Beruflich Dokumente
Kultur Dokumente
Diastereoselective Corey Chaykovsky 9 Epoxymethylation of Cinchona Alkaloids: Access To Chiral Scaffolds With Diverse Functionalities
Hochgeladen von
Ana BrunoOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Diastereoselective Corey Chaykovsky 9 Epoxymethylation of Cinchona Alkaloids: Access To Chiral Scaffolds With Diverse Functionalities
Hochgeladen von
Ana BrunoCopyright:
Verfügbare Formate
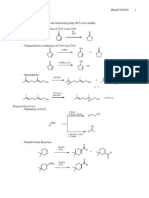
Article
pubs.acs.org/joc
Diastereoselective CoreyChaykovsky 9Epoxymethylation of
Cinchona Alkaloids: Access to Chiral Scaolds with Diverse
Functionalities
Przemysaw J. Boratynski* and Jacek Skarze wski
Department of Organic Chemistry, Faculty of Chemistry, Wrocaw University of Technology, 50-370 Wrocaw, Poland
S Supporting Information
*
ABSTRACT: Reaction of dimethylsulfonium methylide with Cinchona alkaloid ketones proceeds with complete
diastereoselectivity to give epoxides of 8,9-like conguration. The reaction of dimethylsulfoxonium methylide gives dierent
isomers, albeit with lower (4:1) selectivity. -Epimerization of the alkaloid ketones resulted in formation of two separable
diasteromeric products. The congurations of the epoxides were elucidated on the basis of NMR data combined with DFT
calculations. Models explaining observed selectivity are discussed. The epoxides were eciently transformed to a number of
derivatives through selective SN2-type ring-opening reactions with various nucleophiles, often without the need of additional
purication steps.
1. INTRODUCTION
Cinchona alkaloids, privileged transition metal ligands and
organocatalysts, enjoy continued interest for their numerous
applications in asymmetric synthesis.1 Nevertheless, there have
been relatively few successful modications to the alkaloid
carbon skeleton at the C-9 center. Few examples include the
introduction of additional CF3,2 simple alkyl, aryl and vinyl
groups,3 and the corresponding ring rearrangements.4 An
attractive approach to the extension of carbon framework is
oered by the CoreyChaykovsky reaction of sulfur ylides5
with the respective 9-carbonyl compounds. Generally, the
stereochemistry of epoxides resulting from the Corey
Chaykovsky reaction can be controlled by the use of stabilized
chiral sulfur ylides6 or chiral lithiumlanthanide complexes.7
Highly diastereoselective reaction was also noted for the
adequately substituted cyclic ketones.8 However, in the case of
acyclic ketones with a stereogenic center at the -position,
rather fair diastereoselectivities have been found.9
The feasibility of epoxides for further transformations makes
them valuable synthetic intermediates. As a result, a library of
multifunctional derivatives of Cinchona alkaloids relevant to
organocatalysis1,10 and metalorganic frameworks11 can be
envisaged.
Similar results were also obtained for other solvent and base
combinations13 (like potassium tert-butoxide in THF). After an
aqueous workup, the epoxides were obtained as a mixture of
two diastereomers in very high (8090%) yield. No other
isomeric products could be detected by NMR. However, when
instead of trimethylsulfonium, respective sulfoxonium iodide
was used, all of the four possible isomers were identied in the
crude mixture (Scheme 2). This is in contrast to the usually
observed higher selectivity of the sulfoxonium reagents.14
The epoxides from the reaction of trimethylsulfonium iodide
were formed with essentially invariable diastereomeric ratio of
7:6, regardless of the base, solvent, and addition sequence.
Chromatography of the initial mixtures on silica gel did not
separate the isomers; however, fractional crystallization allowed
for isolation of pure diastereomers. Crystallization from tertbutyl methyl ether was particularly ecient for cinchoninederived epoxides CN-2 and CD-2 (Scheme 1). In the rst crop
pure CN-2 was isolated in 30% yield. Pure CD-2 was obtained
by sequential crystallization or a chromatography of the
enriched mother liquor followed by recrystallization from
diethyl ether. Unfortunately this separation procedure did not
translate well to the quinidine-series epoxides. There, only QD2 could be isolated in high diastereomeric purity by
crystallization from diethyl ether. However, each of the two
additional isomers (QD-3 and QN-3) formed in the reaction of
trimethylsulfoxonium iodide (Scheme 2) was separated by
chromatography.
2. RESULTS AND DISCUSSION
2.1. Synthesis of Epoxides. Cinchona alkaloids (quinine
and cinchonine) were converted to the corresponding ketones
QD-1 and CN-1 by Oppenauer oxidation and crystallization.12
The ketones treated with trimethylsulfonium iodide and
potassium hydroxide in DMSO were cleanly transformed to
the epoxides 2 (Scheme 1).
XXXX American Chemical Society
Received: March 5, 2013
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
The Journal of Organic Chemistry
Article
Scheme 1. Synthesis of Epoxides 2
of the observed NOESY correlations, involving quinolines H-5
and H-3 atoms as well as the epoxide CH2 group are much
better explained assuming 9R conguration for CN-2 and 9S
for CD-2 (Figure 2). In the molecular models of CN-2 and
CD-2, but not of their 9-epimers, the interatomic distances
corresponding to the observed NOESY cross-peaks were
relatively short (2.23.7 ) and exhibited good correlation
with the measured signal intensities (see Section S3.3,
Supporting Information). The assignment of conguration
was further augmented by comparison of the experimental and
calculated (GIAO/B3LYP/6-31G(d,p)) chemical shifts. Both
the calculated 1H and 13C NMR shifts are in consistently better
correlation with the experimental data for the assigned 8,9-like
conguration (see Section S3.4, Supporting Information).
2.3. Stereochemistry of CoreyChaykovsky Reaction.
Identication of (8R,9R)-CN-2 and (8S,9S)-CD-2 as diastereomeric pair diering in conguration at both C-8 and C-9
stereocenters indicates that the CoreyChaykovsky reaction
using the sulfonium ylide is diastereoselective. The compositions of the obtained CN-2/CD-2 and QD-2/QN-2 product
mixtures correspond to the compositions of the respective
ketone mixtures equilibrated in the presence of base.
Furthermore, identical product mixtures were obtained both
from crystalline QD-1 and the previously equilibrated mixture
of QD-1 and QN-1. The isomerization of the ketones (QD-1
and CN-1) in solution is long documented,15 and here it
appears to be much faster than the nucleophilic addition of the
ylide (Scheme 3).
The observed addition of the sulfonium ylide occurred from
the Re face of cinchoninone (CN-1) and quinidinone (QD-1)
Scheme 2. Reaction of Dimethylsulfoxonium Methylide
2.2. Conguration of Epoxides. Investigation of the
NOESY spectra of the separated CN-2 and CD-2 allowed for
the unequivocal assignment of the conguration at the C-8
stereogenic center. The correlation of H-8 with H-6 for CN-2
identies 8R conguration. For CD-2 the correlation of H-8
with H-2 conrms its 8S conguration (Figure 1). The overall
patterns of the correlations suggest 8,9-like conguration.
Figure 1. Nuclear Overhauser eect experiments for CN-2 and CD-2.
A molecular model (Figure 2) was used to verify the
conguration at C-9. The lowest energy conformers for both
CN-2 and CD-2 and their 9-epimers were determined at the
DFT/B3LYP/6-31G(d,p) level of theory. The essential pattern
Figure 2. DFT/B3LYP/6-31G(d,p) optimized structures and selected NOESY interactions (dashed lines) for CN-2 (left) and CD-2 (right). Strong
interactions are marked in red, weak in magenta (for details, see Section S3.3, Supporting Information).
B
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
The Journal of Organic Chemistry
Article
Scheme 3. Stereochemistry of CoreyChaykovsky Reaction of Dimethylsulfonium Methylide
Figure 3. DFT/B3LYP/6-31G(d,p) low energy conformations and reactivity of CN-1. Re and Si faces of carbonyl group are marked.
heterocyclic ketone with dimethylsulfonium methylide,18 the
face of the nucleophilic attack was opposite to the heteroatom
lone pair. Thus, in our case the electrostatic and stereoelectronic eects appear to be dominant and result in the
formation of the observed kinetic products from either of the
two lowest energy conformers of the starting material.
In the case of reaction of dimethylsulfoxonium methylide, a
dierent set of isomers ((8R,9S)-QD-3 and (8S,9R)-QN-3)
was dominant (Scheme 2). There, unlike in the reactions of
dimethylsulfonium methylide, the addition step to form
intermediate betaines is known to be reversible.19 Our
preliminary calculations indicate that the betaines leading to
the epoxides of 8,9-unlike conguration are of lower energy (see
Section S3.2, Supporting Information). Thus thermodynamic
equilibration of the betaines seems to dene the stereochemical
outcome.
2.4. Epoxide Ring-Opening. In order to examine possible
applications of the epoxides as synthetic intermediates, several
ring-opening reactions with various nucleophiles were studied.
Hydrolysis of the epoxide CN-2 under mildly acidic conditions
(tartaric acid) delivered a single diol CN-4. The reaction of
epoxide CN-2 with thiophenolates under ambient conditions
gave the respective phenylsulfanyl derivatives CN-5 and CN-6
with excellent yields (Scheme 4).
On the other hand the reaction of CN-2 with ammonium
thiocyanate resulted predominantly in deoxygenation.20 The
most likely course of the reaction involves thiirane21 that
undergoes spontaneous desulfurization22 to give the 9methylidene derivative CN-7. The reaction of epoxide CN-2
with NaN3 in the presence of NH4Cl in MeOH gave the
and Si face for cinchonidinone and quininone (QN-1) (Scheme
3). Similar facial selectivity was previously reported for the
addition of TMS-CF3 to qunidinone.2 Conversely, addition at
the opposite face occurred in cases where metal chelates were
involved, like in the addition of Grignard reagents,3 and lithium
aluminum hydride reduction.16
We hypothesized that the conformation of the starting
material determines the outcome of the reaction. The
optimization of CN-1 in vacuum at the DFT/B3LYP/631G(d,p) level of theory indicates two low energy conformations (Figure 3). They dier in the OC9C8N1
dihedral angle of +103 and +10 for the closed and open
conformers, respectively. The closed conformer is lowest in
energy and is similar to the observed in the crystal structure of
QD-1.2 In this conformation the Re face is exposed, while the
quinuclidine ring sterically hinders the nucleophilic attack from
the Si-face (Figure 3). Nevertheless, the calculated energy
dierence between the conformers is rather small (3 and 1
kcal/mol for calculations in vacuum and DMSO (PCM),
respectively). Thus, the steric factors alone cannot be
responsible for the observed selectivity in the nucleophilic
additions. In both conformations the nitrogen lone pair is
directed from the Si face. It is more pronounced for the open
conformer, where the lone electron pair is nearly perpendicular
to the plane of the carbonyl group. The overlap of the lled
orbital at the nitrogen atom with the orbitals of the carbonyl
group causes a stereoelectronic eect that prevents the
approach of the reacting nucleophile from the side of the
overlap17 (see Section S3.1, Supporting Information). Similarly,
in the recently reported CoreyChaykovsky reaction of
C
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
The Journal of Organic Chemistry
Article
In all the described epoxide ring-openings only one isomer of
product was formed from a single isomer of the epoxide.
Likewise in both the hydrolysis and azidolysis the use of CD-2
instead of CN-2 led to the single diol CD-4 and azide CD-8,
respectively (Scheme 7).
All the compounds with free hydroxyl group exhibit
quaternary 13C NMR signals at 7983 ppm attributed to the
C-9 of tertiary alcohols. As expected, these signals are aected
only to a little extent by the nucleophiles attached at the
neighboring CH2 group. The structures of the products were
additionally conrmed by further NMR experiments. In the
HMBC spectrum of CN-18, the introduced CH2 group (but
not the quaternary C-9) correlates with the atoms of benzyl and
acetyl groups (Figure 4). Additionally, the 13C NMR shifts
observed for CN-14 also agree with those observed for 5,5disubstituted oxazolidinethiones and not for the 4,4-disubstituted derivatives (for the details see Figure S5, Supporting
Information). Thus the ring-opening reaction must have
occurred at the less substituted carbon atom through the SN2
mechanism. The unlikely SN1-type reaction at the more
substituted carbon would not lead to a clean conversion
observed in most of the reactions here. Involvement of a
carbocation at C-9 had previously resulted in two diastereomers
of the products and a series of rearranged compounds (cf.
