Beruflich Dokumente
Kultur Dokumente
Alkanes: HH H H H H H H Staggered Eclipsed
Hochgeladen von
Camille AdleOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Alkanes: HH H H H H H H Staggered Eclipsed
Hochgeladen von
Camille AdleCopyright:
Verfügbare Formate
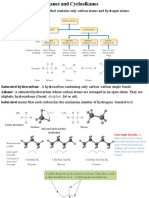
ALKANES Hydrocarbons General formula CnH2n+2 All C atoms are sp3-hybridized. All single bonds ( bonds).
ds). Saturated hydrocarbons HOMOLOGOUS SERIES Compounds that differ from each other in their molecular formulas by the unit CH2(methylene) unit. The boiling points of alkanes increases steadily with increase in molecular weight within a homologous series: C1 C4, gases; C5 C17, liquids; C18 and up, solids. The boiling points of different alkanes with the same MW increases as the chain length increases. Melting points of different alkanes with the same MW depend on the compactness of the molecular shape. Spherical molecules pack better in crystal lattices and will have higher melting points.
Methane, CH4 simplest hydrocarbon
H H H a 3-D representation C H H H Lewis structure H C H CH4 condensed formula
Ethane
H H C C H H H H C H C H CH3CH3 condensed formula
H H perspective drawing
H H Lewis strcuture
CONFORMATIONS OF A MOLECULE: Different arrangements in space of the atoms within the molecule due to rotations around single bonds.
NEWMAN PROJECTIONS View of a molecule down the axis of a C-C bond. The C atom toward the front is represented by a dot, and the C atom toward the rear by a circle. The atoms or groups of atoms on the C atoms are shown as being bonded to the dot or circle.
H H H H H H H
HH H H
H Staggered
Eclipsed
Propane
C3H8 CH3CH2CH3 Contains 2 different kinds of Cs: o Primary C (1 C) attached to only 1 other C o Secondary C (2 C) attached to 2 other Cs
Butane 2 possible structures for C4H10:
H H H H H C C C C H H H H H H H C H H H H C C C H H H H 2-methylpropane (or isobutane) n-butane
Tertiary (3o) carbon bonded to three other C atoms. Staggered Conformations of Butane: Anti conformation methyl groups are farthest from each other; most stable conformation of butane. Gauche conformation methyl groups are 60o apart; less stable than anti conformation by 0.9 kcal/mol. Line structures for organic compounds: A.k.a. skeletal structural formulas Each junction of 2 straight lines or the end of a straight line represents a C atom and the necessary number of H atoms to give the C atom 4 bonds. Functional groups are represented by their usual symbols. Alkanes may be: Acyclic hydrocarbons open-chain alkanes Cycloalkanes
Cycloalkanes: Hydrocarbons in which some or all of the C atoms form a ring. General formula: CnH2n. Named by adding the prefix cyclo- to the name of the alkane having the same number of C atoms as are in the ring.
Cyclopropane: Smallest cycloalkane. All three Cs define a plane. Internal bond angles of 60o; External C-C-H bond angle of 116o. H-C-H bond angle of 118o. All the bonds are eclipsed.
Contains bent bonds, i.e., the orbitals overlap at a slight angle bonds are weaker and more reactive than other alkane bonds. Has a lot of angle strain.
Cyclobutane: One of the atoms in the ring is bent out of plane of the other three by about 20o. Internal bond angle is 88o instead of 90o. This minimizes the eclipsing of H atoms on adjacent C atoms increases angle strain but decreases torsional strain. Has less angle strain than cyclopropane but more torsional strain because of its larger number of Hs.
Angle strain the strain introduced into a molecule when a bond angle is deformed from its ideal value. Torsional strain or eclipsing strain; the strain in a molecule caused by electron repulsion between eclipsed bonds.
