Beruflich Dokumente
Kultur Dokumente
1
Hochgeladen von
70123Originalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
1
Hochgeladen von
70123Copyright:
Verfügbare Formate
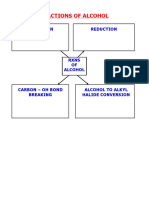
1. To differentiate different types of alcohol.
Introduction:
In organic chemistry, alcohols from the functional group of hydroxyl groups are organic compounds which ar hydroxyl (OH) groups attached to a carbon atom of an alkyl group (hydrocarbon chain). Alcohols have the g represent the number of carbon atom in the compound) or R-OH (where R represent an alkyl group).
Alcohols is usually classified into three classes, the primary alcohols (1), secondary alcohols (2) and tertiary different classes depending on how the -OH group is positioned on the chain of carbon atoms. In a primary (1 the -OH group is only attached to one alkyl group. In a secondary (2) alcohol, the carbon with the -OH group alkyl groups. In a tertiary (3) alcohol, the carbon atom holding the -OH group is attached directly to three alk may be from the combination of same or different groups. R = alkyl group
The boiling points of the normal alcohols increase regularly with the increase of the molecular weights. The p temperature than the secondary alcohols that are isomeric with them. Similarly, the latter have higher boiling Among isomeric alcohols, the boiling point decreases with increase in branching in the alkyl group. Boiling p those of alkenes, halo alkenes or ethers of comparable molecular masses. This is because in alcohols intermol which a large amount of energy is required to break these bonds. Smaller molecules of alcohols are soluble in carbon reaches to four and beyond, its solubility in water reduced and will form two obvious layers.
The chemical properties of alcohols generally involve the reactions of -OH group. They can undergo substitu this experiment, few tests including Lucas test (substitution by halogens) and oxidation test is carried out to d classes of alcohols. For Lucas test, the reagents are a mixture of concentrated hydrochloric acid and zinc chlo oxidation test is sodium dichromate. The end product formed can determine the types of alcohol each reactan consideration includes the colour of product formed and also the time taken for reaction. Besides, esterificatio properties of esters by reacting alcohol with a carboxylic acid. Ester generally will give out a fruity smell. Materials:
Ethanol, n-butanol, glacial acetic acid, alcohol X, Lucas reagent, 2-Methyl-2-propanol, concentrated H2SO4, Apparatus: Corks, dropper, test tubes, stopwatch, water bath, measuring cylinder (10mL). Procedure: A. Lucas test 1. 2mL of Lucas reagent was added to 1mL of 2-methyl-2-propanol. 2. The tube was stopped and shacked. 3. The observation and the time taken for the reaction to occur were recorded. 4. The steps above were repeated using 2-butanol, n-butanol and alcohol X. B. Oxidation test 1. One drop of concentrated H2SO4 was added to 5mL 0.04 M Na2Cr2O7 solution in a test tube in the fume
2. Two drops of n-butanol was added to the mixture and heated. 3. The steps above were repeated using 2-butanol, 2-methyl-2-propanol and alcohol X. 4. The colour change was recorded. C. Esterification test
1. 1mL of glacial acetic acid and 3 drops of concentrated H2SO4 was added to 2mL ethanol in the fume cupb 2. The mixture was shacked and warmed in a water bath.
3. 3mL of distilled water was added and the whiff of the vapour released was taken. The smell wad described Results: A. Lucas test Reactants Reagents Time taken Observations 2-methyl-2-propanol
Lucas reagent (concentrated hydrochloric acid, HCl and Zinc chloride, ZnCl) Immediately Solution turns clou 2-butanol 7 minutes Solution turns cloudy with 2 layers formed n-butanol >15 minutes No reaction observed after 15 minutes Alcohol X >15 minutes No reaction observed after 15 minutes B. Oxidation test Reactants Reagents Observations 2-methyl-2-propanol Sodium dichromate, Na2Cr2O7 No observation observed, yellow colour remains 2-butanol Solution turns from yellow to greenish colour after heating n-butanol Solution turns from yellow to pale greenish colour after heating Alcohol X Solution turns from yellow to pale greenish colour after heating C. Esterification test Reactants reagents Observations Ethanol
Concentrated sulphuric acid (H2SO4) + glacial acetic acid A rubbery smell is detected from the product form Discussion:
A. Lucas test
The Lucas reagent is an aqueous solution which consists of strong acid (hydrochloric acid, HCl) and zinc chlo to determine the class of alcohols. The reactants used to react with Lucas reagent must be soluble in water for compound with more than six carbons is to large to be dissolved in the reagent and therefore will not react in soluble alcohols of low molecular weight will provide positive results in this test. Hence only four different lo which are the 2-methyl-2-propanol, 2-butanol, n-butanol and an unknown alcohol X.
