumatophore at a lenticel (A, B). and complementary cells (C, D). Mass of complementary cells (cc) make regularly alignment and there is a intercellular space (arrows) between them and there is no intercellular matrix on this space.
In the pneumatophore describe in the present study, lenticels arise further down the pneumatophore than most subrisules and are a more permanent site of conductance. Lenticels seem to be rejuvenated several times throughout their functional life. Some lenticels are probably non functional, especially in an older pneumatophores on which the growth of algae is profuse. Pneumatophores which are covered in algae have lenticels which grow out in a fashion which is termed hypertrophy. Hypertrophic lenticels are functional and evident on regions of pneumatophores which are inundated most of the time. Hypertrophic lenticels were not studied here since the interest was mostly with aerenchyma development. The periderm of pneumatophores is both water and air tight resulting in gases being kept in the root system and water out. The periderm covers all of the penumatophore except at the sites conductance, lenticels. Since lenticels are large and complex organs of gas exchange, they take time to produce. Therefore lenticels are not found in the tip most section of pneumatophores. If the pneumatophore is actively growing, then the dividing cells at the tip have a high oxygen demand. This demand could be a significant sink of the oxygen which the pneumatophore is supplying to the rest of the isolated root system. It is likely that this demand is met by the order structure like subrisules or horizontal structures in the pneumatophores (Allaway et al., 2001; 7
Hovenden and Allaway, 1994). The observation that functional pneumatophores which are not actively growing do not posses subrisules supports this hypothesis. The suberized layers whish are produced by the phellogen along with the complementary tissue in lenticels have been termed closing layers (Fahn, 1974). The production of a closing layer in the lenticels has been described previously (Fahn, 1974). Most authors postulate that the function of the closing layer is to hold together the rather loose aggregations of complementary cells. This may be the case in plant species which produce closing layers regularly alternating with layers of complementary cells. The production of closing layer in the lenticels of A. marina is infrequent and may be a seasonal phenomenon. A closing layer is absent in many lenticels and I didnt found more than one intact closing layer in a lenticel such as was described for some species by Haberlandt (1914) although some lenticels have several rings of scar tissue which probably represent ruptured closing layer. The closing layer of A. marina is heavily suberized and it is likely that a lenticel with an intact closing layer is non-functional. In this species, the production of closing layer may function as an anti-fouling mechanism whereby the lenticels clears all setting algae and exposes a fresh conducting surface. Chapman (1940) referred to a closing layer at the point where the penumatophore emerges from cable root. This is a very different structure and the name should not be used for it. Three stages in the development of lenticels have been identified by Gordon and Dubinsky (1992), viz, immature, partially-mature and mature. I suggest an extension of this developmental series to include the production of a closing layer and its eventual rupturing. These two steps may occur many times during the life of lenticels. The importance of lenticels for gas exchange has been demonstrated by measuring O 2 and CO2 concentrations in the aerenchyma of Rhizophora roots. When the lenticels are occluded by smearing grease over the aerial potion of the root, O2 declines continuously and CO2 rises. Control roots showed fluctuations related to tidal level (Scholander et al., 1955 and Hogarth 1999).
Conclusion
The development stages for lenticels of A. Marina may be similar in some or all the species in the genus. The complementary cells structures of mature lenticels help more effective in the aeration process, as its larger opening and the larger amount of complementary cells inside 8
the lenticels, allow more air to diffuse into the lenticels and to more rapidly. This structure of lenticels fully supports the air conducting on the root system of A. marina to adapt on their habitat.
Akcnowledgments
We thank to Prof. Tokushiro Takaso, University of Ryukyus and Prof. Mitsuo Suzuki, University of Tohoku for their great support in our field and laboratory research. We also thank Kazutaka Kobayashi, Takahisa Tanaka, Tomohiro Hasebe, Youichi Hasegawa, Masanori Seki and Hiroaki Terasawa for their field sampling assistance. Great appreciate for our colleague in Department of Biology, Faculty of Sciences and Technology, Universitas Airlangga for their supporting and pray.