Cinchona rearrangements).4
Scheme 4. Reaction of Epoxide CN-2 with Chalcogen
Nucleophiles
3. CONCLUSIONS
The diastereoselective CoreyChaykovsky reaction of the
Cinchona alkaloid ketones led to the corresponding 9epoxymethyl derivatives in good yields. The reaction with
dimethylsulfonium methylide occurred at the face opposite to
the nitrogen lone pair, resulting exclusively in the 8,9-like
conguration. Particularly, (9R)-9-epoxymethyl-cinchonine
(CN-2) was easily prepared from the commercially available
alkaloids, without the use of chromatography. The reaction of
dimethylsulfoxonium methylide gave predominantly the
epoxides of 8,9-unlike conguration. The selective SN2 ringopening of epoxides with various oxygen, sulfur, and nitrogen
nucleophiles gave multifunctional aminoalcohols, chiral building blocks for prospective catalysts.
respective azide CN-8. In this case the crude product did not
require further purication, and the yield was nearly
quantitative (Scheme 5).
The azide CN-8 reacted with phenylacetylene in the
copper(I)-catalyzed 1,3-dipolar cycloaddition click reaction
giving the corresponding triazole CN-9. Also, the azide CN-8
was reduced to the corresponding amine CN-10 with LiAlH4.23
Although the isolation of pure aminoalcohol CN-10 was
ineective, the crude product was used for further transformations. Alternatively, the azide CN-8 was rst converted to
the corresponding silyl ether CN-11, which was reduced under
the Staudinger conditions to the more tangible aminoether CN12. The reaction of crude CN-10 with 3,5-bis(triuoromethyl)phenyl isothiocyanate gave the corresponding thiourea CN-13.
Apart from the desired thiourea, also oxazolidinethione CN-14
was isolated from the reaction mixture. Oxazolidinethione CN14 was independently obtained in the reaction of CN-10 and
carbon disulde. It was also found to form in the heated sample
of CN-13 in DMSO. However, under similar conditions
thiourea with silyl ether group CN-15 was stable. Amine CN10 was also converted to the amide CN-16 with acetic
anhydride.
The epoxide CN-2 reacted with benzylamine in the presence
of lithium perchlorate at 100 C, giving the corresponding
secondary amine CN-17. Treatment of the aminoalcohol CN17 with an excess of acetic anhydride gave the N-acetylated
product CN-18 with unmodied 9-hydroxyl group.
Hydrogenation of CN-2 on 10% Pd/C occurred solely at the
vinyl group of the alkaloid. The product DHCN-2 was identical
to one of the isomers obtained in the epoxidation of 10,11dihydrocinchoninone (DHCN-1, Scheme 6). The epoxides
could not be eciently opened by Grignard reagents or by
LiAlH4.
4. EXPERIMENTAL SECTION
NMR spectra were internally referenced to solvent signals: 1H 7.26 for
CHCl3, 2.50 for DMSO-d5, and 13C 77.16 for CDCl3, 39.52 for
DMSO-d6. High resolution mass spectra (TOF) were obtained in
electrospray ionization mode. All reagents were purchased from
commercial suppliers and used as received, CN-1, and QD-1 were
prepared according to literature procedures.3,13 DHCN-1 was
prepared following the literature procedure used for CN-13b from
10,11-dihydrocinchonidine and recrystallized from Et2O in 62% yield.
10,11-Dihydrocinchonanone (DHCN-1). Yellow crystalline
solid: mp 121124 C (Et2O), 122125 C (EtOH) (lit.15 mp 138
C (EtOH)); 1H NMR (CDCl3, 300 MHz) 8.97 (d, J = 4.4 Hz, 1H),
8.20 (d, J = 8.4 Hz, 1H), 8.12 (d, J = 8.5 Hz, 1H), 7.72 (ddd, J = 8.5,
6.9, 1.4 Hz, 1H), 7.64 (d, J = 4.4 Hz, 1H), 7.58 (ddd, J = 8.4, 6.9, 1.3
Hz, 1H), 4.16 (m, 1H), 3.12 (dd, J = 13.6, 9.2 Hz, 1H), 2.77 (m, 1H),
2.502.74 (m, 2H), 2.07 (m, 1H), 1.83 (m, 1H), 1.77 (m, 1H), 1.63
(m, 1H), 1.331.51 (m, 4H), 0.92 (t, J = 7.2 Hz, 3H); 13C NMR
(CDCl3, 75.5 MHz) 203.2, 149.8, 149.2, 143.4, 130.2, 129.7, 128.0,
125.1, 124.6, 119.5, 63.4, 57.4, 43.5, 37.4, 28.0, 27.6, 25.2, 21.6, 12.2.
CoreyChaykovsky Reaction of CN-1 and Dimethylsulfonium Methylide. In a 100 mL round-bottom ask were placed
recrystallized cinchoninone3 (11.9g, 40.8 mmol), trimethylsulfonium
iodide (8.67 g, 42.4 mmol, 1.04 equiv), and DMSO (64 g). The
suspension was stirred for 5 min, and potassium hydroxide (akes
D
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
The Journal of Organic Chemistry
Article
Scheme 5. Synthesis of Nitrogen Derivatives
Scheme 6. Synthesis of 10,11-Dihydroepoxides
Scheme 7. Selected Reactions of Epoxide CD-2
90%, 2.65 g, 41.8 mmol, 1.03 equiv) was added. Within a few minutes
the mixture turned deep red and became temporarily homogeneous.
After a few hours a precipitate formed and the mixture turned light
orange. The mixture was stirred for a total of 52 h, and then it was
diluted with diethyl ether (450 mL),24 washed with brine (8 50 mL),
and dried over Na2SO4. On evaporation, a mixture of CN-2 and CD-2
(11.1 g, 90%) was obtained. Repeated procedures on 0.132 g scale
gave 8090% yields.
The mixture of isomers was recrystallized from tert-butyl methyl
ether giving 4.17 g of pure CN-2 (33%). On slow evaporation of
solvent from the mother liquor crystalline material containing mostly
CD-2 was obtained. Chromatography of CD-2-enriched fractions on
E
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
The Journal of Organic Chemistry
Article
reaction of CN-1. Starting from QN-1 (5.56 g, 17.2 mmol),
trimethylsulfonium iodide (3.62 g, 17.7 mmol, 1.03 equiv) and
KOH (akes 90%, 2.16 g, 34.1 mmol, 1.98 equiv) in DMSO (25 mL),
4.50 g (78%) of QN-2/QD-2 mixture was obtained. Repeated
procedures on 0.053 g scale gave 7785% yields: HRMS (ESI)
calcd. for [C21H24N2O2 + H]+ 337.1911, found 337.1918. A sample
was recrystallized in diethyl ether at 0 C to give pure QD-2.
9R-Epoxymethylquinidine (QD-2). White crystalline solid: mp
102106 C (Et2O); []D21= +59.6 (c 0.78, EtOH 96%); 1H NMR
(CDCl3, 300 MHz) 8.74 (d, J = 4.3 Hz, 1H), 8.04 (d, J = 9.2 Hz,
1H), 7.47 (br., 1H), 7.37 (dd, J = 9.2, 2.6 Hz, 1H), 7.16 (br., 1H), 6.03
(m, 1H), 5.056 (d, J = 16.5 Hz, 1H), 5.047 (d, J = 11.0 Hz, 1H), 3.94
(s, 3H), 3.59 (t, J = 8.9 Hz, 1H), 3.08 (d, J = 4.9 Hz, 1H), 3.01 (dd, J =
13.7, 7.9 Hz, 1H), 2.80 (m, 1H), 2.532.74 (m, 3H), 2.18 (q, J = 8.6
Hz, 1H), 1.85 (m, 1H), 1.77 (m, 1H), 1.66 (m, 1H), 1.481.57 (m,
2H), 1.33 (m, 1H); 13C NMR (CDCl3, 75.5 MHz) 157.6, 147.9,
144.8, 144.3, 140.9, 132.1, 126.8, 120.6, 120.4, 114.7, 102.6, 59.4, 57.7,
55.7, 49.6, 49.2, 47.7, 40.2, 28.5, 26.7, 20.3; IR (KBr) 2928, 2869,
1621, 1506, 1430, 1251, 1229, 1028, 903, 845, 827, 720 cm1.
CoreyChaykovsky Reaction of QD-1 and Dimethylsulfoxonium Methylide. In a 50 mL round-bottom ask were placed
recrystallized quinidinone (1.05 g, 3.26 mmol) and trimethylsulfoxonium iodide (0.746 g, 3.39 mmol, 1.04 equiv) and DMSO (9 mL).
Potassium hydroxide (90%, 0.233 g, 3.73 mmol, 1.15 equiv) was
added. Within a few minutes an orange solution was obtained. The
mixture was stirred for 24 h. Then it was diluted with ethyl acetate (40
mL), washed with brine (5 15 mL), dried over Na2SO4, and
evaporated. Chromatography on silica gel (EtOAc/Et3N 25:1) gave
162 mg (15%) of 7:6 QD-2/QN-2 mixture, 459 mg (42%) of QD-3,
and 177 mg (15%) of QN-3.
9S-Epoxymethylquinidine (QD-3). White solid: mp 107111 C
(CH2Cl2), []D24= +114.5 (c 1.1, EtOH 96%); 1H NMR (CDCl3, 300
MHz, 318 K) 8.69 (d, J = 4.4 Hz, 1H), 8.00 (d, J = 9.3 Hz, 1H), 7.38
(d, J = 4.4 Hz, 1H), 7.33 (dd, J = 9.3, 2.7 Hz, 1H), 7.30 (br., 1H), 5.95
(ddd, J = 17.4, 10.0, 7.0 Hz, 1H), 5.005.08 (m, 2H), 3.90 (s, 3H),
3.65 (d, J = 5.8 Hz, 1H), 3.32 (t, J = 9.5 Hz, 1H), 3.01 (ddd, J = 13.7,
8.0, 2.2 Hz, 1H), 2.672.92 (m, 3H), 2.77 (d, J = 5.8 Hz, 1H), 2.16
(m, 1H), 1.84 (m, 1H), 1.70 (m, 1H), 1.401.52 (m, 2H), 1.18 (m,
1H); 13C NMR (CDCl3, 75.5 MHz, 318 K) 158.1, 147.5, 144.7,
143.9, 140.5, 131.9, 127.0, 121.7, 120.2, 114.5, 102.6, 63.1, 56.2, 55.6,
51.8, 50.5, 49.3, 39.8, 28.5, 26.6, 23.5; IR (KBr) 2930, 2869, 1619,
1504, 1475, 1429, 1229, 1023, 913, 904, 850, 828, 719 cm1; HRMS
(ESI) calcd. for [C21H24N2O2 + H]+ 337.1911, found 337.1918.
9R-Epoxymethylquinine (QN-3). Light brown oil: []D24= 6.7
(c 1.1, EtOH 96%); 1H NMR (CDCl3, 300 MHz, 318 K) 8.70 (d, J =
4.5 Hz, 1H), 8.01 (d, J = 9.1 Hz, 1H), 7.37 (d, J = 4.4 Hz, 1H), 7.35
(dd, J = 9.1, 2.6 Hz, 1H), 7.32 (d, J = 2.6 Hz, 1H), 5.68 (ddd, J = 17.4,
10.4, 7.3 Hz, 1H), 4.91 (dt, J = 17.4, 1.5 Hz, 1H), 4.86 (dt, J = 10.4,
1.5 Hz, 1H), 3.92 (s, 3H), 3.63 (d, J = 5.6 Hz, 1H), 3.44 (t, J = 9.2 Hz,
1H), 3.21 (m, 1H), 3.06 (dd, J = 13.8, 10.1 Hz, 1H), 2.81 (d, J = 5.6
Hz, 1H), 2.69 (m, 1H), 2.61 (m, 1H), 2.23 (m, 1H), 1.78 (m, 1H),
1.391.70 (m, 4H); 13C NMR (CDCl3, 75.5 MHz, 318 K) 158.2,
147.5, 144.8, 143.9, 142.0, 131.9, 127.0, 121.8, 120.3, 114.4, 102.5,
62.8, 57.6, 56.4, 55.8, 51.4, 42.9, 40.0, 28.07, 28.04, 24.1; IR (KBr)
2938, 2867, 1620, 1507, 1475, 1432, 1230, 1030, 915, 853, 832 cm1;
HRMS (ESI) calcd. for [C21H24N2O2 + H]+ 337.1911, found
337.1916.