The reaction that occurs in the Lucas test is a nucleophilic substitution. The reaction is a substitution in which group. Only alcohols that can generate stable carbocation intermediates will undergo the reaction. The acid ca (OH group) of the alcohol by protonating the oxygen atom. The C-OH bond breaks to generate the carbocatio chloride ion (nucleophile) to generate an alkyl halide product. Besides, the hydroxyl ions are protonized with reaction involves replacement of hydroxyl group (-OH) in the alcohol by the chloride ions from the Lucas rea haloalkane) and water. Alkyl halide is non-polar compound, while water is polar compound. Hence when the together, they do not mix with each other, forming a cloudy two layers solution, where the less dense colourle denser alkyl halide is at the bottom.
Lucas test can differentiate the classes of alcohol by comparing the product formed and the time taken for rea generally implies that the alcohol used is an primary alcohol. If the product formed turns cloudy and form two the alcohol used is a secondary alcohol. When tertiary alcohol is tested in the other hand, the solution will tur this reaction is proportional to the energy required to form the carbocation. The reaction rate is faster when th stabilized by greater number of electron donating alkyl group (R-) bonded to the positively charged carbon at reacts much more slowly. Thus, a tertiary alcohol reacts fastest followed by secondary alcohol while primary
From the experimental results obtained, we know that 2-methyl-2-propanol is a tertiary alcohol (3 alcohol) b when Lucas reagent is added to form 2 layered-solution which is cloudy. 2-butanol is a secondary alcohol (2 minutes to form a cloudy solution while n-butanol and alcohol X are primary alcohols (1 alcohol) as they did 15minutes. The equations for the reactions of different alcohols used are as below:2-methyl-2-propanol (3 alcohol): ZnCl2 (CH3)3COH + HCl (CH3)3CCl + H2O (2-methyl-2-propanol) (2-chloro-2-methylpropane) 2-butanol (2 alcohol): ZnCl2 CH3CH(OH)CH2CH3 + HCl CH3CHClCH2CH3 + H2O (2-butanol) (2-chlorobutane / 2-butyl chloride) n-butanol (1 alcohol): ZnCl2 CH3CH2CH2CH2OH + HCl CH3CH2CH2CH2Cl + H2O (n-butanol) (1-chlorobutane / n-butyl chloride)
Despite the fact that the experimental results obtained are accurate, 2-butanol took a longer time to react as co where it should react in within 5 minutes. This may due to slight contamination occurred in the reactants and B. Oxidation test
Oxidation test is also another way to determine the class of alcohols. There are a variety of oxidizing agent th permanganate, potassium permanganate, potassium dichromate and hydrogen peroxide. In this experiment, th is sodium dichromate (Na2Cr2O7). Before the sodium dichromate is directly used to oxidize any alcohols, th concentrated sulphuric acid (H2SO4) to become acidified sodium dichromate. The acidic conditions keep the it is able to oxidize the alcohols. If oxidation occurs, the yellow solution containing the dichromate (VI) ions, solution containing chromium (III) ions, Cr3+. Hence the colour of product formed can be used to identify an added to increase the efficiency of the reaction. Cr2O72- + 14 H+ + 6 e- 2 Cr3+ + 7 H2O (orange / yellow) (green)
Oxidation reaction involve the removal of the hydroxyl group(-OH group) and a hydrogen group from the alc forming a carbon-oxygen double bond. Theoretically, only primary and secondary alcohol will undergo oxida undergo any oxidation process. Primary alcohols will be oxidized to form a pale green solution while seconda greenish solution. The end product of oxidation of alcohols will produce different functional group depends o of primary alcohol usually will form carboxylic acid with an aldehyde group as the intermediate. Secondary a alcohol when oxidized.