References
Allaway, W.G., Curran, M., Hollington, L.M., Ricketts, M.C. and Skelton, N.J. 2001. Gas space and oxygen exchange in roots of Avicennia marina (Forssk.) Vierh. Var. Australasica (Walp.) Moldenke ex N.C. Duke, the Grey Mangrove. Wetl. Ecol. Manag., 9: 211218. Chapman, V.J. 1939. Cambridge University Expedition to Jamaica-Part 3. The morphology of Avicennia nitida Jacq. And the function of its pneumatophores. J. Linn. Soc., 52: 487533. Chapman, V.J. 1940. The functions of the pneumatophores of Avicennia nitida Jacq. Proc. Linn. Soc., 153: 228233. Chapman, V.J. 1976. Mangrove vegetation. Cramer, Vaduz. Fahn, A. 1974. Plant Anatomy 4th Ed. Pergamon Press. New York. Gordon, N.I.S. and Dubinsky, Z. 1992. Ultrastructure of the pneumatophores of the mangrove A. marina. South Afr. J. Bot., 58: 358-362. Gordon, N.I.S. and Z. Dubinsky, 1992. Ultrastructure of the pneumatophores of the mangrove A. marina. South Afr. J. Bot., 58: 358362. Haberlandt, G. 1914. Physiology Plant Anatomy. London. Hogarth, P.J. 1999. The biology of the mangrove. Oxford University Press. Oxford-New York.
Hovenden, M.J. and Allaway, W.G. 1994. Horizontal structures on the pneumatophores of Avicennia marina (Forsk.) Vierh. A new site of oxygen conductance. Ann. Bot., 73: 377383. Mauseth, J.D. and Fujii, T. 1994. Resin-casting: A method for investigating apoplastic spaces. Amer. J. Bot., 81: 104110. Purnobasuki, H. and Suzuki, M. 2004. Aerenchyma formation and porosity in root of a mangrove plan, Sonneratia alba J. Smith. J. Plant Res., 117: 465472. Purnobasuki, H. and Suzuki, M. 2005a. Functional anatomy of air conducting network on the pneumatophores of a mangrove plant, Avicennia marina (Forsk.) Vierh. Asian J. Plant Sci., 4: 334347. Purnobasuki, H. and Suzuki, M. 2005b. Aerenchyma tissue development and gas pathway structure in root of Avicennia marina (Forsk.) Vierh. 285-294 ISSN: 0918-9440. Rosner, S. and Kartusch, B. 2003. Structural changes in primary lenticels of Norway Spruce over the seasons. IAWA J. 24 (2): 105116. Scholander, P.F., Van Dam, L. and Scholander, S.I. 1955. Gas exchange in the roots of mangroves. Amer. J. Bot. 42: 9298. Tomlinson, P.B. 1986. The botany of mangroves. Cambridge University Press. New York. Journal of Plant Research 118:
10
Das könnte Ihnen auch gefallen
- 113 234 2 PBDokument7 Seiten113 234 2 PBputrisss 26Noch keine Bewertungen
- Bio Summary T2 Wk1Dokument7 SeitenBio Summary T2 Wk1LilyNoch keine Bewertungen
- 1.2.3 Transport in PlantsDokument11 Seiten1.2.3 Transport in PlantsMo_Bash1Noch keine Bewertungen
- Mariano Marcos State University College of Arts and Sciences Batac City Water Uptake and Movement in PlantsDokument13 SeitenMariano Marcos State University College of Arts and Sciences Batac City Water Uptake and Movement in PlantsFrancis Dave FloresNoch keine Bewertungen
- Internal Structure of The LeafDokument27 SeitenInternal Structure of The LeafLenaNoch keine Bewertungen
- Gas exchange and transport in plantsDokument59 SeitenGas exchange and transport in plantsKai Yung ChingNoch keine Bewertungen
- AS Biology Unit 2 NotesDokument34 SeitenAS Biology Unit 2 NotesMehreenSaeedNoch keine Bewertungen