9R-Hydroxymethyl-cinchonine (CN-4). Epoxide CN-2 (800 mg,
2.61 mmol) and L-tartaric acid (610 mg, 4.06 mmol, 1.56 equiv) were
suspended in water (40 mL) and heated under reux for 4 h. Then the
mixture was cooled to room temperature and alkalized with solid
NaHCO3, extracted with CHCl3 (7 10 mL), and dried over Na2SO4.
Column chromatography on silica gel (CHCl3/MeOH/Et3N 40:1:4)
gave unreacted starting material and 239 mg of CN-4 (29%) as white
crystalline solid: mp 168171 C; 1H NMR (CDCl3, 300 MHz, 313
K) 8.54 (d, J = 4.4 Hz, 1H), 8.46 (br. d, 1H), 7.91 (d, J = 8.4 Hz,
1H), 7.517.62 (m, 2H), 7.44 (m, 1H), 5.98 (ddd, J = 17.2, 10.7, 7.3
Hz, 1H), 5.03 (dt, J = 10.7, 1.5 Hz, 1H), 5.01 (dt, J = 17.2, 1.5 Hz,
1H), 4.32 (d, J = 11.1 Hz, 1H), 4.00 (d, J = 11.1 Hz, 1H), 4.0 (br.
2H), 3.45 (t, J = 9.7 Hz, 1H), 2.98 (ddd, J = 13.8, 8.0, 2.2 Hz, 1H),
Figure 4. Selected 1H, 13C HMBC correlations for CN-18.
silica gel with ethyl acetate followed by crystallization from diethyl
ether gave pure CD-2. Two additional crops of crystallizations yielded
pure CN-2 (total 5.68 g, 45%) and pure CD-2 (2.52 g, 20%).
9R-Epoxymethylcinchonine (CN-2). White crystalline solid: mp
173177 C (MTBE); []D24= +92.5 (c 1, EtOH 96%); 1H NMR
(CDCl3, 300 MHz) 8.80 (d, J = 4.4 Hz, 1H), 8.13 (d, J = 8.3 Hz,
1H), 7.94 (br., 1H), 7.69 (ddd, J = 8.4, 7.0, 1.3 Hz, 1H), 7.55 (ddd, J =
8.3, 7.0, 1.3 Hz, 1H), 7.51 (br., 1H), 6.04 (m, 1H), 5.035.10 (m,
2H), 3.63 (t, J = 8.9 Hz, 1H), 3.07 (d, J = 4.8 Hz, 1H), 2.963.05 (m,
1H), 2.78 (dd, J = 13.4, 10.2 Hz, 1H), 2.67 (d, J = 4.7 Hz, 1H), 2.52
2.64 (m, 2H), 2.18 (q, J =8.4 Hz, 1H), 1.77 (m, 1H), 1.66 (m, 1H),
1.471.57 (m, 2H), 1.34 (m, 1H); 13C NMR (CDCl3, 151 MHz)
150.3, 148.1, 146.4, 140.9, 130.6, 129.0, 126.6, 125.9, 123.4, 120.1,
114.7, 59.5, 58.1, 49.5, 49.1, 47.6, 40.3, 28.5, 26.7, 20.2; IR (KBr) 2925,
2865, 1633, 1597, 1569, 1507, 1448, 1297, 1223, 937, 914, 844, 802,
764; HRMS (ESI) calcd. for [C20H22N2O + H]+ 307.1805, found
307.1806.
9S-Epoxymethylcinchonidne (CD-2). Large transparent crystals:
mp 124127 C (Et2O); []D24= 35.6 (c 1, EtOH 96%); 1H NMR
(CDCl3, 600 MHz) 8.88 (d, J = 4.5 Hz, 1H), 8.13 (d, J = 8.4 Hz,
1H), 7.93 (br. d, J = 8.1 Hz, 1H), 7.70 (ddd, J = 8.2, 6.9, 1.2 Hz, 1H),
7.56 (ddd, J = 8.2, 6.9, 1.1 Hz, 1H), 7.52 (br.d, J = 4.5 Hz, 1H), 5.88
(ddd, J = 17.2, 10.6, 7.3 Hz 1H), 5.01 (d, J = 10.6 Hz, 1H), 5.00 (d, J =
17.2 Hz, 1H), 3.70 (t, J = 8.5 Hz, 1H), 3.27 (m, 1H), 3.06 (d, J = 4.9
Hz, 1H), 2.90 (d, J = 13.4, 10.5 Hz, 1H), 2.64 (d, J = 4.5 Hz, 1H), 2.55
(m, 1H), 2.41 (m, 1H), 2.20 (m, 1H), 1.83 (m, 1H), 1.651.75 (m,
2H), 1.44 (m, 1H), 1.29 (m, 1H); 13C NMR (CDCl3, 151 MHz)
150.3, 148.1, 146.4, 142.2, 130.7, 129.0, 126.6, 125.9, 123.3, 119.7,
114.4, 59.3, 58.2, 56.4, 47.3, 42.5, 39.6, 27.9, 27.5, 20.3; IR (KBr) 2932,
2860, 1636, 1593, 1567, 1507, 1445, 1307, 1215, 1002, 938, 914, 850,
825, 769 cm1; HRMS (ESI) calcd. for [C20H22N2O + H]+ 307.1805,
found 307.1796.
9R-Epoxymethyl-10,11-dihydrocinchonine (DHCN-2). Method
A: Epoxide CN-2 (144 mg, 0.47 mmol) was dissolved in AcOEt (10
mL) and palladium on activated charcoal (10%, 11.6 mg, 2.5 mol %)
was added. Hydrogen was passed through the stirred suspension for 18
h, and the catalyst was ltered o. On evaporation, 145 mg (99%) of
DHCN-2 was obtained. Method B: DHCN-1 (1.17 g, 4.00 mmol) and
trimethylsulfonium idodide (821 mg, 4.02 mmol, 1.01 equiv) were
dissolved in DMSO (7.5 mL), and KOH was added (akes, 90%, 0.29
g, 4.6 mmol, 1.1 equiv). The mixture was stirred at room temperature
for 80 h and diluted with diethyl ether (50 mL), washed with brine (5
20 mL), dried over Na2SO4, and evaporated giving 0.993 g (81%) of
a 1:1 mixture of DHCN-2/DHCD-2. Crystallization from tert-butyl
methyl ether at 0 C gave DHCN-2 as yellowish crystals: 1H NMR
(CDCl3, 300 MHz) 8.88 (d, J = 4.5 Hz, 1H), 8.12 (d, J = 8.6 Hz,
1H), 7.93 (br. d, J = 8 Hz, 1H), 7.68 (ddd, J = 8.3, 6.8, 1.3 Hz, 1H),
7.54 (ddd, J = 8.3, 7.0, 1.3 Hz, 1H), 7.51 (br., 1H), 3.60 (t, J = 9.2 Hz,
1H), 3.06 (d, J = 4.9 Hz, 1H), 2.672.80 (m, 1H), 2.62 (d, J = 4.9 Hz,
1H), 2.462.64 (m, 2H), 1.70 (m, 1H), 1.221.63 (m, 7H), 0.89 (t, J
= 7.3 Hz, 3H); 13C NMR (CDCl3, 75.5 MHz) 150.3, 148.1, 146.5,
130.6, 129.0, 126.5, 125.9, 123.4, 120.2, 59.3, 58.2, 50.7, 49.6, 47.5,
37.4, 27.5, 26.4, 25.4, 20.0, 12.2; IR (KBr) 2957, 2932, 2864, 1597,
1509, 1451, 1298, 936, 844, 762 cm1; HRMS (ESI) calcd. for
[C20H24N2O + H]+ 309.1961, found 309.1960.
CoreyChaykovsky Reaction of QN-1 and Dimethylsulfonium Methylide. Reaction was performed analogously to the
F
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
The Journal of Organic Chemistry
Article
2.552.82 (m, 3H), 2.102.25 (m, 2H), 1.341.64 (m, 3H); 13C
NMR (CDCl3, 75.5 MHz) 151.8 (br.), 149.4, 148.2, 140.9, 130.0,
128.6, 126.4, 125.8, 125.4, 120.3, 114.5, 80.4, 66.2, 61.8, 50.4, 49.7,
40.2, 29.0, 26.6, 22.4; IR (KBr) 3452, 3254, 3101, 2916, 2865, 1579,
1513, 1454, 1110, 1070, 1050, 909, 826, 770 cm1; HRMS calcd. for
[C20H24N2O2 + H]+ 325.1911, found 325.1905.
9S-Hydroxymethyl-cinchonidine (CD-4). Compound was obtained analogously to CN-4. Starting from 625 mg (2.04 mmol) of
CD-2, 189 mg (28%) of CD-4 was obtained as white solid: mp 150
156 C; 1H NMR (CDCl3, 300 MHz, 313 K) 8.34 (m, 1H), 8.26 (d,
J = 8.2 Hz, 1H), 7.73 (d, J = 7.9 Hz, 1H), 7.59 (d, J = 4.7 Hz, 1H),
7.347.48 (m, 2H), 5.89 (ddd, J = 17.4, 9.8, 7.3 Hz, 1H), 4.965.05
(m, 2H), 4.35 (br., 2H), 4.32 (d, J = 11.1 Hz, 1H), 3.81 (d, J = 11.1
Hz, 1H), 3.53 (t, J = 8.9 Hz, 1H), 3.16 (m, 1H), 2.84 (dd, J = 13.6,
10.4 Hz, 1H), 2.52 (m, 1H), 2.30 (m, 1H), 2.20 (m, 1H), 2.03 (m,
1H), 1.86 (m, 1H), 1.661.84 (m, 2H), 1.38 (m, 1H); 13C NMR
(CDCl3, 75.5 MHz, 313 K) 152.0, 149.4, 148.4, 142.4, 130.2, 128.5,
126.7, 125.8, 125.3, 120.3 114.2, 80.5, 66.5, 62.0, 57.6, 43.4, 40.0, 28.6,
27.7, 22.9; IR (KBr) 3465, 3102, 2934, 2872, 1583, 1512, 1386, 1070,
921, 820, 767 cm1; HRMS calcd. for [C20H24N2O2 + H]+ 325.1911,
found 325.1907.
9R-Phenylsulfanylmethyl-cinchonine (CN-5). Epoxide CN-2
(110 mg, 0.36 mmol) and thiophenol (0.10 mL, 0.96 mmol, 2.7 equiv)
were dissolved in ethanol (96%, 5 mL), and sodium hydroxide (12.5
mg, 0.31 mmol, 0.86 equiv) was added. The mixture was stirred for 18
h at room temperature and diluted with diethyl ether (35 mL), washed
with 10% aqueous NaOH (2 20 mL), and extracted with 2 M HCl
(30 mL). Acidic extracts were treated with excess Na2CO3 and
extracted with CH2Cl2, dried over Na2SO4 and evaporated to give 136
mg (90%) of CN-5 as amorphous solid: 1H NMR (CDCl3, 300 MHz,
318 K) 8.76 (d, J = 4.7 Hz, 1H), 8.38 (br, 1H), 8.06 (dd, J = 8.4, 1.3
Hz, 1H), 7.72 (br. 1H), 7.57 (t, J = 7.5 Hz, 1H), 7.63 (br. 1H), 7.57
(ddd, J = 8.4, 6.9, 1.3 Hz, 1H), 7.42 (ddd, J = 8.7, 6.9, 1.5 Hz, 1H),
7.017.06 (m, 2H), 6.947.00 (m, 3H), 5.99 (ddd, J = 17.2, 10.6, 7.3
Hz, 1H), 5.01 (ddd, J = 10.6, 1.6, 1.3 Hz), 4.98 (ddd, J = 17.2, 1.6, 1.4
Hz, 1H), 4.1 (br., 1H), 4.09 (d, J = 13.4 Hz, 1H), 3.55 (t, J = 9.4 Hz,
1H), 3.48 (d, J = 13.4 Hz, 1H), 3.04 (ddd, J = 13.9, 7.9, 2.2 Hz, 1H),
2.462.71 (m, 3H), 2.27 (t, J = 10.8 Hz, 1H), 2.11 (q, J = 8.3 Hz, 1H),
1.82 (m, 1H), 1.411.63 (m, 3H); 13C NMR (CDCl3, 75.5 MHz, 318
K) 150.6, 149.5, 149.3, 140.8, 134.9, 131.1, 130.7, 128.6, 128.0,
126.8, 125.62, 125.58, 125.3, 120.2, 114.2, 79.9, 63.4, 50.8, 49.7, 45.8,
40.1, 29.2, 26.7, 22.3; IR (KBr) 3434, 3071, 2933, 2869, 1582, 1510,
1439, 1301, 1103, 1089, 759, 741, 690 cm1; HRMS (ESI) calcd. for
[C26H28N2OS + H]+ 417.1995, found 417.1985.