From the experiment, it is observed that 2-methyl-2-propanol remains yellow in colour. This proves that 2-me This is because tertiary alcohols don't have a hydrogen atom attached to that carbon. Since hydrogen atom is reaction will occur. Hence, tert-butanol is stable towards oxidation reaction.
As for 2-butanol, a greenish solution is formed after heating. One hydrogen from the hydroxyl group (-OH) a from the carbon where hydroxyl functional group is attached and replaced by a carbon-hydrogen double bond hydrogen from OH group and the hydrogen atom is removed) is attached to two alkyl group, hence the prod butanone. Besides, the two removed hydrogen atom will react with oxygen to form a water molecule.
For n-butanol and alcohol X, a pale green solution is formed. If excess alcohol is used, an aldehyde group wil butanal and water. However, because excess oxidising agent is used, n-butanol is further oxidised by adding o carboxylic acid. Hence the end product is butanoic acid. As for alcohol X, it is determined as an primary alco oxidation test. The equations of the chemical reactions of n-butanol (1 alcohol) and 2-butanol (2 alcohol) ar n-butanol (1 alcohol): Na2Cr2O7 / H+ CH3CH2CH2CH2OH + [O] CH3CH2CH2CHO + H2O (n-butanol) (butanal) Na2Cr2O7 / H+ CH3CH2CH2CHO + [O] CH3CH2CH2COOH (butanal) (butanoic acid)
Overall: Na2Cr2O7 / H+ CH3CH2CH2CH2OH + 2[O] CH3CH2CH2COOH + H2O (n-butanol) (butanoic acid) 2-butanol (2 alcohol): Na2Cr2O7 / H+ CH3CH(OH)CH2CH3 + [O] CH3COCH2CH3 + H2O (2-butanol) (2-butanone) 2-methyl-2-propanol (3 alcohol): Na2Cr2O7 / H+ (CH3)3COH + [O] No reaction (2-methyl-2-propanol)
Overall, primary alcohols are oxidized to carboxylic acids or aldehydes, where in the experiment n-butanol w usage of the strong oxidising agent like Na2Cr2O7 in excess. On the other hand, secondary alcohols are oxidi oxidised to 2-butanone. Tertiary alcohols show no reaction due to their stable structure where the carbon atom stable alkyl groups. C. Esterification test
Esters are derived from carboxylic acids and alcohols. A carboxylic acid contains the -COOH group while alc are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst just like in this experiment. The esterification reaction is both slow and reversible. Hydroxyl group from the a alcohol is removed to form water and ester. Due to the reaction of removal of water, esterification is also kno general equation is shown as below:
In this test, ethanol, CH3CH2OH react with ethanoic acid, CH3COOH, in the presence of a few drops of conc bath, to form an ester called ethyl ethanoate or ethyl acetate. The chemical composition of acids, alcohols and reaction. In general, ester produces pleasant scent. However in this experiment, a rubbery scent is detected. T slow and reversible; hence not much ester is produced. The smell might be slightly distorted or covered by th need to be added to the ester to produce a stronger pleasant, often fruit smell. Esters are widely used in the fra used in the organic chemistry for the test of alcohols and carboxylic acid. The equation of the chemical reaction that occurred is shown as follows: Concentrated HCl CH3CH2OH + CH3COOH CH3COOCH2CH3 + H2O (Ethanol) (Ethanoic acid) (Ethyl ethanoate) Precautionary steps:
1. Keep all the alcohols from any source of fire as alcohols are highly flammable.
2. All the unused or used waste materials must be poured into a specific waste container prepared in the fume environment. 3. Cautions must be taken when pouring concentrated sulphuric acid as it is toxic and is destructive to mucus 4. 2-methyl-2-propanol is harmful if inhaled. May cause skin and respiratory irritant. Severe eye irritant. 5. All transfer of chemicals should be done in the fume chamber to avoid inhalation of any chemicals vapour as cancer. Conclusions:
From the experiment, it can be concluded that n-butanol and the unknown alcohol X are primary alcohol, 2-b methyl-2-propanol is a tertiary alcohol. Primary alcohol will undergo oxidation but react slowest in halogen s alcohol in the other hand undergoes oxidation reaction but will react with a medium speed under halogen sub not undergo oxidation reaction but will react almost immediately in halogen substitution. Alcohols will react References: 1. From the internet:
"boiling point of alcohols and phenols", retrieved on 1 February 2011 from http://www.tutorvist iv/oxygen-i/alcohols-and-phenols-boiling-point.php
"Lucas test for alcohols", retrieved on 1 February 2011 from http://jchemed.chem.wisc.edu/JCESoft/CCA/CCA5/MAIN/1ORGANIC/ORG11/TRAM11/C/0362502/MOV
"oxidation of alcohols", retrieved on 1 February 2011 from http://www.chemguide.co.uk/organi
"introducing alcohols", retrieved on 2 February 2011 from http://www.chemguide.co.uk/organic
"reactin of alcohols", retrieved on 2 February 2011 from http://www.cliffsnotes.com/study_guid Alcohols.topicArticleId-23297,articleId-23272.html "chapter 15, alcohols, diols and triols", retrieved on 2 February 2011 from http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch15/ch15-4.html
Das könnte Ihnen auch gefallen
- CHM1024 Report 4: Reactions of AlcoholsDokument15 SeitenCHM1024 Report 4: Reactions of AlcoholsAkmal Adib Fadzil83% (18)
- Ketones and AldehydesDokument10 SeitenKetones and AldehydesManjeeta Mandlik0% (1)
- Classification Tests For Hydroxyl - and Carbonyl - Containing CompoundsDokument6 SeitenClassification Tests For Hydroxyl - and Carbonyl - Containing CompoundsShaira Jhann L. Rosales50% (2)
- Experiment 17 AlcoholDokument23 SeitenExperiment 17 AlcoholChongZYNoch keine Bewertungen
- Intro & Abstract of AlcoholDokument6 SeitenIntro & Abstract of AlcoholgunaNoch keine Bewertungen
- Alcohols and Phenols (ROH, Functional GRP - OH.)Dokument24 SeitenAlcohols and Phenols (ROH, Functional GRP - OH.)MadhureemaNoch keine Bewertungen
- 1B Carbonyl CompoundsDokument14 Seiten1B Carbonyl CompoundsRida Nadeem SheikhNoch keine Bewertungen
- The Hydrogenation of AlkenesDokument8 SeitenThe Hydrogenation of AlkenesMuhamad Nazrul BoyoteenNoch keine Bewertungen
- Chemistry of The Alcohols Alcohols: Monohydric C H OHDokument23 SeitenChemistry of The Alcohols Alcohols: Monohydric C H OHAyodele AdeyonuNoch keine Bewertungen
- The Oxidation of AlcoholsDokument3 SeitenThe Oxidation of AlcoholsZul FahmiNoch keine Bewertungen
- ALKANOLSDokument25 SeitenALKANOLSKoki KingNoch keine Bewertungen
- Qualitative Analysis Formal Report - AlcoholsDokument3 SeitenQualitative Analysis Formal Report - AlcoholsPrincess Alyssa Abid100% (1)