- Report SheetDokument11 SeitenReport SheetMikhail LandichoNoch keine Bewertungen
- Exercise 3Dokument8 SeitenExercise 3Melvy June Balasa100% (1)
- 1 s2.0 S017616171930015X Main PDFDokument7 Seiten1 s2.0 S017616171930015X Main PDFwahyuNoch keine Bewertungen
- 1 s2.0 S017616171930015X Main PDFDokument7 Seiten1 s2.0 S017616171930015X Main PDFwahyuNoch keine Bewertungen
- Respiratory System: StructureDokument29 SeitenRespiratory System: StructureDr. Abir Ishtiaq100% (1)
- 9.1 Plant Structure and Growth: Topic 9: Plant Science (HL)Dokument6 Seiten9.1 Plant Structure and Growth: Topic 9: Plant Science (HL)Morgan LockeNoch keine Bewertungen
- Mechanisms of Gaseous Exchange in Humans and PlantsDokument23 SeitenMechanisms of Gaseous Exchange in Humans and PlantsSaif ullah SalafiNoch keine Bewertungen
- Transport in Plants AnswersDokument3 SeitenTransport in Plants AnswersEncik Amir Kamal20% (5)
- Crop Physiology Exercise-2Dokument7 SeitenCrop Physiology Exercise-2Ravikant MishraNoch keine Bewertungen
- Gaseousexchange: Animation 10: Gaseous Exchange Source & Credit: WikispacesDokument23 SeitenGaseousexchange: Animation 10: Gaseous Exchange Source & Credit: WikispacesWorld of FunNoch keine Bewertungen
- Toe Pad Litoria ArticleDokument9 SeitenToe Pad Litoria Articlegummy25Noch keine Bewertungen
- Aerenchyma Formation and Porosity in Root of A Mangrove Plant, Sonneratia Alba (Lythraceae)Dokument8 SeitenAerenchyma Formation and Porosity in Root of A Mangrove Plant, Sonneratia Alba (Lythraceae)Hery PurnobasukiNoch keine Bewertungen
- Transport in plant SDADokument5 SeitenTransport in plant SDAkmakabe78Noch keine Bewertungen
- Report SheetDokument6 SeitenReport SheetMikhail LandichoNoch keine Bewertungen
- Ann Bot-1965-EVANS-205-17Dokument13 SeitenAnn Bot-1965-EVANS-205-17Vrushali PatilNoch keine Bewertungen
- Tonsil Anatomy Review PDFDokument3 SeitenTonsil Anatomy Review PDFElisabethLisyeKonnyNoch keine Bewertungen
- 2.2. Gas ExchangeDokument6 Seiten2.2. Gas ExchangeZarmina KhanNoch keine Bewertungen
- 04 Respiration 2022 - AnswersDokument10 Seiten04 Respiration 2022 - Answersria khanNoch keine Bewertungen
- Porifera: Sally P. Leys and Nathan FarrarDokument9 SeitenPorifera: Sally P. Leys and Nathan FarrarFaradilla Aqidatul IzzahNoch keine Bewertungen
- Exercise 2-InvertsDokument10 SeitenExercise 2-InvertsMilkah PedrosaNoch keine Bewertungen
- Carbon FixationDokument6 SeitenCarbon FixationMridul Kumar BarmanNoch keine Bewertungen
- Tsou & Fu 2007 - Octad Pollen Formation in Cymbopetalum (Annonaceae) - The Binding Mechanism PDFDokument11 SeitenTsou & Fu 2007 - Octad Pollen Formation in Cymbopetalum (Annonaceae) - The Binding Mechanism PDFMárcio BazanteNoch keine Bewertungen
- Leaves: Mesomorphic, Xeromorphic & Hydromorphic TypesDokument7 SeitenLeaves: Mesomorphic, Xeromorphic & Hydromorphic TypesHailey CoutuNoch keine Bewertungen
- Gas ChangeDokument20 SeitenGas ChangeBleebs CreigsNoch keine Bewertungen
- Research Report 2018Dokument7 SeitenResearch Report 2018api-267834315Noch keine Bewertungen
- Paranasal Sinus AnatomyDokument35 SeitenParanasal Sinus AnatomyGeorge DaviesNoch keine Bewertungen