9R-(2-Aminophenyl)-sulfanylmethyl-cinchonine (CN-6). Lithium hydride (26 mg, 2.6 mmol, 2.3 equiv) was suspended in a solution
of 2-aminothiophenol (0.26 mL, 2.43 mmol, 2.2 equiv) in DMF (1
mL), and stirred until a clear solution was obtained (ca. 15 min). Then
epoxide CN-2 (356 mg, 1.11 mmol) was added, the suspension was
stirred for 5 min, and additional DMF (1 mL) was added. The mixture
was stirred for 18 h at room temperature, evaporated in vacuo (50 C),
and dried in a vacuum desiccator over H2SO4. The residue was
dissolved in ethyl acetate (50 mL) and washed with brine (3 10
mL), dried over Na2SO4 and evaporated. Chromatography on silica gel
(CHCl3/MeOH 10:1) gave 442 mg (93%) of CN-6 as a yellowish
amorphous solid: 1H NMR (CDCl3, 300 MHz, 313 K) 8.79 (d, J =
4.8 Hz, 1H), 8.39 (br., 1H), 8.08 (dd, J = 8.5, 1.3 Hz, 1H), 7.65 (br.
1H), 7.58 (ddd, J = 8.5, 7.0, 1.1 Hz, 1H), 7.41 (ddd, J = 8.4, 7.0, 1.3
Hz, 1H), 7.00 (d, J = 7.5 Hz, 1H), 6.90 (t, J = 7.6 Hz, 1H), 6.44 (d, J =
7.7 Hz, 1H), 6.41 (t, J = 7.5 Hz, 1H), 5.94 (m, 1H), 4.98 (dt, J = 10.2,
1.3 Hz, 1H), 4.95 (dd, J = 17.3, 1.2 Hz, 1H), 4.1 (br., 2H), 4.04 (d, J
= 13.1 Hz, 1H), 3.44 (t, J = 8.3 Hz, 1H), 3.24 (d, J = 13.1 Hz, 1H),
3.00 (m, 1H), 2.452.70 (m, 3H), 2.042.24 (m, 2H), 1.78 (m, 1H),
1.371.58 (m, 3H); 13C NMR (CDCl3, 75.5 MHz, 318 K) 149.7,
149.4, 148.0, 142.4, 140.8, 135.7, 131.1, 130.0, 128.2, 127.0, 125.7 (2C
overlap), 120.4, 119.0, 117.8, 115.2, 114.4, 80.6, 63.9, 51.0, 49.9, 46.1,
40.2, 29.3, 26.7, 22.5; IR (KBr) 3434, 3302, 3175, 3066, 2934, 2870,
1609, 1581, 1479, 1449, 1303, 910, 752 cm1; HRMS (ESI) calcd. for
[C26H29N3OS + H]+ 432.2104, found 432.2097.
9-Deoxy-9-methylidene-cinchonine (CN-7). In a resealable
tube epoxide CN-2 (434 mg, 1.42 mmol) and ammonium thiocyanate
(470 mg, 6.17 mmol, 4.3 equiv) were suspended in methanol (6 mL).
The mixture was heated at 5060 C for 144 h. Then the mixture was
cooled to room temperature, NaOH (10 mL, 10% aqueous) was
added, and the mixture was extracted with CH2Cl2 and evaporated.
Chromatography on silica gel (CHCl3/MeOH 20:1) gave 109 mg
(26%) of CN-7 as solidifying oil: mp 8487 C; 1H NMR (CDCl3,
300 MHz) 8.84 (d, J = 4.4 Hz, 1H), 8.10 (d, J = 8.3 Hz, 1H), 8.04
(d, J = 8.6 Hz, 1H), 7.69 (ddd, J = 8.3, 7.0, 1.4 Hz, 1H), 7.52 (ddd, J =
8.4, 7.0, 1.3 Hz, 1H), 7.21 (d, J = 4.4 Hz, 1H), 5.84 (ddd, J = 17.1, 9.9,
7.0, 1H), 5.74 (dd, J = 2.0, 1.3 Hz, 1H), 5.30 (dd, J = 2.0, 1.3 Hz, 1H),
5.005.07 (m, 2H), 3.82 (t, J = 9.4 Hz, 1H), 2.743.09 (m, 4H), 2.26
(q, J = 8.0 Hz, 1H), 1.72 (m, 1H), 1.541.65 (m, 3H), 1.37 (m, 1H);
13
C NMR (CDCl3, 75.5 MHz) 149.8, 149.1, 148.7, 145.6, 140.3,
130.0, 129.3, 127.3, 126.5, 125.5, 120.1, 117.2, 114.7, 59.2, 49.0, 48.1,
40.1, 28.2, 26.6, 25.8; IR (KBr) 3064, 2922, 2862, 1637, 1583, 1504,
1018, 909, 830, 763 cm1; HRMS calcd. for [C20H22N2 + H]+
291.1856, found 291.1852.
9S-Azidomethyl-cinchonine (CN-8). Epoxide CN-2 (1.31 g, 4.28
mmol), sodium azide (1.05 g, 16.2 mmol, 3.8 equiv) and ammonium
chloride (0.537 g, 10.0 mmol, 2.34 equiv) were suspended in methanol
(10 mL) in a resealable tube and stirred vigorously for 3 days at 6065
C. The solvent was removed in vacuo, and the residue was treated
with aqueous NaOH (2%, 5 mL), extracted with CHCl3 and dried
over Na2SO4. After evaporation 1.49 g (99%) of CN-8 was obtained as
slowly crystallizing oil: 1H NMR (CDCl3, 300 MHz) 8.86 (d, J = 4.7
Hz, 1H), 8.59 (br. 1H), 8.15 (dd, J = 8.4, 1.3 Hz, 1H), 7.69 (ddd, J =
8.3, 6.9, 1.3 Hz, 1H), 7.53 (ddd, J = 8.6, 6.9, 1.5 Hz, 1H), 7.50 (br.
1H), 5.92 (ddd, J = 17.3, 10.4, 7.0 Hz, 1H), 5.07 (dt, J = 10.4, 1.4 Hz,
1H), 5.03 (dt, J = 17.2, 1.5 Hz, 1H), 4.07 (d, J = 12.6 Hz, 1H), 3.88 (d,
J = 12.6 Hz, 1H), 3.40 (t, J = 9.8 Hz, 1H), 2.652.95 (m, 4H), 2.21 (q,
J = 8.3 Hz, 1H), 2.00 (br. 1H), 1.84 (m, 1H), 1.501.59 (m, 2H), 1.43
(br. 1H); 13C NMR (CDCl3, 75.5 MHz) 149.6, 149.5 (br.), 149.4,
140.0, 130.9, 128.6, 126.5, 126.1, 125.8 (br.), 119,9, 114.8, 80.2, 62.6,
57.6, 50.8, 49.9, 39.5, 28.7, 26.0, 22.3; IR (KBr) 3196, 3076, 2939,
2872, 2103, 1582, 1510, 1454, 1302, 1284, 911, 763, 733 cm1; HRMS
(ESI) calcd. for [C20H23N5O + H]+ 350.1975, found 350.1971.
9R-Azidomethyl-cinchonidine (CD-8). Compound was prepared
analogously to CN-8. Starting from CD-2 (324 mg, 1.06 mmol) after
chromatography on silica gel (CHCl3/MeOH 20:1) 278 mg of CD-8
(80%) was obtained: 1H NMR (CDCl3, 300 MHz) 8.87 (d, J = 4.7
Hz, 1H), 8.44 (br. 1H), 8.16 (dd, J = 8.5, 1.2 Hz, 1H), 7.68 (ddd, J =
8.3, 6.8, 1.3 Hz, 1H), 7.57 (br. 1H), 7.53 (ddd, J = 8.3, 6.8, 1.5 Hz,
1H), 5.79 (ddd, J = 17.2, 10.3, 7.2 Hz, 1H), 4.99 (dt, J = 17.2, 1.4 Hz,
1H), 5.97 (d, J = 10.3 Hz, 1H), 4.2 (br. 1H), 4.11 (d, J = 12.4 Hz,
1H), 3.81 (d, J = 12.4 Hz, 1H), 3.55 (t, J = 9.3 Hz, 1H), 3.16 (m, 1H),
2,98 (m, 1H), 2.482.72 (m, 2H), 2.26 (m, 1H), 1.91 (m, 1H), 1.75
1.88 (m, 2H), 1.69 (m, 1H), 1.49 (m, 1H); 13C NMR (CDCl3, 75.5
MHz) 149.7, 149.6 (br.), 149.2, 141.8, 131.0, 128.6, 126.4, 126.1,
125.2 (br.), 119.9, 114.5, 80.1, 62.2, 57.8, 57.6, 43.4, 39.6, 28.3, 27.5,
23.2; IR (neat) 3393, 2935, 2864, 2102, 1582, 1509, 1284, 912, 761
cm1; HRMS (ESI) calcd. for [C20H23N5O + H]+ 350.1975, found
350.1961.
9S-(4-Phenyl-1,2,3-triazol-1-yl-methyl)-cinchonine (CN-9).
Azide CN-8 (74.2 mg, 0.214 mmol) and phenylacetylene (45 L,
0.41 mmol, 1.9 equiv) were dissolved in THF (2 mL), and a solution
of sodium ascorbate (45 mg, 0.23 mmol, 106 mol %) in water (0.6
mL) was added, followed by aqueous CuSO4 (18 L, 0.78 M, 0.014
mmol, 6 mol %). The mixture was stirred for 24 h at room
temperature. Pyridine (0.07 mL) was added, and the mixture was
ltered through a pad of silica gel, washed with CHCl3/MeOH 5:1,
and evaporated. Purication on silica gel (CHCl3/MeOH 20:1)
aorded 82.6 mg of CN-9 (86%) as amorphous solid: 1H NMR
(DMSO-d6, 300 MHz, 343 K) 8.75 (br., 1H), 8.73 (d, J = 4.7 Hz,
1H), 8.03 (s, 1H), 8.01 (dd, J = 8.5, 1.4 Hz, 1H), 7.617.70 (m, 4H),
7.56 (ddd, J = 8.7, 6.8, 1.5 Hz, 1H), 7.347.41 (m, 2H), 7.28 (m, 1H),
5.90 (m, 1H), 5.74 (br., 1H), 5.17 (d, J = 14.4 Hz, 1H), 5.03 (d, J =
14.4 Hz, 1H), 4.834.94 (m, 2H), 3.72 (m, 1H), 2.84 (m, 1H), 2.68
(m, 1H), 2.322.48 (m, 2H), 2.17 (m, 1H), 2.06 (m, 1H), 1.73 (m,
G
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
The Journal of Organic Chemistry
Article
1H), 1.62 (m, 1H), 1.381.51 (m, 2H); 1H NMR (CDCl3, 600 MHz,
273K, mixture of conformers 1:1) 9.06 (d, J = 8.8 Hz, 1H), 8.68 (d,
J = 4.7 Hz, 1H), 8.67 (d, J = 4.7 Hz, 1H), 8.58 (br. m, 1H), 8.25 (d, J =
8.6 Hz, 1H), 8.15 (d, J = 8.3 Hz, 1H), 8.06 (br., 1H), 8.04 (d, J = 8.3
Hz, 1), 7.72 (t, J = 7.4 Hz, 1H), 7.70 (d, J = 4.7 Hz, 1H), 7.627.68
(m, 4H), 7.50 (t, J = 7.7 Hz, 1H), 7.45 (d, J = 7.5 Hz, 2H), 7.35 (d, J =
4.7 Hz, 1H), 7.267.32 (m, 4H), 7.217.24 (m, 1H), 6.07 (ddd, J =
17.6, 10.0, 7.6 Hz, 1H), 6.0 (br, 1H), 5.85 (ddd, J = 17.3, 10.5, 6.6
Hz, 1H),), 5.10 (d, J = 10.5 Hz, 1H), 5.005.07 (m, 3H), 4.98 (d, J =
14.2 Hz, 1H), 3.77 (t, J = 9.0 Hz, 1H), 3.34 (t, J = 9.9 Hz, 1H), 2.99
3.09 (m, 3H), 2.87 (m, 1H), 2.642.74 (m, 2H), 2.522.62 (m, 2H),
2.452.50 (m, 1H), 2.27 (q, J = 8.0 Hz, 1H), 2.15 (q, J = 8.1 Hz, 1H),
1.781.90 (m, 3H), 1.471.66 (m, 5H), 1.30 (m, 1H); 13C NMR
(DMSO-d6, 75.5 MHz, 333 K) 149.1, 148.9, 148.2, 145.2, 140.7,
130.4, 129.8, 128.3, 127.5, 127.2, 126 (br.), 125.9, 125.1, 124.7,
121.9, 119.7, 113.5, 80.3, 60.5, 57.0, 49.3, 48.4, 39.2, 28.2, 25.7, 20.7;
IR (KBr) 3408, 2934, 2871, 1581, 1510, 1456, 1232, 1201, 1047, 764,
695 cm1; HRMS calcd. for [C28H29N5O + H]+ 452.2445, found
452.2434.