Cyclopentane: Bond angles are close to the tetrahedral bond angle. Most stable conformation when one C is out of plane of the other 4 Cs envelop Cyclohexane: C6H12 Bond angles: 109o Exist in two conformations: Chair and boat Axial hydrogens in cyclohexane 3Hs are above the plane while the other 3 are below the plane. Equatorial hydrogens in cyclohexane lie roughly in the plane of the molecule
Axial H H H H H H H H H H H Equatorial H H H
Newman Projections of Chair and Boat Forms
Chair form
H H CH2 H H
Boat form H H
H H
HH
H H CH2 H H
H H
Methylcyclohexane
H H CH2 CH3 H H H CH2 H CH3
H H
CH2
H H
CH2
The more stable conformation has the methyl group at the equatorial position. In general, the larger the substituent, the greater the repulsive interaction when it occupies an axial position. 1,2-Dimethylcyclohexane May be cis or trans Cis-1,2-dimethylcyclohexane has both methyls on the same side of the plane of the ring
H H H H
CH3 CH3 H H H H
CH3 CH3
In trans-1,2-dimethylcyclohexane, the 2 methyl groups are on opposite side of the plane of the ring.
H H H H
CH3 H H CH3 H H
H CH3
ISOMERS: Different compounds with the same molecular formula May be: 1. Structural or constitutional isomers differ in the way the atoms are bonded together. 2. Stereoisomers differ in the way the atoms are oriented in space but the attachment of atoms is the same Geometric Isomerism: Due to restricted rotation about a rigid structure such as a multiple bond or a ring structure. Examples: cis and trans isomers of alkenes and cycloalkanes. Stereoisomers can also be present in compounds with at least one chiral center. Chiral center carbon which is bonded to 4 different atoms or groups of atoms. Enantiomers compounds which are non-superimposable mirror images of each other Diastereomers non-superimposable compounds which are not mirror images For a molecule having n chiral centers, the maximum number of stereoisomers is 2n. Meso compound compound that has chiral centers but is superimposable on its mirror image. This happens because there is a symmetry plane within the molecule Example: Tartaric acid
CH3
COOH H H OH OH COOH
Reactions of Alkanes: 1. Combustion 2. Halogenation. Reaction with halogens at high temperatures or under the influence of light (h). Gives a mixture of products.
CH4
+ Cl2
CH3Cl + CH2Cl2
+ CHCl3 + CCl4 Intermediates unstable short-lived species Reactive intermediate free radicals. Free radical a highly reactive intermediate which has an unpaired electron and no charge Chain reaction type of reaction in which the product of one step is a reactant in the next step. Free radical chain reaction the reactive intermediate formed in different steps of the reaction are free radicals.
Steps in a chain reaction: 1. Initiation Step in which the first reactive intermediate is formed. In halogenation, homolytic cleavage of the halogen molecule is the initiation step.
.. : Cl ..
2.
.. Cl .. :
.. : Cl . ..
.. . Cl : ..
Propagation Consists of steps that are repeated many times to carry the reaction forward. A radical is present in the reactant side, and a radical is also formed as product.
H H C H H
H H C. H
3.
.. + . Cl .. :
H H C. H
H .. . Cl : ..
+ HCl
Cl2
H C Cl H
Termination Consists of steps that occur when two free radicals collide with each other and form a covalent bond, thereby stopping the chain of reactions.
H H C. H .. : Cl . .. H H C. H + + .. . Cl : .. H H C. H .. : Cl .. + .. . Cl : .. H H C Cl H .. : Cl .. H H H C C H H H
Stability of Free Radicals:
Most stable
3o > 2o > 1o > H3C .