- Experiment 4 ChemDokument9 SeitenExperiment 4 ChemLogen WolverineNoch keine Bewertungen
- Experiment 2: Organic Chemistry For Environmental HealthDokument7 SeitenExperiment 2: Organic Chemistry For Environmental HealthASYRANI ZULAIKHANoch keine Bewertungen
- Alcohol Notes 2014Dokument4 SeitenAlcohol Notes 2014chenjudeNoch keine Bewertungen
- Experiment 9 Formal ReportDokument5 SeitenExperiment 9 Formal ReportTrishaNoch keine Bewertungen
- Alcohols 1Dokument13 SeitenAlcohols 1Suresh VedpathakNoch keine Bewertungen
- Alco and PhenoDokument5 SeitenAlco and PhenofastrackeNoch keine Bewertungen
- EXPERIMENT 4 (Organic Chemistry II) Properties of Alcohols: Structure, Reactions and Identification of AlcoholsDokument11 SeitenEXPERIMENT 4 (Organic Chemistry II) Properties of Alcohols: Structure, Reactions and Identification of AlcoholsNor Ashikin IsmailNoch keine Bewertungen
- 11.3B AlcoholsDokument34 Seiten11.3B AlcoholsЕлнур ИкимбаевNoch keine Bewertungen
- Chemistry Form 6 Sem 3 Chapter 5Dokument51 SeitenChemistry Form 6 Sem 3 Chapter 5Yuzamrah Awang Noh100% (1)
- F334 - What's in A Medicine?Dokument11 SeitenF334 - What's in A Medicine?Becky Tenney100% (1)
- Topic 4.5 Compounds Containing The Carbonyl GroupDokument30 SeitenTopic 4.5 Compounds Containing The Carbonyl GroupimaniceguyNoch keine Bewertungen
- Alcohol, Phenol and EtherDokument21 SeitenAlcohol, Phenol and EtherAditya NandaNoch keine Bewertungen
- Experiment 4Dokument9 SeitenExperiment 4AdliHakimNoch keine Bewertungen
- Fig. 1: Redox ReactionDokument9 SeitenFig. 1: Redox ReactionsabrinacruzNoch keine Bewertungen
- Group 1 - chm132 - Lab Report1 - An'nur Najwa Binti Abd Bayan - 2021463836Dokument10 SeitenGroup 1 - chm132 - Lab Report1 - An'nur Najwa Binti Abd Bayan - 2021463836AN'NUR NAJWA ABD BAYANNoch keine Bewertungen
- Alcohol Phenols and EthersDokument13 SeitenAlcohol Phenols and EthersShivaanee SK100% (1)
- Synthesis of Cyclohexene Lab ReportDokument3 SeitenSynthesis of Cyclohexene Lab ReportImani London Smith67% (3)
- Classification Test For HydroxylDokument2 SeitenClassification Test For HydroxylJ.c. RimorinNoch keine Bewertungen
- CHM 121 Lecture NoteDokument13 SeitenCHM 121 Lecture NoteOyedotun TundeNoch keine Bewertungen
- Reactions of Alcohols, Phenols, Aldehydes and KetonesDokument44 SeitenReactions of Alcohols, Phenols, Aldehydes and KetonesGlen Mangali100% (4)
- Detection of Functional Groups in Organic CompoundsDokument6 SeitenDetection of Functional Groups in Organic CompoundsKiran PatroNoch keine Bewertungen
- Carbonyl CompoundsDokument40 SeitenCarbonyl CompoundsMiguelNoch keine Bewertungen
- AlcoholDokument25 SeitenAlcoholKING RAFINoch keine Bewertungen
- Chemistry Form 6 Sem 3 04Dokument44 SeitenChemistry Form 6 Sem 3 04Ng Swee Loong StevenNoch keine Bewertungen
- Revised Organic ChemistryDokument90 SeitenRevised Organic ChemistryMinh TieuNoch keine Bewertungen
- The Properties of Alcohols I.: C H OH H R C R' OH H R C R' OH R'' RDokument10 SeitenThe Properties of Alcohols I.: C H OH H R C R' OH H R C R' OH R'' RWimbo TrionoNoch keine Bewertungen
- Identification of AlcoholsDokument26 SeitenIdentification of Alcoholspupu_wowNoch keine Bewertungen
- Unit 7-10 SM Theory Book 2 EM For 2022GRDokument19 SeitenUnit 7-10 SM Theory Book 2 EM For 2022GRThilanka LiyanageNoch keine Bewertungen
