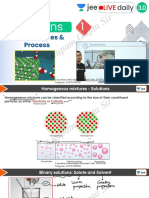
Beruflich Dokumente
Kultur Dokumente
Bitch Niggas Be Hating On My Fresh
Hochgeladen von
Richard SmithOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Bitch Niggas Be Hating On My Fresh
Hochgeladen von
Richard SmithCopyright:
Verfügbare Formate
Development of New Controllable Hydrogen Sulfide Donors
Powell Bagdon, Nelmi O. Devarie-Baez, Chung-Min Park, Yu Zhao, Bo Peng, Ming Xian* Department of Chemistry, Washington State University, Pullman , WA 99164
Background
It has long been known that hydrogen sulfide gas (H2S) is harmful and sometimes fatal at high concentrations, but recent studies have suggested that it is also plays an important role in cell biology and signaling. Mammals are able to produce H2S in a controllable manner. This production is attributed to several sources: three enzymes (cystathionine--synthetase, cystathionine-lysase and 3-mercaptopyruvate sulfurtransferase), FeS clusters and bound sulfane sulfur. In biological systems H2S has been attributed to a number of important tasks including neuromodulation, vasodilation, antiagrregatory activity against platelets, anti-inflammatory processes, the angiogenesis of wound healing [1-3]. Currently the most commonly used H2S donors are solutions of NaSH, Na2S and CaS. These donors are none-controllable and do not accurately emulate biological levels of H2S. In contrast to these inorganic donors, organic donors have been proven to produce H2S controllably and at concentrations much closer to those observed in cells. Organic donors can be triggered to release H2S upon exposure to a number of external stimuli including light, pH, and through activation by biothiol [4], but at this time only a few such donors, shown below, have been created and as such there is a large need for the development of new, controllable donors with varied activation mechanisms. Here the development of two such organic, controllable H2S donors is presented.
Scheme
A B
H2S Release of Donors in Cells
C
S NO2
7b
Figure 5. Fluorescent image of cells incubated in donor 1d and fluorescent H2S probe WSP-1 [6] before and after irradiation with UV light.
w/o light
w/ light
Synthesis of Light Activated Donors
Synthesis of Phosphorodithiolate Based Donors
Figure 6. Fluorescent image of cells incubated in donor 2a and WSP-1. A: 0 M donor B: 100 M. C: 200 M.
Conclusion
Both donor templates controllably release H2S in aqueous solution. Phosphorodithiolate based donors 2a-c were found to be as effective as commonly used GYY4137 at controllably releasing H2S. Light activated donors 1a-f were found to controllably release H2S upon irradiation with UV light. It is expected that this new class of donor can allow for both spatial and temporal control of H2S release, and be used in the study real time of H2S activities.
Measurement of Light Activated Donors
Measurement of Phosphorodithiolate Based Donors
1.2 1 0.8
Acknowledgement
We would like to thank the Washington State University College of Arts and Sciences, American Chemical Society-Teva USA Scholar Grant and the NIH (R01GM088226) for support of this research.
H2S (uM)
0.6 0.4 0.2 0 0 30 60 90 120 150 180
References
Time (min)
Design of H2S Donors
We proposed two new, controllable H2S donor molecule templates. Gem-dithiols have been known to produce H2S in aqueous solutions. We proposed that installing a photo-labile protecting group on this template would provide us with a controllable method in which to initiate H2S production. The second is based on a phosphorodithiolate structure. These molecules are known to slowly hydrolyze in aqueous solutions allowing for closer and better control of H2S production.
Figure 1. The release profile of donor 1a before and after activation with UV radiation (=365nm).
GYY4137
1a
2b
2c
Figure 3. The release profiles of donors 2a-c compared with the known donor GYY4137 in 1:1 ACN/PBS (pH=7.4) measured with the selective H2S fluorescent probe Dz-N3 [5].
Use of Donors in the Protection Against ROS in Cells
1. Li, L.; Rose, P. and Moore, P. K. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169-187. 2. Vandiver, M. S. and Snyder, S. H. J. Mol. Med. 2012, 90, 255 263 3. Wang, R. Physiol. Rev. 2012, 92, 791 896 4. Zhao, Y.; Wang, H. and Xian, M. J. Am. Chem. Soc., 2011, 133, 15-17. 5. Peng, Y.; Cheng, C.; Dai, A. L.; King, B. L.; Predmore, D. J.; Lefer and Wang, B. Angew. Chem. Int. Ed. 2011, 50, 9672-9675. 6. Liu, C.; Pan, J.; Li, S.; Zhao, Y.; Wu, L. Y.; C. E. Berkman,; Whorton, A. R. and Xian, M. Angew. Chem. Int. Ed. 2011, 50, 10327-10329.
Figure 2. The release profiles of donors 1a-f after irradiation with UV light (=365nm).
Figure 4. H9c2 cells viability after oxidative injury by H2O2 in the presence of donors 2a, 2b, GYY4137 and NaSH .