- Air Tissue Sifton 1945Dokument36 SeitenAir Tissue Sifton 1945Pilar7100Noch keine Bewertungen
- 28 Arzani Idesia 31 1Dokument8 Seiten28 Arzani Idesia 31 1Septian RizkyNoch keine Bewertungen
- Transport in Flowering Plants: 1995 Paper Question 6Dokument11 SeitenTransport in Flowering Plants: 1995 Paper Question 6Kin Long Chris WongNoch keine Bewertungen
- Section 3Dokument10 SeitenSection 3Aaron RajeshNoch keine Bewertungen
- Sistem Pernapasan JunqueiraDokument33 SeitenSistem Pernapasan JunqueiraFiya AfivaNoch keine Bewertungen
- Anatomy Phalaenopsis AmabilisDokument8 SeitenAnatomy Phalaenopsis AmabilisGenee Guevara MoonNoch keine Bewertungen
- Ultrastructural Organization of Two Tapetal Types in AngiospermsDokument8 SeitenUltrastructural Organization of Two Tapetal Types in AngiospermsEnma AiNoch keine Bewertungen
- Transport in PlantsDokument20 SeitenTransport in PlantsLeon XuNoch keine Bewertungen
- Anatomical Patterns of Aerenchyma in Aquatic Plants 2008Dokument12 SeitenAnatomical Patterns of Aerenchyma in Aquatic Plants 2008Gilberto Aleman SancheschulzNoch keine Bewertungen
- EVOLUTION OF RESPIRATORY SYSTEMS FROM ANCIENT TIMESDokument10 SeitenEVOLUTION OF RESPIRATORY SYSTEMS FROM ANCIENT TIMESWwwanand111Noch keine Bewertungen
- CAPE PhotosynthesisDokument20 SeitenCAPE PhotosynthesisIsheba Warren100% (24)
- Chapter 6 Anatomy of Flowering PlantsDokument8 SeitenChapter 6 Anatomy of Flowering Plantsswayamdey71Noch keine Bewertungen
- 1st Lecture of Respiratory Histology by DR RoomiDokument24 Seiten1st Lecture of Respiratory Histology by DR RoomiMudassar Roomi100% (1)
- Jurnal MikrometerDokument5 SeitenJurnal MikrometerDwi AgustinaNoch keine Bewertungen
- Gaseous Exchange: Short Question AnswersDokument5 SeitenGaseous Exchange: Short Question AnswersNadeem ArainNoch keine Bewertungen
- Laporan 3 StomataDokument18 SeitenLaporan 3 StomataRizkaHasanahNoch keine Bewertungen
- Leaf Vascular Systems in C3 and C4 GrassesDokument11 SeitenLeaf Vascular Systems in C3 and C4 Grassesduy_dương_41Noch keine Bewertungen
- Respirasi IkanDokument6 SeitenRespirasi IkanPebrianti putriNoch keine Bewertungen
- Respiratory System 2Dokument8 SeitenRespiratory System 2Shawn Gaurav JhaNoch keine Bewertungen
- Gills and Trachea. Moist Outer SkinDokument4 SeitenGills and Trachea. Moist Outer SkinirisNoch keine Bewertungen
- Respiratory Epithelium: Human Histology Lecture Chapter 17 Ï The Respiratory SystemDokument16 SeitenRespiratory Epithelium: Human Histology Lecture Chapter 17 Ï The Respiratory SystemEvandie OngNoch keine Bewertungen
- Lab Report Plant PhysiologyDokument5 SeitenLab Report Plant Physiologysarahyahaya100% (1)
- Chapter 9 Respiratory Systems NotesDokument11 SeitenChapter 9 Respiratory Systems NotesCaleb DodgeNoch keine Bewertungen
- Plant and Animal Organ System and Their FunctionDokument21 SeitenPlant and Animal Organ System and Their FunctionJovon GuyoNoch keine Bewertungen
- 18 OCTOBER, 2021 Monday. Biology Respiratory SystemsDokument9 Seiten18 OCTOBER, 2021 Monday. Biology Respiratory SystemsOyasor Ikhapo AnthonyNoch keine Bewertungen