Least stable
Das könnte Ihnen auch gefallen
- Schaum's Easy Outline of Organic Chemistry, Second EditionVon EverandSchaum's Easy Outline of Organic Chemistry, Second EditionBewertung: 3.5 von 5 Sternen3.5/5 (2)
- Chapter1 and 2 HandoutDokument20 SeitenChapter1 and 2 HandoutsugNoch keine Bewertungen
- Unit 2 - Functional GroupsDokument59 SeitenUnit 2 - Functional GroupsNico MendezNoch keine Bewertungen
- A-Level Chemistry Revision: Cheeky Revision ShortcutsVon EverandA-Level Chemistry Revision: Cheeky Revision ShortcutsBewertung: 4 von 5 Sternen4/5 (5)
- AlkenesDokument15 SeitenAlkenesKamrul Alam MasumNoch keine Bewertungen
- Organic Chemistry Study Guide: Key Concepts, Problems, and SolutionsVon EverandOrganic Chemistry Study Guide: Key Concepts, Problems, and SolutionsBewertung: 3.5 von 5 Sternen3.5/5 (10)
- AlkenesDokument12 SeitenAlkenesDoc_CrocNoch keine Bewertungen
- Strained Organic Molecules: Organic Chemistry: A Series of Monographs, Vol. 38Von EverandStrained Organic Molecules: Organic Chemistry: A Series of Monographs, Vol. 38Noch keine Bewertungen
- Nomenclature & Isomerism (1-77)Dokument77 SeitenNomenclature & Isomerism (1-77)deepakkr08088% (8)
- PR Revision NotesDokument29 SeitenPR Revision Notesabdurahman1234Noch keine Bewertungen
- Physical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974Von EverandPhysical Organic Chemistry—Ii: Specially Invited Lectures Presented at the Second IUPAC Conference on Physical Organic Chemistry Held at Noordwijkerhout, Netherlands, 29 April–2 May 1974Th. J. De BoerNoch keine Bewertungen
- Introduction To Orgnic ChemistryDokument27 SeitenIntroduction To Orgnic ChemistryladybugNoch keine Bewertungen
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionVon EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionNoch keine Bewertungen
- Organic Chemistry NotesDokument3 SeitenOrganic Chemistry NotesHalimaNoch keine Bewertungen
- Eg Ethylene (IUPAC: Ethene), CDokument16 SeitenEg Ethylene (IUPAC: Ethene), CVansh JindalNoch keine Bewertungen
- 226 Dienes Alkynes LecDokument12 Seiten226 Dienes Alkynes LecHari PrasathNoch keine Bewertungen
- Reaction MechanismDokument68 SeitenReaction MechanismSiddarth Singh73% (11)
- GOC (Hints)Dokument2 SeitenGOC (Hints)hchawla421Noch keine Bewertungen
- Chemistry Unit 2Dokument8 SeitenChemistry Unit 2sashabelleNoch keine Bewertungen
- IB Topic 10: Organic Chemistry Practice QuestionsDokument36 SeitenIB Topic 10: Organic Chemistry Practice Questionshunarsandhu50% (2)
- c2 AlkenesDokument4 Seitenc2 Alkenesapi-247243068Noch keine Bewertungen
- Hydrocarbon:: A Compound That Contains Only Carbon Atoms and Hydrogen AtomsDokument37 SeitenHydrocarbon:: A Compound That Contains Only Carbon Atoms and Hydrogen Atomsmohammad 3yaNoch keine Bewertungen
- Organic Chemistry: A Brief IntroductionDokument33 SeitenOrganic Chemistry: A Brief IntroductionWai Kwong ChiuNoch keine Bewertungen
- Chapter 11Dokument30 SeitenChapter 11kanilkadianNoch keine Bewertungen
- CH 03Dokument24 SeitenCH 03Ummi Khairani UrfaNoch keine Bewertungen
- Ch221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedDokument11 SeitenCh221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedSanjay Mani TripathiNoch keine Bewertungen
- OrganicDokument93 SeitenOrganicPatel MswaziNoch keine Bewertungen
- ChemistryDokument70 SeitenChemistryKieran SangheraNoch keine Bewertungen
- Chemistry 10006Dokument27 SeitenChemistry 10006Shai Shazza GrossNoch keine Bewertungen
- Complete Unit 2 Notes PDFDokument77 SeitenComplete Unit 2 Notes PDFSan Siddz100% (1)
- Alkenes and AlkynesDokument39 SeitenAlkenes and Alkynesrajeevbansal1807Noch keine Bewertungen
- Chapter 3 Alkanes and Their Stereochemistry-1-1Dokument23 SeitenChapter 3 Alkanes and Their Stereochemistry-1-1eas111Noch keine Bewertungen
- Aromatic Compounds: NamingDokument13 SeitenAromatic Compounds: NamingTanzimNoch keine Bewertungen