9S-Aminomethyl-cinchonine (CN-10). Azide CN-8 (1.42 g, 4.07
mmol) was dissolved in THF (15 mL) and cooled to 0 C. LiAlH4
(159 mg, 4.18 mmol, 1.03 equiv) was added, the mixture was stirred
for 15 min at 0 C followed by another portion of LiAlH4 (15.4 mg,
0.40 mmol, 0.10 equiv), and the mixture was stirred for additional 10
min. The reaction was quenched by addition of brine, extracted with
CH2Cl2, dried over Na2SO4, and evaporated. The crude mixture was
used without further purication. A sample was puried on silica gel
with CHCl3/MeOH (5:1) followed by MeOH: 1H NMR (CDCl3, 300
MHz, 313 K) 8.85 (d, J = 4.6 Hz, 1H), 8.39 (br., 1H), 8.14 (d, J =
8.3 Hz, 1H), 7.78 (br., 1H), 7.64 (m, 1H), 7.48 (m, 1H), 6.06 (m,
1H), 4.975.05 (m, 2H), 3.61 (d, J = 12.4 Hz, 1H), 3.55 (m, 1H),
3.13 (m, 1H), 3.00 (d, J = 12.4 Hz, 1H), 1.972.90 (m, 7H), 1.82 (m,
1H), 1.401.67 (m, 3H); HRMS (ESI) calcd. for [C20H25N3O + H]+
324.2072, found 324.2082.
O-tert-Butyldimethylsilyl-9S-azidomethyl-cinchonine (CN11). Azide CN-8 (544 mg, 1.56 mmol) was dissolved in dichloromethane (9 mL), and triethylamine (0.25 mL, 1.79 mmol, 1.15 equiv)
was added. The mixture was cooled to 0 C, a rst portion of TBDMS
triate (0.50 mL) was added, and then the mixture was allowed to
attain room temperature, and after 2.5 h another portion of TBDMS
was added (0.25 mL; total 3.27 mmol, 2.1 equiv). The mixture was
stirred at room temperature for 18 h, and ltered through a pad of
silica gel with CHCl3/MeOH 10:1. After evaporation, the residue
(1.40 g) was triturated with 10% aqueous NaOH, extracted with
CH2Cl2, dried over Na2SO4, evaporated, and washed with 0.3 mL of
hexane to give 656 mg (90%) of product as o-white crystalline solid:
1
H NMR (CDCl3, 300 MHz) 8.87 (d, J = 4.8 Hz, 1H), 8.72 (d, J =
8.8 Hz, 1H), 8.12 (dd, J = 8.4, 1.1 Hz, 1H), 7.66 (ddd, J = 8.1, 6.8, 1.1
Hz, 1H), 7.58 (br., 1H), 7.50 (ddd, J = 8.3, 6.8, 1.2 Hz, 1H), 5.31
(ddd, J = 17.0, 10.4, 6.2 Hz, 1H), 4.72 (d, J = 13.4 Hz, 1H), 4.68 (d, J
= 10.5 Hz, 1H), 4.44 (d, J = 17.1 Hz, 1H), 4.06 (d, J = 13.4 Hz, 1H),
3.21 (t, J = 9.5 Hz, 1H), 2.96 (m, 1H), 2.68 (dt, J = 13.1, 8.8 Hz, 1H),
2.42 (m, 1H), 1.882.00 (m, 2H), 1.521.76 (m, 3H), 1.391.52 (m,
2H), 0.99 (s, 9H), 0.28 (s, 3H), 0.03 (s, 3H); 13C NMR (CDCl3, 75.5
MHz) 149.3, 149.2, 147.6, 139.4, 130.6, 128.7, 127.6, 127.4, 125.9,
122.1, 114.4, 84.0, 64.5, 58.9, 51.5, 49.6, 39.5, 28.9, 26.5, 26.0, 22.5,
19.4, 1.8, 2.2; IR (KBr) 3065, 2955, 2860, 2108, 1512, 1472, 1259,
1160, 1030, 838, 779, 638 cm 1 ; HRMS (ESI) calcd. for
[C26H37N5OSi + H]+ 464.2840, found 464.2837.
O-tert-Butyldimethylsilyl-9S-aminomethyl-cinchonine (CN12). Azide CN-11 (80.6 mg, 0.173 mmol) was dissolved in THF (4
mL), and triphenylphosphine (69.9 mg, 0.267 mmmol, 1.54 equiv)
was added. The mixture was stirred at 45 C for 18 h and then allowed
to attain room temperature. Water (0.5 mL) was added, and the
mixture was stirred for additional 24 h, and extracted with CHCl3,
dried over Na2SO4, and evaporated. Chromatography on silica gel
(CHCl3/MeOH 10:1) gave 46 mg (61%) of CN-12 as colorless oil: Rf
0.38 (CHCl3/MeOH, 10:1); 1H NMR (CDCl3, 300 MHz) 8.86 (d, J
= 4.6 Hz, 1H), 8.79 (d, J = 8.7 Hz, 1H), 8.10 (dd, J = 8.3, 1.2 Hz, 1H),
7.64 (ddd, J = 8.3, 6.8, 1.3 Hz, 1H), 7.58 (br., 1H), 7.48 (ddd, J = 8.7,
6.8, 1.5 Hz, 1H), 5.44 (ddd, J = 17.2, 10.5, 7.1 Hz, 1H), 4.75 (d, J =
10.5 Hz, 1H), 4.55 (d, J = 17.2 Hz, 1H), 4.04 (br. m, 1H), 3.153.29
(m, 2H), 2.94 (m, 1H), 2.66 (ddd, J = 13.2, 8.8, 8.6 Hz, 1H), 2.48 (m,
1H), 2.13 (m, 1H), 1.96 (q, J = 7.9 Hz, 1H), 1.64 (m, 1H), 1.111.62
(m, 6H), 0.95 (s, 9H), 0.32 (s, 3H), 0.17 (s, 3H); 13C NMR (CDCl3,
75.5 MHz) 149.5, 149.4, 149.3, 139.8, 132.1, 130.6, 128.6, 127.2,
126.0, 122.2, 114.2, 87.1, 64.3, 51.5, 49.9, 49.2, 39.5, 28.9, 26.7, 26.0,
22.9, 19.6, 1.9, 2.1; IR (neat) 3394, 3299, 2930, 2857, 2576, 1508,
1472, 1249, 1120, 1093, 1055, 982, 834, 776 cm1; HRMS (ESI) calcd.
for [C26H39N3OSi + H]+ 438.2935, found 438.2949.
N-3,5-Bis(triuoromethyl)phenyl-N-(8R,9S-9-hydroxy-cinchonan-9-yl-methyl)-thiourea (CN-13). Crude mixture containing
CN-10 (1.12 g) was dissolved in DCM (20 mL), and 3,5bis(triuoromethyl)phenyl isothiocyanate (934 mg, 3.44 mmol) was
added. The mixture was stirred for 18 h, concentrated to about 10 mL,
and subjected to chromatography on silica gel (CHCl3/MeOH 10:1 to
5:1). Obtained was 800 mg (41% over 2 steps) of CN-13. Additionally
30 mg (3%) of CN-14 was isolated. CN-13: mp 120128 C; 1H
NMR (CDCl3, 300 MHz, 313 K) 9.5 (br., 1H), 8.5 (br. 1H),
8.25 (br. 1H), 7.87 (s, 2H), 7.527.76 (br. m, 3H), 7.55 (s, 1H),
7.407.49 (m, 2H), 6.00 (ddd, J = 17.1, 10.5, 7.0 Hz 1H), 5.10 (d, J =
10.5 Hz, 1H), 5.07 (d, J = 17.1 Hz, 1H), 4.74 (br. 1H), 4.39 (br. d, J =
13 Hz, 1H), 3.48 (br., 1H), 3.03 (dd, J = 13.0, 7.9 Hz, 1H), 2.55
3.81 (br. m, 3H), 2.152.29 (m, 2H), 1.89 (m, 1H), 1.471.66 (m,
3H); 13C NMR (CDCl3, 75.5 MHz, 313 K) 183.6, 151 (br.), 149.0,
148.3 (br.), 140.4, 139.6, 132.2 (q JCF = 34 Hz), 129.3, 129.2, 126.7,
126 (br.), 123.7, 123.1 (q JCF = 273 Hz), 118.6 (sept J = 4 Hz),
115.1, 81.5, 62.8, 51.7, 50.9, 49.8, 39.7, 28.9, 26.3, 22.4 (2 sp2 C
missing due to coalescence and overlaps); IR (KBr) 3366, 2940, 1576,
1513, 1471, 1381, 1279, 1177, 1133, 759 cm1; HRMS (ESI) calcd. for
[C29H28F6N4OS + H]+ 595.1961, found 595.1946.
5S-5-(Quinolin-4-yl)-5-((1S,2R,5R)-5-vinyl-quinuclidin-2-yl)oxazolidine-2-thione (CN-14). Crude mixture containing CN-10
(28 mg) was dissolved in THF (1.5 mL). Triethylamine (0.11 mL,
0.79 mmol) and carbon disulde (0.06 mL, 0.99 mmol) were added,
and the mixture was stirred at 5060 C for 20 h. The mixture was
briey evaporated and puried on silica gel (CHCl3/MeOH, 10:1)
giving 18.6 mg (58% over 2 steps) of white crystalline solid After
chromatography the sample became insoluble in CHCl3: mp 273275
C (dec); 1H NMR (DMSO-d6/H2O, 600 MHz) 10.26 (br, 1H),
8.88 (d, J = 4.6 Hz, 1H), 8.08 (dd, J = 8.4, 0.9 Hz, 1H), 7.96 (br. d, J =
8.4 Hz, 1H), 7.77 (ddd, J = 6.7, 8.1, 0.9 Hz, 1H), 7.597.62 (m,
2H), 6.19 (ddd, J = 17.0, 10.4, 8.2 Hz, 1H), 5.06 (dd, J = 17.0, 1.9 Hz,
1H), 4.99 (dd, J = 10.4, 1.9 Hz, 1H), 4.35 (d, J =10.8 Hz, 1H), 3.93 (d,
J =10.8 Hz, 1H), 3.83 (t, J = 8.2 Hz, 1H), 2.89 (dd, J = 12.9, 7.4 Hz,
1H), 2.45 (t, J = 12.0 Hz, 1H), 2.232.31 (m, 2H), 2.09 (q, J = 8.6 Hz,
1H), 1.83 (m, 1H), 1.721.78 (m, 2H), 1.56 (q, J = 10.6 Hz, 1H),
1.41 (m, 1H); 13C NMR (DMSO-d6/H2O, 151 MHz) 186.1, 150.0,
148.8, 148.4, 141.0, 130.5, 129.0, 126.7, 123.5, 124.2, 117.2, 115.0,
93.9, 62.0, 53.2, 49.6, 48.4, 40.1, 28.7, 25.8, 20.4; IR (KBr) 2942, 2872,
1547, 1511, 1317, 1277, 1173, 1132, 912, 776, 759 cm1; HRMS (ESI)
calcd. for [C21H23N3OS + H]+ 366.1636, found 366.1651.