- Primary Structure and Intercelullar Space Formation of Feeding Root in Sonneratia Alba J. SmithDokument6 SeitenPrimary Structure and Intercelullar Space Formation of Feeding Root in Sonneratia Alba J. SmithHery PurnobasukiNoch keine Bewertungen
- PERKEMBANGAN KULTUR DAUN Aglaonema Sp. Var Siam Pearl, Aglaonema Sp. Var. Lady Valentin Dan Aglaonema Sp. Var. Lipstik DENGAN PERLAKUAN ZAT PENGATUR TUMBUH IAA DAN BAPDokument7 SeitenPERKEMBANGAN KULTUR DAUN Aglaonema Sp. Var Siam Pearl, Aglaonema Sp. Var. Lady Valentin Dan Aglaonema Sp. Var. Lipstik DENGAN PERLAKUAN ZAT PENGATUR TUMBUH IAA DAN BAPHery PurnobasukiNoch keine Bewertungen
- Ornamental Shrubs As Plant Palletes Elements and Bioindicators Based On Air Pollution Tolerance Index in Surabaya City, Indonesia.Dokument5 SeitenOrnamental Shrubs As Plant Palletes Elements and Bioindicators Based On Air Pollution Tolerance Index in Surabaya City, Indonesia.Hery PurnobasukiNoch keine Bewertungen
- Aerenchyma Tissue Development and Gas-Pathway Structure in Root of Avicennia Marina (Forsk.) Vierh.Dokument10 SeitenAerenchyma Tissue Development and Gas-Pathway Structure in Root of Avicennia Marina (Forsk.) Vierh.Hery PurnobasukiNoch keine Bewertungen
- Porang 01Dokument7 SeitenPorang 01Hery PurnobasukiNoch keine Bewertungen
- Characteristics of Root Caps in Four Root Types of Avicennia Marina (Forsk.) Vierh.Dokument6 SeitenCharacteristics of Root Caps in Four Root Types of Avicennia Marina (Forsk.) Vierh.Hery PurnobasukiNoch keine Bewertungen
- Effect of Nitrogen Supply and Genotypic Variation For Nitrogen Use Efficiency in MaizeDokument18 SeitenEffect of Nitrogen Supply and Genotypic Variation For Nitrogen Use Efficiency in MaizeHery PurnobasukiNoch keine Bewertungen
- Aerenchyma Formation and Porosity in Root of A Mangrove Plant, Sonneratia Alba (Lythraceae)Dokument8 SeitenAerenchyma Formation and Porosity in Root of A Mangrove Plant, Sonneratia Alba (Lythraceae)Hery PurnobasukiNoch keine Bewertungen
- Sonneratia Purnobasuki02Dokument8 SeitenSonneratia Purnobasuki02Hery PurnobasukiNoch keine Bewertungen
- Avicennia Purnobasuki02Dokument14 SeitenAvicennia Purnobasuki02Hery PurnobasukiNoch keine Bewertungen
- Certification Cutting TreesDokument7 SeitenCertification Cutting TreesBarangay AmasNoch keine Bewertungen
- Video Worksheet Modern Marvels CornDokument2 SeitenVideo Worksheet Modern Marvels CornNathan MoyerNoch keine Bewertungen
- EFFECT OF ORGANIC MANURES AND BIO-FERTILIZERS ON GROWTH, YIELD, QUALITY AND ECONOMICS OF BROCCOLI (Brassica Oleracea L. Var. Italica PLENCK) Cv. GREEN HEAD UNDER HIGH-HILL CONDITIONS OF UTTARAKHANDDokument5 SeitenEFFECT OF ORGANIC MANURES AND BIO-FERTILIZERS ON GROWTH, YIELD, QUALITY AND ECONOMICS OF BROCCOLI (Brassica Oleracea L. Var. Italica PLENCK) Cv. GREEN HEAD UNDER HIGH-HILL CONDITIONS OF UTTARAKHANDDr Sandeep KumarNoch keine Bewertungen
- The Loom of Life Unravelling Ecosystems PDFDokument173 SeitenThe Loom of Life Unravelling Ecosystems PDFKamelNoch keine Bewertungen
- CGCL CatalogDokument24 SeitenCGCL CatalogMNoch keine Bewertungen
- Ansi Wdma - Is 1a 2013 FlushDokument53 SeitenAnsi Wdma - Is 1a 2013 FlushJoseph Allan TolentinoNoch keine Bewertungen