- CHM 1321 Assignment #2 - : AnswersDokument11 SeitenCHM 1321 Assignment #2 - : AnswersSara YuenNoch keine Bewertungen
- הרצאה 2- קבוצות פונקציונאליות, אלקאנים ואיזומריםDokument51 Seitenהרצאה 2- קבוצות פונקציונאליות, אלקאנים ואיזומריםGal ZeevNoch keine Bewertungen
- Alkanes, Alkenes, Alkynes, and CycloalkanesDokument162 SeitenAlkanes, Alkenes, Alkynes, and CycloalkanesNurtasha AtikahNoch keine Bewertungen
- Level3 Organic So FarDokument0 SeitenLevel3 Organic So Farapi-218511741Noch keine Bewertungen
- f322 Mod1Dokument16 Seitenf322 Mod1api-275024237Noch keine Bewertungen
- Chapter 3 McmurryDokument26 SeitenChapter 3 Mcmurrymuhammad_asim_10Noch keine Bewertungen
- 1 - Introduction To HydrocarbonsDokument56 Seiten1 - Introduction To HydrocarbonsPaul NderebaNoch keine Bewertungen
- GOC NotesDokument154 SeitenGOC Notessamay gujratiNoch keine Bewertungen
- H CL H H H CL: (Bonding Implicit) (Bonding Explicit) (C and Connected H Implicit)Dokument2 SeitenH CL H H H CL: (Bonding Implicit) (Bonding Explicit) (C and Connected H Implicit)Chutneyc4064Noch keine Bewertungen
- Organic ChemistryDokument9 SeitenOrganic ChemistrySundaram ShuklaNoch keine Bewertungen
- Lab 01 Structure and BondingDokument19 SeitenLab 01 Structure and BondingynottripNoch keine Bewertungen
- Organic Compounds EditedDokument52 SeitenOrganic Compounds EditedbrendonNoch keine Bewertungen
- Structure and FormulaeDokument64 SeitenStructure and FormulaeLoveena SteadmanNoch keine Bewertungen
- Introduction To Organic ChemistryDokument34 SeitenIntroduction To Organic ChemistryEll LynaNoch keine Bewertungen
- Organic Chemistry: Muh. Yanis Musdja The Study of The Compounds of CarbonDokument58 SeitenOrganic Chemistry: Muh. Yanis Musdja The Study of The Compounds of CarbonAgung Nugroho OteNoch keine Bewertungen
- AlkyneDokument13 SeitenAlkyneJueeli More100% (1)
- Unit 11 Fundamentals Org ChemDokument35 SeitenUnit 11 Fundamentals Org ChemKavisha AshaNoch keine Bewertungen
- Hydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEDokument1 SeiteHydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEjaindhruv850Noch keine Bewertungen
- 12 - Organic Chemistry - Some Basic Principles and TechniquesDokument9 Seiten12 - Organic Chemistry - Some Basic Principles and TechniquesmohammedNoch keine Bewertungen
- CHE 112 - Lecture 2Dokument103 SeitenCHE 112 - Lecture 2Martias WambiNoch keine Bewertungen
- Alkanes PDFDokument11 SeitenAlkanes PDFDinesh BijalwanNoch keine Bewertungen
- Organic ChemistryDokument40 SeitenOrganic ChemistryGlenda ResultayNoch keine Bewertungen
- 51a Chapter 1 2014 Copy 2Dokument37 Seiten51a Chapter 1 2014 Copy 2Efrain AnayaNoch keine Bewertungen
- Introduction To Organic ChemistryDokument57 SeitenIntroduction To Organic ChemistryBehzod ShoraimovNoch keine Bewertungen
- Organic Reaction Mechanisms (Lecture 2) : Understanding, As Opposed To Memorizing, Mechanisms Is Critical To Mastering Organic ChemistryDokument18 SeitenOrganic Reaction Mechanisms (Lecture 2) : Understanding, As Opposed To Memorizing, Mechanisms Is Critical To Mastering Organic ChemistryDoris GladiaNoch keine Bewertungen
- Self AwarenessDokument8 SeitenSelf AwarenessCamille AdleNoch keine Bewertungen
- Aromatic Compounds: Y Y Y YDokument9 SeitenAromatic Compounds: Y Y Y YCamille AdleNoch keine Bewertungen
- Adult LearningDokument16 SeitenAdult LearningCamille Adle100% (1)
- Organic Nomeclature of Organic CompoundsDokument15 SeitenOrganic Nomeclature of Organic CompoundsNgoVietCuongNoch keine Bewertungen
- Al KynesDokument3 SeitenAl KynesCamille AdleNoch keine Bewertungen
- Amines: + HCL RC O N R' H R'NH + RC O CLDokument7 SeitenAmines: + HCL RC O N R' H R'NH + RC O CLCamille AdleNoch keine Bewertungen
- Alkyl HalidesDokument8 SeitenAlkyl HalidesCamille AdleNoch keine Bewertungen
- Cis-Trans Isomerism: AlkenesDokument4 SeitenCis-Trans Isomerism: AlkenesCamille AdleNoch keine Bewertungen