N-((8R,9S)-9-(tert-Butyldimethylsilyloxy)-cinchonan-9-ylmethyl)-N-3,5-bis(triuoromethyl)phenyl-thiourea (CN-15).
Amine CN-12 (44.0 mg, 0.10 mmol) was dissolved in CH2Cl2 (4
mL), and 3,5-bis(triuoromethyl)phenyl isothiocyanate (19 L, 0.10
mmol, 1.0 equiv) was added. The mixture was stirred for 28 h and
then chromatographed on silica gel (CHCl3/MeOH 20:1) to give 56.5
mg (80%) of product as solidifying oil: 1H NMR (CDCl3, 300 MHz,
318 K) 8.79 (d, J = 8.8 Hz, 1H), 8.72 (br., 1H), 8.62 (br., 1H),
7.90 (br., 1H), 7.70 (s, 2H), 7.69 (m, 1H), 7.5 (br. 1H), 7.57 (s,
1H), 7.50 (m, 1H), 7.47 (br. 1H), 5.36 (m, 1H), 4.724.83 (m, 2H),
4.59 (d, J = 17.2 Hz, 1H), 4.33 (d, J = 14.4 Hz, 1H), 3.45 (t, J = 9.6
Hz, 1H), 2.94 (t, J = 11.0 Hz, 1H), 2.312.59 (m, 2H), 1.892.07 (m,
2H), 1.63 (m, 1H), 1.381.59 (m, 3H), 1.06 (m, 1H), 0.86 (s, 9H),
0.34 (s, 3H), 0.02 (s, 3H); 13C NMR (CDCl3, 75.5 MHz, 318 K)
181.6, 149.1, 148.1 (br.), 140.0, 139.2, 132.9 (q, J = 32 Hz), 129.7,
128.7 (br.), 127.7, 127.2, 126.4, 123.3, 123.0 (q, J = 273 Hz), 121.0
(br.), 118.7, 114.6 (br.), 83.1 (br.), 65.3, 55.0 (br.), 51.5, 49.6, 39.1,
29.0, 26.5, 25.9, 23.4, 19.3, 1.9, 2.5 (1 sp2 C missing due to
H
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
The Journal of Organic Chemistry
coalescence and overlaps); IR (KBr) 3384, 3337, 2933, 2860, 1514,
1472, 1385, 1279, 1180, 1136, 838, 778 cm1; HRMS (ESI) calcd. for
[C35H42N4OF6SSi + H]+ 709.2826, found 709.2827.
9S-(N-Acetylaminomethyl)-cinchonine (CN-16). Crude mixture containing CN-10 (88.5 mg) was dissolved in dichloromethane (3
mL), and acetic anhydride (0.040 mL, 0.42 mmol) was added. The
mixture was stirred at room temperature for 24 h, and saturated
aqueous NaHCO3 was added, extracted with dichloromethane, and
dried over Na2SO4. The mixture was applied to silica gel column with
CHCl3/MeOH 10:1 and then eluted from with methanol providing 40
mg (38% over 2 steps) of white amorphous solid: 1H NMR (CDCl3,
300 MHz, 313 K) 8.73 (d, J = 4.6 Hz, 1H), 8.56 (br., 1H), 8.04 (d,
J = 8.5 Hz, 1H), 7.61 (m, 1H), 7.46 (ddd, J = 8.6, 6.9, 1.4 Hz, 1H),
7.64 (br., 1H), 6.08 (br., 1H), 5.94 (ddd, J = 17.5, 10.4, 7.6 Hz, 1H),
5.04 (dt, J = 10.4, 1.2 Hz, 1H), 5.00 (dt, J = 17.4, 1.5 Hz, 1H), 7.57
(br., 1H), 4.19 (br., 1H), 3.85 (br. 1H), 3.48 (t, J = 9.2 Hz, 1H), 2.94
(m, 1H), 2.592.89 (m, 3H), 2.042.25 (m, 2H), 1.83 (m, 1H), 1.73
(s, 3H), 1.351.64 (m, 3H); 13C NMR (CDCl3, 75.5 MHz, 313 K)
171.9, 150.0, 149.7, 139.9, 131.2, 128.6, 126.2, 126 (br.), 120.6,
115.1, 81.1, 63.1, 51.1, 50.1, 47.5, 39.8, 29.0, 26.3, 22.9, 22.2 (2 sp2 C
missing due to coalescence and overlaps); IR (KBr) 3261, 3069, 2934,
2670, 1637, 1570, 1510, 1376, 1302, 1116, 761; HRMS (ESI) calcd.
for [C22H27N3O2 + H]+ 366.2176, found 366.2192.
9S-(N-Benzyl-aminomethyl)-cinchonine (CN-17). Epoxide
CN-2 (219 mg, 0.72 mmol) and benzylamine (0.25 mL, 2.29 mmol,
3.2 equiv) were placed in a sealable tube and suspended in acetonitrile
(1.5 mL), and a solution of LiClO4 (0.15 mL, 4 M in Et2O, 0.60 mmol,
0.83 equiv) was added. The tube was sealed and stirred for 5 days at
105 C. Then the mixture was diluted with CH2Cl2, washed with 10%
NaOH, dried over Na2SO4, and evaporated. Residual benzylamine was
removed in a vacuum desiccator over H2SO4 to give 262 mg (88%) of
yellow amorphous solid: 1H NMR (CDCl3, 300 MHz, 323 K) 8.83
(d, J = 4.7 Hz, 1H), 8.33 (br., 1H), 8.14 (dd, J = 8.5, 1.5 Hz, 1H), 7.81
(br., 1H), 7.63 (ddd, J = 8.5, 6.8, 1.5 Hz, 1H), 7.45 (ddd, J = 8.7, 6.8,
1.5 Hz, 1H), 7.187.30 (m, 3H), 7.027.07 (m, 2H), 6.08 (ddd, J =
17.8, 9.5, 7.6, 1H), 4.975.04 (m, 2H), 3.453.64 (m, 4H), 3.12 (ddd,
J = 13.8, 7.7, 2.3 Hz, 1H), 2.92 (d, J = 12.0 Hz, 1H), 2.432.67 (m,
3H), 2.34 (t, J = 10.6 Hz, 1H), 2.10 (q, J = 8.5 Hz, 1H), 1.80 (m, 1H),
1.371.64 (m, 3H); 13C NMR (CDCl3, 75.5 MHz, 318 K) 152.7,
150.2, 149.4, 141.6, 139.6, 131.3, 128.6, 128.2, 128.0, 127.9 (br.),
127.3, 125.5, 125.4 (br.), 120.3, 114.0, 79.3, 64.5, 55.1, 54.0, 50.5, 49.7,
40.7, 29.4, 27.2, 22.2; IR (KBr) 3259, 2932, 2868, 1580, 1509, 1457,
1300, 1110, 909, 760, 699 cm1; HRMS (ESI) calcd. for [C27H31N3O
+ H]+ 414.2540, found 414.2548.
9S-(N-Acetyl-N-benzyl-aminomethyl)-cinchonine (CN-18).
Amine CN-17 (76.7 mg, 0.18 mmol) was dissolved in CH2Cl2, then
triethylamine (0.15 mL, 1.1 mmol, 6 equiv) and acetic anhydride (70
L, 0.74 mmol, 4.1 equiv) were added, and the mixture was stirred at
room temperature for 20 h. Saturated aqueous NaHCO3 was added,
and the mixture was extracted with CH2Cl2, dried over Na2SO4, and
ltered through silica gel (CHCl3/MeOH, 10:1) to give 85 mg (99%)
of CN-18 as brown oil: 1H NMR (CDCl3, 600 MHz) 8.90 (d, J = 4.7
Hz, 1H), 8.19 (dd, J = 8.4, 0.8 Hz, 1H), 8.15 (d, J = 4.7 Hz, 1H), 7.95
(d, J = 8.6 Hz, 1H), 7.67 (dd, J = 8.4, 6.9 Hz, 1H), 7.43 (ddd, J = 8.6,
6.9, 0.8 Hz, 1H), 7.187.22 (m, 3H), 6.546.56 (m, 2H), 6.31 (br,
0.7 H), 6.16 (ddd, J = 16.9, 10.5, 8.2 Hz, 1H), 5.00 (d, J = 16.9 Hz,
1H), 4.99 (d, J = 10.5 Hz, 1H), 4.45 (d, J = 14.6 Hz, 1H), 3.98 (d, J =
16.9 Hz, 1H), 3.65 (t, J = 8.9 Hz, 1H), 3.50 (d, J = 14.7 Hz, 1H), 3.17
(ddd, J = 13.6, 7.5, 1.9 Hz, 1H), 2.92 (d, J = 16.9 Hz, 1H), 2.56 (m,
1H), 2.52 (m, 1H), 2.5 (br. 0.3H), 2.362.43 (m, 2H), 2.06 (q, J =
8.2 Hz, 1H), 1.99 (s, 3H), 1.80 (m, 1H), 1.501.55 (m, 2H), 1.45 (m,
1H); 13C NMR (CDCl3, 151 MHz) 175.5, 151.5, 150.8, 148.6,
141.4, 135.1, 131.6, 128.9, 128.2, 127.9, 126.4, 126.14, 126.05, 124.2,
121.3, 114.1, 82.8, 61.7, 54.3, 53.4, 50.2, 48.9, 40.6, 29.0, 26.9, 22.4,
21.5; IR (neat) 3271, 2935, 2871, 1622, 1454, 1419, 1363, 1112, 911,
762, 734, 699 cm1; HRMS (ESI) calcd. for [C29H33N3O2 + H]+
456.2646, found 456.2666.
Article
ASSOCIATED CONTENT
S Supporting Information
*
Supporting tables and gures. Additional spectral and computational details including tables of atom coordinates and absolute
energies. This material is available free of charge via the
Internet at http://pubs.acs.org.
AUTHOR INFORMATION
Corresponding Author
*E-mail: przemyslaw.boratynski@pwr.wroc.pl.
Notes
The authors declare no competing nancial interest.
ACKNOWLEDGMENTS
The work was nanced by a statutory activity subsidy from the
Polish Ministry of Science and Higher Education for the
Faculty of Chemistry of Wrocaw University of Technology.
We thank Wrocaw Networking and Supercomputing Center
for allotment of computer time and Mr. ukasz Toma for
synthesis of DHCN-1.
REFERENCES
(1) (a) Cinchona Alkaloids in Synthesis & Catalysis; Song, C. E., Ed.;
Wiley-VCH: Weinheim, 2009. (b) Wu, Y.; Deng, L. J. Am. Chem. Soc.
2012, 134, 1433414337. (c) Melchiorre, P. Angew. Chem., Int. Ed.
2012, 51, 97489770. (d) Marcelli, T.; Hiemstra, H. Synthesis 2010,
12291279. (e) Vakulya, B.; Varga, S.; Csampai, A.; Soos, T. Org. Lett.
2006, 7, 19671969. (f) Hayashi, M.; Shiomi, N.; Funahashi, Y.;
Nakamura, S. J. Am. Chem. Soc. 2012, 134, 1936619369. (g) Molleti,
N.; Rana, N. K.; Singh, V. K. Org. Lett. 2012, 14, 43224325.
(2) Prakash, G. K. S.; Wang, F.; Ni, C.; Shen, J.; Haiges, R.; Yudin, A.
K.; Mathew, T.; Olah, G. A. J. Am. Chem. Soc. 2011, 133, 99929995.