Das könnte Ihnen auch gefallen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- IB Chemistry 1 SL QuestionsDokument36 SeitenIB Chemistry 1 SL QuestionsCamilla0% (3)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Module 3 PDFDokument33 SeitenModule 3 PDFPathi YashNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Adsorption Engineering, Suzuki (1990)Dokument278 SeitenAdsorption Engineering, Suzuki (1990)Nigel Mitchell80% (5)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- An Overview of Green Corrosion Inhibitors For Sustainable and Environment Friendly Industrial DevelopmentDokument19 SeitenAn Overview of Green Corrosion Inhibitors For Sustainable and Environment Friendly Industrial DevelopmentAbdinasir Mohamed AdanNoch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Reciprocating CompressorDokument28 SeitenReciprocating CompressorBalaji Kalai71% (7)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Heat Transfer: Revision - ExamplesDokument37 SeitenHeat Transfer: Revision - ExamplesYasser HendyNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- 5 Internal Energy and First Law of ThermodynamicsDokument7 Seiten5 Internal Energy and First Law of Thermodynamicsstuart3390Noch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Development of Self-Heating Concrete Using Low-Temperature Phase Change MaterialsDokument37 SeitenDevelopment of Self-Heating Concrete Using Low-Temperature Phase Change MaterialsRoel PlmrsNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Heat of Neutralization - Lab ReportDokument7 SeitenHeat of Neutralization - Lab ReportJasmeetSingh56% (9)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Atoms Molecules and IonsDokument3 SeitenAtoms Molecules and Ionsapi-304350501Noch keine Bewertungen
- Xcell Ammonia (NH) Sensor: Technical Data SheetDokument2 SeitenXcell Ammonia (NH) Sensor: Technical Data SheetPaulo HeideckeNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- (PDF) Chemistry NTSE Stage-1 - CompressDokument7 Seiten(PDF) Chemistry NTSE Stage-1 - CompressQWERTY111Noch keine Bewertungen
- Combined Liquid Solutions FileDokument357 SeitenCombined Liquid Solutions FileMaloth ShruthiNoch keine Bewertungen
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- 8462 1H MS Chemistry Specimen (Set 2) v1.1Dokument21 Seiten8462 1H MS Chemistry Specimen (Set 2) v1.1humbee0404Noch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- L - 01 - Energy Transfer Concepts DetailDokument32 SeitenL - 01 - Energy Transfer Concepts DetailMUHAMMAD LUQMAN HAKIMI MOHD ZAMRINoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Rigorous Steady-State Simulation of Acetone Production Using Aspen Hysys®Dokument9 SeitenRigorous Steady-State Simulation of Acetone Production Using Aspen Hysys®eva apriliaNoch keine Bewertungen
- 1Dokument6 Seiten1Jake ArbutanteNoch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Question Bank - Part ADokument5 SeitenQuestion Bank - Part AvaishaliNoch keine Bewertungen
- EquilibriumDokument7 SeitenEquilibriumPassmore DubeNoch keine Bewertungen
- U15 S4 HW Packet 13-20Dokument27 SeitenU15 S4 HW Packet 13-20Rohith GudatiNoch keine Bewertungen
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Jan 1aa SPH4C Properties-Of-FluidsDokument31 SeitenJan 1aa SPH4C Properties-Of-Fluidsssrames7282Noch keine Bewertungen
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- 1.1 English and Communication Skills - I L T P 3 - 2 RationaleDokument20 Seiten1.1 English and Communication Skills - I L T P 3 - 2 RationaleSehna SerajNoch keine Bewertungen
- Dr. Sami El-Khatib American University of SharjahDokument1 SeiteDr. Sami El-Khatib American University of SharjahAmro HeshamNoch keine Bewertungen
- Thermo Chemistry of Fuel-Air MixturesDokument34 SeitenThermo Chemistry of Fuel-Air Mixturesmahmudul adilNoch keine Bewertungen
- Water Investigation Stations Part IDokument8 SeitenWater Investigation Stations Part Iapi-232803009Noch keine Bewertungen
- PPoMP 36 2002 PDFDokument322 SeitenPPoMP 36 2002 PDFAnonymous OnoowoNoch keine Bewertungen
- Unit 1 Chemistry ReviewDokument16 SeitenUnit 1 Chemistry ReviewFirmino GonçalvesNoch keine Bewertungen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Applied Energy: Contents Lists Available atDokument14 SeitenApplied Energy: Contents Lists Available atAhmad YaniNoch keine Bewertungen
- Cold Flow Hydrate TechnologyDokument6 SeitenCold Flow Hydrate TechnologyAloisio NunesNoch keine Bewertungen
- Material Balance Project Styrene Manufacture: H CHCH H C CH CH H CDokument4 SeitenMaterial Balance Project Styrene Manufacture: H CHCH H C CH CH H CMhd SakerNoch keine Bewertungen
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)