- Hand AlcoholsDokument3 SeitenHand AlcoholsMarxlen EndicoNoch keine Bewertungen
- Aldehydes and KetonesDokument9 SeitenAldehydes and KetonesCamille AdleNoch keine Bewertungen
- Farmer Business School HandbookDokument305 SeitenFarmer Business School HandbookCamille Adle83% (6)
- The Empath's Guidebook Version 1.1Dokument191 SeitenThe Empath's Guidebook Version 1.1Camille Adle100% (6)
- How To Do Recipe CostingDokument5 SeitenHow To Do Recipe CostingCamille Adle100% (1)
- 0 ArtStudRdgDokument10 Seiten0 ArtStudRdgCamille AdleNoch keine Bewertungen
- DAS On StigmaDokument9 SeitenDAS On StigmaCamille AdleNoch keine Bewertungen
- Taxes, Financing Decisions, and Firm Value: Eugene F. Fama and Kenneth R. FrenchDokument25 SeitenTaxes, Financing Decisions, and Firm Value: Eugene F. Fama and Kenneth R. FrenchDiploma IV KeuanganNoch keine Bewertungen
- Inorganic ChemistryDokument194 SeitenInorganic ChemistryClarice Jenn Ramirez Malto67% (3)
- Procurement Plan URSIP - Newform - 20181101 (DRAFT)Dokument22 SeitenProcurement Plan URSIP - Newform - 20181101 (DRAFT)Abib Ansari100% (2)
- The Term Structure of Interest Rates: Investments - Bodie, Kane, MarcusDokument37 SeitenThe Term Structure of Interest Rates: Investments - Bodie, Kane, MarcusMohammed Al-YagoobNoch keine Bewertungen
- The Chemistry of Fullerenes PDFDokument285 SeitenThe Chemistry of Fullerenes PDFsadfsdafNoch keine Bewertungen
- Global Capital Market NotesDokument10 SeitenGlobal Capital Market NotesAbdulrahman AlotaibiNoch keine Bewertungen
- Adhesives in Wood IndustryDokument70 SeitenAdhesives in Wood IndustryBrenda Maria Monterroso WaightNoch keine Bewertungen
- CMBS World - Reremic PhenomenonDokument14 SeitenCMBS World - Reremic PhenomenonykkwonNoch keine Bewertungen
- Collusive HomeworkDokument5 SeitenCollusive HomeworkJacopo SalmistrariNoch keine Bewertungen
- Appendix 12ADokument4 SeitenAppendix 12ACheng LiNoch keine Bewertungen
- Enzyme ImmobilizationDokument73 SeitenEnzyme ImmobilizationDaniel Pulido RojasNoch keine Bewertungen
- SAT II Chemistry Practice Test 1Dokument4 SeitenSAT II Chemistry Practice Test 1Jaime DianzonNoch keine Bewertungen
- JACS, Vol. 108, 1986, 452Dokument10 SeitenJACS, Vol. 108, 1986, 452rrgodboleNoch keine Bewertungen
- Bond ValuationDokument78 SeitenBond ValuationSarikaNoch keine Bewertungen
- Materi Ke 9 Polimer Matriks CompositeDokument11 SeitenMateri Ke 9 Polimer Matriks CompositeErdi Sofyandra AdikriNoch keine Bewertungen
- Band TheoryDokument19 SeitenBand TheoryTwishaNoch keine Bewertungen
- Enzyme TerminologyDokument3 SeitenEnzyme TerminologycapricornchrissNoch keine Bewertungen
- 6CH01 01 Rms 20170816 PDFDokument24 Seiten6CH01 01 Rms 20170816 PDFMr-Mohamed AliNoch keine Bewertungen
- Glossary of Construction Cost EstimatingDokument8 SeitenGlossary of Construction Cost EstimatingFaty BercasioNoch keine Bewertungen
- Le Chimie Invitation LetterDokument6 SeitenLe Chimie Invitation LetterAaron John BalanaNoch keine Bewertungen
- BNK601 Short NotesDokument7 SeitenBNK601 Short NotesNasir MuhammadNoch keine Bewertungen
- Organic Sub-Part 1Dokument16 SeitenOrganic Sub-Part 1ahsaanahmadNoch keine Bewertungen
- Past Paper Agha Khan Board Class IX Chemistry 2013Dokument12 SeitenPast Paper Agha Khan Board Class IX Chemistry 2013Muhammad Mohsin100% (1)
- Markets and Commodity Figures: Total Market Turnover StatisticsDokument3 SeitenMarkets and Commodity Figures: Total Market Turnover StatisticsTiso Blackstar GroupNoch keine Bewertungen
- Past Paper Chemistry 1st Year BISE Lahore 2012 Group 1Dokument5 SeitenPast Paper Chemistry 1st Year BISE Lahore 2012 Group 1Mian EjazNoch keine Bewertungen
- Brazil ListDokument2 SeitenBrazil ListLuiz Bonacin NettoNoch keine Bewertungen
- FINS 2624 Quiz 2 AnswersDokument2 SeitenFINS 2624 Quiz 2 Answerssagarox7Noch keine Bewertungen
- Poly Tank Repair Kit InstrutionsDokument2 SeitenPoly Tank Repair Kit InstrutionsRgonz79Noch keine Bewertungen