(3) (a) Woodward, R. B.; Wendler, N. L.; Brutschy, F. J. J. Am. Chem.
Soc. 1945, 67, 14251429. (b) Boratynski, P. J.; Turowska-Tyrk, I.;
Skarzewski, J. Tetrahedron: Asymmetry 2012, 23, 876883.
(4) (a) Franz, H. M.; Roper, S.; Wartchow, R.; Hoffmann, H. M. R. J.
Org. Chem. 2004, 69, 29832991. (b) Braje, W. M.; Wartchow, R.;
Hoffmann, H. M. R. Angew. Chem., Int. Ed. 1999, 38, 25392543.
(5) (a) Aggarwal, V. K.; Alonso, E.; Fang, G.; Ferrara, M.; Hynd, G.;
Porcelloni, M. Angew. Chem., Int. Ed. 2001, 40, 14331436.
(b) Aggarwal, V. K.; Charmant, J. P. H.; Fuentes, D.; Harvey, J. N.;
Hynd, G.; Ohara, D.; Picoul, W.; Robiette, R. I.; Smith, C.; Vasse, J.-L.;
Winn, C. L. J. Am. Chem. Soc. 2006, 128, 21052114. (c) Aggarwal, V.
K; Hebach, C. Org. Biomol. Chem. 2005, 3, 14191427. (d) Okazaki,
R.; Tokitoh, N. Dimethylsulfonium Methylide. In e-EROS Encyclopedia
of Reagents for Organic Synthesis; Wiley: New York, 2009.
(6) (a) Aggarwal, V. K.; Richardson, J. Chem. Commun. 2003, 2644
2651. (b) Aggarwal, V. K.; Winn, C. L. Acc. Chem. Res. 2004, 37, 611
620. (c) Illa, O.; Arshad, M.; Ros, A.; McGarrigle, E. M.; Aggarwal, V.
K. J. Am. Chem. Soc. 2010, 132, 18281830.
(7) (a) Sone, T.; Yamaguchi, A.; Matsunaga, S.; Shibasaki, M. J. Am.
Chem. Soc. 2008, 130, 1007810079. (b) Sone, T.; Yamaguchi, A.;
Matsunaga, S.; Shibasaki, M. Molecules 2012, 17, 16171634.
(8) (a) Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1965, 87,
13531364. (b) Szostak, M.; Abue, J. J. Am. Chem. Soc. 2009, 131,
1324613247. (c) Rodeschini, V.; Boiteau, J.-G.; Van de Weghe, P.;
Tarnus, C.; Eustache, J. J. Org. Chem. 2004, 69, 357373.
(9) (a) For review, see: Li, A.-H.; Dai, L.-X.; Aggarwal, V. K. Chem.
Rev. 1997, 97, 23412372. (b) Konosu, T.; Miyaoka, T.; Tajima, Y.;
Oida, S. Chem. Pharm. Bull. 1991, 39, 22412246. (c) Gala, D.;
DiBenedetto, D. J.; Clark, J. E.; Murphy, B. L.; Schumaher, D. P;
Steinman, M. Tetrahedron Lett. 1996, 37, 611614. (d) Pesti, J.; Chen,
C.-K.; Spangler, L.; DelMonte, A. J.; Benoit, S.; Berglund, D.; Bien, J.;
Brodfuehrer, P.; Chan, Y.; Corbett, E.; Costello, C.; DeMena, P.;
Discordia, R. P.; Doubleday, W.; Gao, Z.; Gingras, S.; Grosso, J.; Haas,
O.; Kacsur, D.; Lai, C.; Leung, S.; Miller, M.; Muslehiddinoglu, J.;
I
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
The Journal of Organic Chemistry
Article
Nguyen, N.; Qiu, J.; Olzog, M.; Reiff, E.; Thorval, D.; Totleben, M.;
Vanyo, D.; Vemishetti, P.; Wasylak, J.; Wei, C. Org. Process Res. Dev.
2009, 13, 716728.
(10) Lee, J. H.; Deng, L. J. Am. Chem. Soc. 2012, 134, 1820918212.
(11) Lewinski, J.; Kaczorowski, T.; Prochowicz, D.; Lipinska, T.;
Justyniak, I.; Kaszkur, Z.; Lipkowski, J. Angew. Chem., Int. Ed. 2010, 49,
70357039.
(12) Hutchison, D. R.; Khau, V. V.; Martinelli, M. J.; Nayyar, N. K.;
Peterson, B. C.; Sullivan, K. A. Org. Synth. 1998, 75, 223234.
(13) When sodium hydride was used as a base, the results were not
reproducible between dierent batches of the hydride giving from 0%
(complete starting material recovery) to 85% yield.
(14) Ashton, W. T.; Cantone, C. L.; Meurer, L. C.; Tolman, R. L.;
Greenlee, W. J.; Patchett, A. A.; Lynch, R. J.; Schorn, T. W.; Strouse, J.
F.; Siegl, P. K. S. J. Med. Chem. 1992, 35, 21032112.
(15) Rabe, P.; Naumann, W.; Kuliga, E. Liebigs Ann. Chem. 1909,
364, 330352.
(16) Gutzwiller, J.; Uskokovic, M. R. Helv. Chim. Acta 1973, 56,
14941503.
(17) (a) Cieplak, A. S. Chem. Rev. 1999, 99, 12651336. (b) Galeotti,
N.; Poncet, J.; Chiche, L.; Jouin, P. J. Org. Chem. 1993, 58, 5370
5376.
(18) Roy, A.; Venkateswaran, R. V. Synth. Commun. 2012, 42, 621
626.
(19) Johnson, C. R.; Schroeck, C. W.; Shanklin, J. R. J. Am. Chem.
Soc. 1973, 95, 74247431.
(20) ESI-MS of the reaction mixture revealed, apart from dominant
alkene CN-7, only traces of HSCN addition products (<1% relative
abundance), thiirane (7%), and disulde (<1%); see Figure S21,
Supporting Information.
(21) Kleiner, C. M.; Horst, L.; Wurtele, C.; Wende, R.; Schreiner, P.
R. Org. Biomol. Chem. 2009, 7, 13971403.
(22) Sander, M. Chem. Rev. 1966, 66, 297339.
(23) Hydrogenation of CN-8 on Pd/C required 10 mol % catalyst
loading to give 10,11-dihydroaminoalcohol DHCN-10.
(24) In an up-scale procedure starting from 30 g of CN-1, some of
CN-2 precipitated during extraction; it was then collected by ltration
and washed thoroughly with water.
dx.doi.org/10.1021/jo400465a | J. Org. Chem. XXXX, XXX, XXXXXX
Das könnte Ihnen auch gefallen
- MCAT Review OChem Notes (Full)Dokument74 SeitenMCAT Review OChem Notes (Full)Chris_Barber09Noch keine Bewertungen
- Isomer in Organic ChemistryDokument111 SeitenIsomer in Organic ChemistryyenquynhNoch keine Bewertungen
- Solution Manual Elementary Problems in Organic Chemistry For JEE by M.S. Chouhan (9th Edition)Dokument285 SeitenSolution Manual Elementary Problems in Organic Chemistry For JEE by M.S. Chouhan (9th Edition)Satish Patel100% (18)
- MULTIPLE CHOICE QUESTIONS Part 5: Stereochemistry: Topic: Identifications and ComparisonsDokument13 SeitenMULTIPLE CHOICE QUESTIONS Part 5: Stereochemistry: Topic: Identifications and ComparisonsErica Chang100% (2)
- ch11 PDFDokument96 Seitench11 PDFMau BaraquelNoch keine Bewertungen
- CHEM 210 CH05 Stereochemistry IIDokument6 SeitenCHEM 210 CH05 Stereochemistry IIHelm weuttNoch keine Bewertungen
- 4811Dokument7 Seiten4811zosuaNoch keine Bewertungen
- PD CatDokument7 SeitenPD CatKiss LeviNoch keine Bewertungen
- Tetrahedron 2010 66 10 01902 - 01910 CiclicoDokument9 SeitenTetrahedron 2010 66 10 01902 - 01910 Ciclicoteodoro11Noch keine Bewertungen
- Copper (II) Complexes With Lignin Model Compound VanillinDokument4 SeitenCopper (II) Complexes With Lignin Model Compound VanillinCatelia KulmanNoch keine Bewertungen
- Metal Complexes of Schiff Bases Derived From Dicinnamoylmethane and Aromatic AminesDokument9 SeitenMetal Complexes of Schiff Bases Derived From Dicinnamoylmethane and Aromatic AminesHusham HussanNoch keine Bewertungen
- Mechanistic Study of The Synthesis of Cdse Nanocrystals: Release of SeleniumDokument4 SeitenMechanistic Study of The Synthesis of Cdse Nanocrystals: Release of SeleniumvirparaNoch keine Bewertungen
- G 044028042Dokument15 SeitenG 044028042IOSR Journal of PharmacyNoch keine Bewertungen
- A Simple and Straightforward Approach To Quinoxalines by Iron/Sulfur-Catalyzed Redox Condensation of o Nitroanilines and PhenethylaminesDokument4 SeitenA Simple and Straightforward Approach To Quinoxalines by Iron/Sulfur-Catalyzed Redox Condensation of o Nitroanilines and PhenethylaminesClaudia BocanegraNoch keine Bewertungen
- Heterogeneous Asymmetric Diels-Alder Reactions Using A Copper-Chiral Bis (Oxazoline) Complex Immobilized On Mesoporous SilicaDokument5 SeitenHeterogeneous Asymmetric Diels-Alder Reactions Using A Copper-Chiral Bis (Oxazoline) Complex Immobilized On Mesoporous SilicaJC Jane BarnesNoch keine Bewertungen
- riduan2009Dokument4 Seitenriduan2009Himadri SahaNoch keine Bewertungen
- Spino1998 Journal of Organic Chemistry, 1998, Vol. 63, # 15, P. 5283 - 5287Dokument5 SeitenSpino1998 Journal of Organic Chemistry, 1998, Vol. 63, # 15, P. 5283 - 5287SpazzaturaNoch keine Bewertungen
- 1 s2.0 S0031942299002393 MainDokument9 Seiten1 s2.0 S0031942299002393 MainDr-Muhammad Imran TousifNoch keine Bewertungen
- Poly EneDokument3 SeitenPoly EneMohammed TarekNoch keine Bewertungen
- Homo - and Heterodinuclear Head-to-Head or Head-to-Tail Complexes of Rhodium (I) and Iridium (I) With C2, N3 or C8, N9 Bridging Azolato LigandsDokument9 SeitenHomo - and Heterodinuclear Head-to-Head or Head-to-Tail Complexes of Rhodium (I) and Iridium (I) With C2, N3 or C8, N9 Bridging Azolato LigandsTristan TanNoch keine Bewertungen
- Soh Weicong U052333W AbstractDokument4 SeitenSoh Weicong U052333W AbstractSoh WeicongNoch keine Bewertungen
- Rhodium ComplexesDokument5 SeitenRhodium ComplexesTavo RodriguezNoch keine Bewertungen
- Arancibia 2007Dokument5 SeitenArancibia 2007Rodrigo Sebastian Arancibia GonzalezNoch keine Bewertungen
- Chemcomm: CommunicationDokument3 SeitenChemcomm: CommunicationImen TalbiNoch keine Bewertungen
- JonesC EndoEndo24Diphosphabicyclo110Butane Orbital Isomers CC 2001 663-4Dokument2 SeitenJonesC EndoEndo24Diphosphabicyclo110Butane Orbital Isomers CC 2001 663-4jazmurdochNoch keine Bewertungen
- Salen Ni (II) ComplexesDokument8 SeitenSalen Ni (II) ComplexesVanadi Helmy NugrohoNoch keine Bewertungen
- Ocando Mavarez1998 PDFDokument7 SeitenOcando Mavarez1998 PDFMateus PinheiroNoch keine Bewertungen
- Synthesis of Iron (0) Complexes Bearing Protic NHC Ligands: Synthesis and Catalytic ActivityDokument5 SeitenSynthesis of Iron (0) Complexes Bearing Protic NHC Ligands: Synthesis and Catalytic ActivityTristan TanNoch keine Bewertungen
- The First Asymmetric Synthesis of Chiral Ruthenium Tris (Bipyridine) From Racemic Ruthenium Bis (Bipyridine) ComplexesDokument4 SeitenThe First Asymmetric Synthesis of Chiral Ruthenium Tris (Bipyridine) From Racemic Ruthenium Bis (Bipyridine) ComplexesKumer SauravNoch keine Bewertungen
- B 3Dokument2 SeitenB 3bijuarNoch keine Bewertungen