- Chem 5-1st Post Lab DiscussionDokument41 SeitenChem 5-1st Post Lab DiscussionJesselie SalayaNoch keine Bewertungen
- Classification of Carbonyl and Hydroxyl Containing CompoundsDokument7 SeitenClassification of Carbonyl and Hydroxyl Containing CompoundsSamantha Hope SyNoch keine Bewertungen
- Separation of The Colored Pigments Found in MalunggayDokument9 SeitenSeparation of The Colored Pigments Found in MalunggayZxyl BasilioNoch keine Bewertungen
- Oxygen Containing Organic CompoundsDokument9 SeitenOxygen Containing Organic CompoundsmNoch keine Bewertungen
- Hydroxyl Compounds: Alcohol & PhenolDokument59 SeitenHydroxyl Compounds: Alcohol & PhenolUMMU MARDHIAH ABDUL HALIMNoch keine Bewertungen
- Exercise 7 (Organic Derivatives of Water)Dokument6 SeitenExercise 7 (Organic Derivatives of Water)Wendell Kim Llaneta0% (1)
- Classification Tests For Carbonyl and Hydroxyl GroupsDokument10 SeitenClassification Tests For Carbonyl and Hydroxyl GroupsJennifer HerediaNoch keine Bewertungen
- Experiment 4 & 5Dokument10 SeitenExperiment 4 & 5Mhi Ismail0% (1)
- Alcohols, Ethers and PhenolsDokument45 SeitenAlcohols, Ethers and Phenolsshivam08Noch keine Bewertungen
- Lab 3 AlcoholDokument11 SeitenLab 3 AlcoholalihusseinNoch keine Bewertungen
- Experiment 12Dokument4 SeitenExperiment 12Rohit BiswasNoch keine Bewertungen
- Mapua Institute of Technology: School of Chemical Engineering and ChemistryDokument12 SeitenMapua Institute of Technology: School of Chemical Engineering and ChemistryVon Joby RomeroNoch keine Bewertungen
- Laboratory 22: Properties of AlcoholsDokument17 SeitenLaboratory 22: Properties of AlcoholsElvis BartingeiNoch keine Bewertungen
- Reactions of Alcohol: Oxidation ReductionDokument18 SeitenReactions of Alcohol: Oxidation ReductioncikguhafidzuddinNoch keine Bewertungen
- Organic Derivatives of WaterDokument6 SeitenOrganic Derivatives of WaterCelyn Ann RamosNoch keine Bewertungen
- P&D AlcoholsDokument5 SeitenP&D AlcoholsBrandon RamkissoonNoch keine Bewertungen
- Classification Tests For Hydroxyl and Carbonyl Containing CompoundsDokument7 SeitenClassification Tests For Hydroxyl and Carbonyl Containing CompoundsSamantha Louise MondonedoNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsVon EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsNoch keine Bewertungen
- Advanced Pharmaceutical analysisVon EverandAdvanced Pharmaceutical analysisBewertung: 4.5 von 5 Sternen4.5/5 (2)
- Corrguard - 95: Key Performance AdvantagesDokument3 SeitenCorrguard - 95: Key Performance AdvantagesTRUNG PHẠM TIẾNNoch keine Bewertungen
- 4-Polyurethanes From Vegetable Oils and Applications A ReviewDokument15 Seiten4-Polyurethanes From Vegetable Oils and Applications A ReviewSolmazNoch keine Bewertungen
- Reactions of AlcoholsDokument49 SeitenReactions of AlcoholsayushNoch keine Bewertungen
- XII Chemistry - Frequently Asked Question Bank PDFDokument175 SeitenXII Chemistry - Frequently Asked Question Bank PDFYASH PATELNoch keine Bewertungen
- IntroductionDokument16 SeitenIntroductionAkhtar RazaNoch keine Bewertungen
- Topic 10.3 2009 Structural Elucidation Prelim Soln PDFDokument21 SeitenTopic 10.3 2009 Structural Elucidation Prelim Soln PDFJustt MeeNoch keine Bewertungen
- Reeves2017 PDFDokument27 SeitenReeves2017 PDFArthur HenriqueNoch keine Bewertungen