- Course Objectives: Course Name: Financial Management - 1Dokument4 SeitenCourse Objectives: Course Name: Financial Management - 1Aninda DuttaNoch keine Bewertungen
- 1.6taxation UK PDFDokument244 Seiten1.6taxation UK PDFAdnan KasmaniNoch keine Bewertungen
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincVon EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincBewertung: 3.5 von 5 Sternen3.5/5 (137)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeVon EverandChemistry for Breakfast: The Amazing Science of Everyday LifeBewertung: 4.5 von 5 Sternen4.5/5 (90)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsVon EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNoch keine Bewertungen
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeVon EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeBewertung: 5 von 5 Sternen5/5 (1)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactVon EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactBewertung: 5 von 5 Sternen5/5 (5)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeVon EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeBewertung: 5 von 5 Sternen5/5 (4)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsVon EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsBewertung: 4 von 5 Sternen4/5 (146)
- Sodium Bicarbonate: Nature's Unique First Aid RemedyVon EverandSodium Bicarbonate: Nature's Unique First Aid RemedyBewertung: 5 von 5 Sternen5/5 (21)
- Guidelines for Chemical Process Quantitative Risk AnalysisVon EverandGuidelines for Chemical Process Quantitative Risk AnalysisBewertung: 5 von 5 Sternen5/5 (1)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideVon EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNoch keine Bewertungen
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolVon EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNoch keine Bewertungen
- Process Plant Equipment: Operation, Control, and ReliabilityVon EverandProcess Plant Equipment: Operation, Control, and ReliabilityBewertung: 5 von 5 Sternen5/5 (1)
- Taste: Surprising Stories and Science About Why Food Tastes GoodVon EverandTaste: Surprising Stories and Science About Why Food Tastes GoodBewertung: 3 von 5 Sternen3/5 (20)
- The Periodic Table: A Very Short IntroductionVon EverandThe Periodic Table: A Very Short IntroductionBewertung: 4.5 von 5 Sternen4.5/5 (3)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeVon EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeBewertung: 4 von 5 Sternen4/5 (1)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeVon EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNoch keine Bewertungen
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsVon EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsBewertung: 5 von 5 Sternen5/5 (3)
- Tribology: Friction and Wear of Engineering MaterialsVon EverandTribology: Friction and Wear of Engineering MaterialsBewertung: 5 von 5 Sternen5/5 (1)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableVon EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableBewertung: 3.5 von 5 Sternen3.5/5 (22)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsVon EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNoch keine Bewertungen
- Bioplastics: A Home Inventors HandbookVon EverandBioplastics: A Home Inventors HandbookBewertung: 4 von 5 Sternen4/5 (2)
- Ingredients: A Visual Exploration of 75 Additives & 25 Food ProductsVon EverandIngredients: A Visual Exploration of 75 Additives & 25 Food ProductsBewertung: 4 von 5 Sternen4/5 (1)
- Nuclear Energy in the 21st Century: World Nuclear University PressVon EverandNuclear Energy in the 21st Century: World Nuclear University PressBewertung: 4.5 von 5 Sternen4.5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeVon EverandChemistry for Breakfast: The Amazing Science of Everyday LifeBewertung: 4.5 von 5 Sternen4.5/5 (14)