- Comparative Effectiveness of Animal Manures on Soil and Crop YieldDokument8 SeitenComparative Effectiveness of Animal Manures on Soil and Crop YieldAnne HathawayNoch keine Bewertungen
- Five-Spice Chicken and RiceDokument2 SeitenFive-Spice Chicken and RiceTiara MillerNoch keine Bewertungen
- Children - S Traditional TalesDokument22 SeitenChildren - S Traditional TalesSidi OmarNoch keine Bewertungen
- Middle Holiday Package - 2022Dokument29 SeitenMiddle Holiday Package - 2022Naki Winfred MaryNoch keine Bewertungen
- Forage Growth, Yield and Quality Responses of Napier Hybrid Grass Cultivars To Three Cutting Intervals in The Himalayan FoothillsDokument9 SeitenForage Growth, Yield and Quality Responses of Napier Hybrid Grass Cultivars To Three Cutting Intervals in The Himalayan FoothillsNaufal ZakiNoch keine Bewertungen
- Golden Ratio in NatureDokument20 SeitenGolden Ratio in NatureRamzen Raphael DomingoNoch keine Bewertungen
- Pests Management COntrolDokument120 SeitenPests Management COntrolStefan AradavoaiceiNoch keine Bewertungen
- Flowers of KasDokument9 SeitenFlowers of KasAmit SayyedNoch keine Bewertungen
- 2005 Meyer, Sugar MechanisationDokument36 Seiten2005 Meyer, Sugar MechanisationrajmysoreNoch keine Bewertungen
- Ecology - 1994 - 75 - BecerraDokument6 SeitenEcology - 1994 - 75 - BecerraWilliams Marcel Caceres FerreiraNoch keine Bewertungen
- Agri Plas GreenhouseDokument6 SeitenAgri Plas GreenhouseBendzGeronaNoch keine Bewertungen
- Propolis Stadarization PaperDokument4 SeitenPropolis Stadarization PaperSeba QussiniNoch keine Bewertungen
- Cocopeat For HorticultureDokument1 SeiteCocopeat For HorticultureDekodemo. EnterpriseNoch keine Bewertungen
- International Journal of Pharma and Bio Sciences: ArticalticleDokument6 SeitenInternational Journal of Pharma and Bio Sciences: ArticalticlevakilNoch keine Bewertungen
- Anatomy and Functional Status of Haustoria in Field Grown Sandalwood Tree Santalum Album L 2168 9776 1000148Dokument4 SeitenAnatomy and Functional Status of Haustoria in Field Grown Sandalwood Tree Santalum Album L 2168 9776 1000148Ajit ChackoNoch keine Bewertungen
- Softwood Rules For GradeDokument64 SeitenSoftwood Rules For GradeGelayel SamiiNoch keine Bewertungen
- 1500a1550 - Dr. Stephen G. DiverDokument67 Seiten1500a1550 - Dr. Stephen G. Diverduga11100% (1)
- Gibberellic AcidDokument2 SeitenGibberellic AcidYara AliNoch keine Bewertungen
- A Collection of Low Protein Recipes Served at Camp Phever 2012Dokument22 SeitenA Collection of Low Protein Recipes Served at Camp Phever 2012Aurel CioteaNoch keine Bewertungen
- CBSE Class 10 Science Previous Year Question Paper 2018Dokument7 SeitenCBSE Class 10 Science Previous Year Question Paper 2018Mohan Singh100% (1)
- Sdre14-12 PNR 1-2-1dec17Dokument3 SeitenSdre14-12 PNR 1-2-1dec17lwin_oo2435Noch keine Bewertungen
- Pioneer Hi-Bred International, Inc.: Dual Column Trial ReportDokument18 SeitenPioneer Hi-Bred International, Inc.: Dual Column Trial Reportapi-27297323Noch keine Bewertungen
- Cu-NP en Varias ExtractosDokument15 SeitenCu-NP en Varias ExtractosDanisbeth QuiñonezNoch keine Bewertungen
- Pruning BookDokument112 SeitenPruning BookAlexandru Bocancia100% (3)