- SYBSc Revised SyllabusDokument21 SeitenSYBSc Revised SyllabusAffan AnsariNoch keine Bewertungen
- SC2889 PDFDokument15 SeitenSC2889 PDFA MahmoodNoch keine Bewertungen
- Esterification Oil of WintergreenDokument8 SeitenEsterification Oil of WintergreenMaria MahusayNoch keine Bewertungen
- Energy Conversion and Management: Dipesh S. Patle, Shivom Sharma, Z. Ahmad, G.P. RangaiahDokument12 SeitenEnergy Conversion and Management: Dipesh S. Patle, Shivom Sharma, Z. Ahmad, G.P. RangaiahsamandondonNoch keine Bewertungen
- Richard Gunawan, Xiang Li, Caroline Lievens, Mortaza Gholizadeh, Weerawut Chaiwat, Xun Hu, Daniel Mourant, John Bromly, Chun-Zhu LiDokument9 SeitenRichard Gunawan, Xiang Li, Caroline Lievens, Mortaza Gholizadeh, Weerawut Chaiwat, Xun Hu, Daniel Mourant, John Bromly, Chun-Zhu LiMaghfira RenandaNoch keine Bewertungen
- Consumer Chemistry Q1Dokument41 SeitenConsumer Chemistry Q1Anisha Shen Tagum100% (1)
- Chapter 23 Functional GroupsDokument81 SeitenChapter 23 Functional GroupsYudi PermanaNoch keine Bewertungen
- FFR 4 PDFDokument7 SeitenFFR 4 PDFCamille Loscalzo100% (1)
- Reaction Kinetics of The Catalytic Esterification of Citric Acid With EthanolDokument8 SeitenReaction Kinetics of The Catalytic Esterification of Citric Acid With EthanolJason SanchezNoch keine Bewertungen
- Chapter 7 Organic Chemistry 2020Dokument39 SeitenChapter 7 Organic Chemistry 2020lavanya.aNoch keine Bewertungen
- Ulangan Bahasa Inggris Kelas Xi Ipa / Ips: A. Choose The Best Answer (A, B, C, D, or E) and Cross in Your Answer SheetDokument8 SeitenUlangan Bahasa Inggris Kelas Xi Ipa / Ips: A. Choose The Best Answer (A, B, C, D, or E) and Cross in Your Answer SheetsindyNoch keine Bewertungen
- Part 1Dokument5 SeitenPart 1eljon18Noch keine Bewertungen
- Degree of Unsaturation.: Prepared By: Efefany Jane H. JumaritoDokument10 SeitenDegree of Unsaturation.: Prepared By: Efefany Jane H. JumaritoJodie Mer DayamaNoch keine Bewertungen
- AQA AS Level Chemistry Data SheetDokument4 SeitenAQA AS Level Chemistry Data SheetA100% (1)
- 32 Organ PDFDokument3 Seiten32 Organ PDFThuvarakaNoch keine Bewertungen
- 6 Hydroxyhexyl 4 E 4 Alkoxy Halostyryl Benzoates Synthesis Characterisation and Study of Mesomorphic and Fluorescent PropertiesDokument16 Seiten6 Hydroxyhexyl 4 E 4 Alkoxy Halostyryl Benzoates Synthesis Characterisation and Study of Mesomorphic and Fluorescent PropertiesKhushi MuhammadNoch keine Bewertungen
- Cubane SynthesisDokument5 SeitenCubane SynthesissquaraineNoch keine Bewertungen
- Ib PPT 10 SL PDFDokument84 SeitenIb PPT 10 SL PDFzarna nirmal rawalNoch keine Bewertungen
- Derivatization Reactions and Reagents For Gas Chromatography AnalysisDokument28 SeitenDerivatization Reactions and Reagents For Gas Chromatography AnalysisangelaNoch keine Bewertungen
- Chemical Cons 01 PL ImrichDokument120 SeitenChemical Cons 01 PL ImrichДеца СрбијеNoch keine Bewertungen
- Production of Sucroesters Using Solvent-FreeDokument8 SeitenProduction of Sucroesters Using Solvent-FreeAlfonso Dominguez GonzalezNoch keine Bewertungen
- Notetaker Organic ChemistryDokument19 SeitenNotetaker Organic ChemistryMike AndersonNoch keine Bewertungen
- Introduction To Organic ChemistryDokument142 SeitenIntroduction To Organic ChemistryHafiz Hamidi100% (1)