- Trends in NMR Chemical Shifts and Ligand Mobility of Tco (V) and Reo (V) Complexes With AminothiolsDokument5 SeitenTrends in NMR Chemical Shifts and Ligand Mobility of Tco (V) and Reo (V) Complexes With AminothiolsElinaNoch keine Bewertungen
- 3-NH Lig ThioureaDokument6 Seiten3-NH Lig ThioureaFarouk El NaggarNoch keine Bewertungen
- International Journals Call For Paper HTTP://WWW - Iiste.org/journalsDokument9 SeitenInternational Journals Call For Paper HTTP://WWW - Iiste.org/journalsAlexander DeckerNoch keine Bewertungen
- Annulation of Imidazolines With Bis-Electrophiles: Synthesis of Imidazo (1,2-A) PyridinesDokument10 SeitenAnnulation of Imidazolines With Bis-Electrophiles: Synthesis of Imidazo (1,2-A) PyridinesboksabbNoch keine Bewertungen
- The reactivity of cobaltocene compared to ferroceneDokument3 SeitenThe reactivity of cobaltocene compared to ferroceneSandipan SahaNoch keine Bewertungen
- 1a PublicacionDokument11 Seiten1a PublicacionRoly RcmNoch keine Bewertungen
- Vrouw, Mar 2011Dokument4 SeitenVrouw, Mar 2011emediageNoch keine Bewertungen
- 2012-Synthesis and Reactivity of 1 2 Methoxy Benzene 3 Benzothiazole Triazene With Copper II or Cobalt II ChlorideDokument7 Seiten2012-Synthesis and Reactivity of 1 2 Methoxy Benzene 3 Benzothiazole Triazene With Copper II or Cobalt II ChlorideELKIN ALFONSO RODRIGUEZ AGUALIMPIANoch keine Bewertungen
- Metal ComplexesDokument2 SeitenMetal Complexeschamp delacruzNoch keine Bewertungen
- Regioselective Hydromethoxycarbonylation of Terminal Alkynes Catalyzed by Palladium (II) Tetraphos ComplexesDokument6 SeitenRegioselective Hydromethoxycarbonylation of Terminal Alkynes Catalyzed by Palladium (II) Tetraphos ComplexesGreciel Egurrola SanchezNoch keine Bewertungen
- Polyhedron: Klaus Beckerle, Andreas Sauer, Thomas P. Spaniol, Jun OkudaDokument6 SeitenPolyhedron: Klaus Beckerle, Andreas Sauer, Thomas P. Spaniol, Jun OkudaAishwarya K NNoch keine Bewertungen
- P 19900000945Dokument10 SeitenP 19900000945Yi-Yan TsaoNoch keine Bewertungen
- Preparation and Pyrolysis of 1 - (Pyrazol-5-Yl) - 1,2,3-Triazoles and Related CompoundsDokument6 SeitenPreparation and Pyrolysis of 1 - (Pyrazol-5-Yl) - 1,2,3-Triazoles and Related CompoundsChandra ReddyNoch keine Bewertungen
- Molecules 12 01796Dokument9 SeitenMolecules 12 01796Kalpesh PatelNoch keine Bewertungen
- Effect of β-Cyclodextrin on the Thermal Cis Trans Isomerization of AzobenzenesDokument6 SeitenEffect of β-Cyclodextrin on the Thermal Cis Trans Isomerization of AzobenzenesnataliaNoch keine Bewertungen
- Paper SakDokument18 SeitenPaper SaknitjsakshiNoch keine Bewertungen
- Synthesis and Regiochemistry of (60) Fullerenyl 2-Methylmalonate Bisadducts and Their Facile Electron-Accepting PropertiesDokument10 SeitenSynthesis and Regiochemistry of (60) Fullerenyl 2-Methylmalonate Bisadducts and Their Facile Electron-Accepting PropertiesDiogo DiasNoch keine Bewertungen
- NitrationDokument27 SeitenNitrationsubhashpithaniNoch keine Bewertungen
- Eur. J, 2010, 16, 6509-6517 Reek Anti-HalpernDokument9 SeitenEur. J, 2010, 16, 6509-6517 Reek Anti-HalpernszbaloghNoch keine Bewertungen
- Cerium-Catalyzed Arylmethanol OxidationDokument7 SeitenCerium-Catalyzed Arylmethanol OxidationMitali PaulNoch keine Bewertungen
- Enantioselective Synthesis of N, O-Psiconucleosides: TetrahedronDokument7 SeitenEnantioselective Synthesis of N, O-Psiconucleosides: Tetrahedronapi-19793040Noch keine Bewertungen
- Coupling of Enamides With Alkynes or Arynes For Synthesis of Substituted Pyridines and Isoquinolines Via Amide ActivationwDokument3 SeitenCoupling of Enamides With Alkynes or Arynes For Synthesis of Substituted Pyridines and Isoquinolines Via Amide ActivationwBalaji ChandrasekharNoch keine Bewertungen
- Awake!, Mar 2011Dokument4 SeitenAwake!, Mar 2011emediageNoch keine Bewertungen
- Synthesis and Characterization of Coordination Compounds of Chelating Ligands Containing Imidazole GroupsDokument11 SeitenSynthesis and Characterization of Coordination Compounds of Chelating Ligands Containing Imidazole GroupsLUKMANNoch keine Bewertungen
- A Covalently Bound Dimeric Derivative of Pyrochlorophyllide A Possible Model For Reaction Center Chlorophyll'Dokument3 SeitenA Covalently Bound Dimeric Derivative of Pyrochlorophyllide A Possible Model For Reaction Center Chlorophyll'Mohammed ZiyadNoch keine Bewertungen
- The Chemistry of Thiocyanic EstersDokument68 SeitenThe Chemistry of Thiocyanic EstersrajdewaanNoch keine Bewertungen
- Highly Selective Oxalate - Membrane Electrode Based On (Cul) (Ac)Dokument10 SeitenHighly Selective Oxalate - Membrane Electrode Based On (Cul) (Ac)Hani KhuludNoch keine Bewertungen
- Advanced Inorganic Lab ExperimentDokument4 SeitenAdvanced Inorganic Lab ExperimentThanhThao TranNoch keine Bewertungen
- Full Paper: Tobias A. Nigst and Herbert MayrDokument10 SeitenFull Paper: Tobias A. Nigst and Herbert Mayrpatybena20Noch keine Bewertungen
- Angew Chem Int Ed - 2020 - Sarkar - A Neutral Three Membered 2 Aromatic Disilaborirane and The Unique Conversion Into ADokument5 SeitenAngew Chem Int Ed - 2020 - Sarkar - A Neutral Three Membered 2 Aromatic Disilaborirane and The Unique Conversion Into ATutu CaiNoch keine Bewertungen
- A New Pyridine Bis N Heterocyclic Carbene Ligand and Its Coordination To RH Synthesis and CharacterizationDokument5 SeitenA New Pyridine Bis N Heterocyclic Carbene Ligand and Its Coordination To RH Synthesis and CharacterizationAbbas WshelNoch keine Bewertungen
- The Chemistry of The Aminochromes. Part Xvi. Proton Magnetic Resonance Spectro CopylDokument4 SeitenThe Chemistry of The Aminochromes. Part Xvi. Proton Magnetic Resonance Spectro CopylNstm3Noch keine Bewertungen
- Research Proposal CorrectedDokument5 SeitenResearch Proposal CorrectedMuraliNaidu100% (2)
- Synthesis and Characterization of Metal Complexes of Novel Schiff's Base Ligands Derived From 4-Carboxy Hydrazide-5,6-Diphenyl-3 (2-H) PyridazoneDokument15 SeitenSynthesis and Characterization of Metal Complexes of Novel Schiff's Base Ligands Derived From 4-Carboxy Hydrazide-5,6-Diphenyl-3 (2-H) PyridazoneScholedge PublishingNoch keine Bewertungen
- Angew. Chem., Int. Ed., 2010, 49, 9229Dokument4 SeitenAngew. Chem., Int. Ed., 2010, 49, 9229rrgodboleNoch keine Bewertungen
- Heterogeneous Catalysis at Nanoscale for Energy ApplicationsVon EverandHeterogeneous Catalysis at Nanoscale for Energy ApplicationsNoch keine Bewertungen
- Introduction To Medicinal Chemistry PDFDokument14 SeitenIntroduction To Medicinal Chemistry PDFعلي الطياريNoch keine Bewertungen
- Organic Chemistry: Third EditionDokument35 SeitenOrganic Chemistry: Third EditionHanaNoch keine Bewertungen
- CH 05Dokument53 SeitenCH 05Mark BakalanNoch keine Bewertungen
- Stereochemistry of Hopanes and TerpenesDokument4 SeitenStereochemistry of Hopanes and TerpenesBushra AliNoch keine Bewertungen
- StereokimiaDokument32 SeitenStereokimiaBrenda GloriaNoch keine Bewertungen
- CY1101 Stereochemistry 290920Dokument209 SeitenCY1101 Stereochemistry 290920Adarsh PriyaranjanNoch keine Bewertungen
- 5 Stereochemistry PDFDokument12 Seiten5 Stereochemistry PDFAppy Zombaa100% (1)
- Ptical Isomerism in Lactic AcidDokument10 SeitenPtical Isomerism in Lactic AcidJay SoniNoch keine Bewertungen
- GPAT Organic Chemistry SyllabusDokument4 SeitenGPAT Organic Chemistry Syllabuskumar HarshNoch keine Bewertungen
- CH 331/1 Midterm Examination/Summer 2013/Dr. Daniel J. T. MylesDokument8 SeitenCH 331/1 Midterm Examination/Summer 2013/Dr. Daniel J. T. MylesAnita MarLaNoch keine Bewertungen
- StereochemistryDokument61 SeitenStereochemistryAndrean ZukempotNoch keine Bewertungen
- Stereoisomers Test Answers+ QuestionsDokument8 SeitenStereoisomers Test Answers+ QuestionsNandakumar SNoch keine Bewertungen
- Additional Problems Final Exam Part 2 AnswersDokument10 SeitenAdditional Problems Final Exam Part 2 AnswersJohn SmithNoch keine Bewertungen
- Stereochemistry 2Dokument21 SeitenStereochemistry 2Evan C BijuNoch keine Bewertungen
- Stereoisomerism TypesDokument30 SeitenStereoisomerism TypesDeepNoch keine Bewertungen
- All India Test Series: Concept Recapitulation Test - IDokument12 SeitenAll India Test Series: Concept Recapitulation Test - IShreya DesaiNoch keine Bewertungen
- CHM 1321 Assignment 3 - : AnswersDokument5 SeitenCHM 1321 Assignment 3 - : AnswersSara YuenNoch keine Bewertungen
- Isomerism KEC 077 Lecture IV BCE A 079-02-17 PST.Dokument26 SeitenIsomerism KEC 077 Lecture IV BCE A 079-02-17 PST.bsarad115Noch keine Bewertungen
- Stereoisomerism Pyqs NsecDokument8 SeitenStereoisomerism Pyqs Nsecmanol sahooNoch keine Bewertungen
- SelectivityDokument4 SeitenSelectivitySamik BiswasNoch keine Bewertungen
- Organic Chemistry 1 Trans for First DepexDokument67 SeitenOrganic Chemistry 1 Trans for First DepexasdfNoch keine Bewertungen
- Chapter 05 Wade 8thDokument66 SeitenChapter 05 Wade 8thanupamgupta112Noch keine Bewertungen
- Monosaccharides:, Dr. Shweta HardiaDokument28 SeitenMonosaccharides:, Dr. Shweta HardiaArzoo RathoreNoch keine Bewertungen
- Organic Chemistry 2nd Edition Klein Test Bank 1Dokument35 SeitenOrganic Chemistry 2nd Edition Klein Test Bank 1carolyn100% (55)