Beruflich Dokumente
Kultur Dokumente
Reaction Kinetics-Ver 2
Hochgeladen von
Ivan Alberto NinaOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Reaction Kinetics-Ver 2
Hochgeladen von
Ivan Alberto NinaCopyright:
Verfügbare Formate
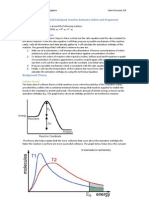
CHM130 Chemical Kinetics Reaction Kinetics: The Bromination of Acetone Objective: The purpose of this experiment is to determine the
rate law and the rate constant for the bromination of acetone. Introduction- Chemical Kinetics is the study of rates (or speeds) of chemical reactions and the mechanisms (sequence of steps) by which they occur. The rate of a reaction tells us how quickly reactants are used up and products are formed. To be useful, chemical reactions must occur at reasonable rates. The Haber process is an important industrial process used to produce millions of tons of ammonia each year. N2(g) + 3H2(g) 2NH3(g) (Haber process) Many fertilizers, explosives, dyes, plastics, and synthetic fibers are then synthesized from the ammonia. At 25 oC, the reaction occurs too slowly to make it economically useful. However, the speed of the reaction can be increased significantly if it is run at high temperatures and pressures with an iron oxide catalyst (usually 450 oC and 200 atmospheres). The aim of this experiment is to determine the rate law for the reaction (CH3)2CO is called acetone. We dont need to worry about its structure or about the organic product BrCH2COCH3 (bromoacetone). The reaction is replacing one of the hydrogens on acetone with a bromine hence it is called a bromination reaction. The bromination of acetone in acid solution proceeds according to
The reaction is catalyzed by hydrogen ion. The rate law is assumed to be of the form
where k is the rate constant and [A] represents concentration of A in moles per liter. The exponents p, q, and r indicate the order of the reaction with respect to acetone, bromine, and hydrogen ion, respectively. The rate law of a reaction shows a relationship between the rate constant and the concentrations of the reactants involved raised to their respective reaction orders. The rate law of the above reaction can be written as: rate = k[acetone]x[Br2]y[H+]z Where k is the rate constant and x, y, and z are the reaction orders. To determine the rate law, we will have to determine the values of k, x, y, and z. Unfortunately, these values CANNOT be determined from the stoichiometric equation. However, they can be determined experimentally. If we determine how fast a reactant is being consumed in the reaction process or how fast a product is formed, then we can generate some useful data that can help us determine the unknown values (k, x,y, and z). Our strategy will be to change the concentration of one reactant at a time, while keeping the other reactant concentrations constant. This will definitely change the reaction rate, however, the rate constant and the reaction orders will remain the same irrespective of how we change the reactant concentrations.
In this experiment you will set up a reaction at known concentrations and time until completion as demonstrated by a color change. The other experiments will involve the doubling of the concentration of one reactant and measuring the change in rate. The most likely effects of doubling a concentration are: 1. rate is the same, takes the same time, then it's a zero order. 2. rate also double, takes half the time, then it's a first order 3. rate increases by four, takes one quarter the time, then it's second order. This laboratory exercise will focus on how changing concentration affects how long to complete a reaction. Materials: This exercise will require the use of the following glassware and hardware: 6 small test tubes and stopper Thermometer Auto-pipetters A piece of white paper This exercise will use the following chemicals: Acetone solution Bromine solution Hydrochloric acid solution Proceudre: This exercise will contain four parts. Each part has a separate page of instructions and a data sheet in Excel to enter collected data and make calculations. The spreadsheet should be down loaded to the laboratory computer and opened from its hard drive during the experiment. The four parts to the experiment are: 1. Determination of the reaction rate at set concentrations. 2. Double one concentration and determine the new rate. 3. Double a different concentration and determine the new rate. 4. Calculate the orders of the reagents. Download kineticsdatapage Spreadsheet. CAUTION: The solutions used are corrosive and hazardous! To obtain a stopwatch on line go to: http://www.online-stopwatch.com/ If you want to run more than one reaction at a time, open multiple stopwatch windows on your computer. Mixture 1: 1. Fill a test tube 10 ml of water. This is a reference. 2. Prepare a mixture of 2.0 ml of 4.0 M acetone, 2.0 ml of 1.0 M hydrochloric acid and 4.0 ml of water in a test tube. 3. Record the temperature of the mixture on the data page. 4. Measure of 2.0 ml of 0.020 M bromine solution into a second test tube. One partner will add this to the other solution, stopper and shake. 5. The initial time is when the bromine solution has been added and the other partner should record this on the data page. 6. Looking down from the top to the bottom of the test tubes, with the mixture and water
against a white background. Watch until all the color in the mixture has disappeared. This is the end of the reaction. Record this as the final time. 7. Repeat steps 1 through 6 two more times. Average the final results. Mixture 2: 1. Prepare a mixture of 4.0 ml of 4.0 M acetone, 2.0 ml of 1.0 M hydrochloric acid and 2.0 ml of water in a test tube. 2. Record the temperature of the mixture on the data page. 3. Measure of 2.0 ml of 0.020 M bromine solution into a second test tube. One partner will add this to the other solution, stopper and shake. 4. The initial time is when the bromine solution has been added and the other partner should record this on the data page. 5. Looking down from the top to the bottom of the test tubes, with the mixture and water against a white background. Watch until all the color in the mixture has disappeared. This is the end of the reaction. Record this as the final time. 6. Repeat steps 1 through 5 two more times. Average the final results. Mixture 3: 1. Prepare a mixture of 2.0 ml of 4.0 M acetone, 4.0 ml of 1.0 M hydrochloric acid and 2.0 ml of water in a test tube. 2. Record the temperature of the mixture on the data page. 3. Measure of 2.0 ml of 0.020 M bromine solution into a second test tube. One partner will add this to the other solution, stopper and shake. 4. The initial time is when the bromine solution has been added and the other partner should record this on the data page. 5. Looking down from the top to the bottom of the test tubes, with the mixture and water against a white background. Watch until all the color in the mixture has disappeared. This is the end of the reaction. Record this as the final time. 6. Repeat steps 1 through 5 two more times. Average the final results. Calculations: The order of the reagents can be determined by first determining the ratio of the rate of disappearance of bromine. -[Br2] = - (0- [Br2]initial) t tfinal - tinitial Dont forget to calculation the concentrations of each reagent used for the experiment. Based on the change in the rate for experiments 2 and 3 resulting from the doubling of concentration fill in the data table for them in the spreadsheet. Remember: Doubling the concentration of a zero order reagent, the rate stays the same. Doubling the concentration of a first order reagent causes the rate to double. Doubling the concentration of a second order reagent causes the rate to become 4 times greater. Optional Mathematical approach: The algebraic method is especially useful for rate laws which have fractional exponents. First, write the general form of rate law, for example rate = k[NO]x[H2]y. Now, write the ratio of rates for experiments 1 and 3 in which [H2] remains constant and [NO] changes.
Now add in numbers from your data table and then solve for x:
2.0 is now the order for the rate law equation.
Laboratory Report: Make sure you explain all of the calculations performed in the lab.
Das könnte Ihnen auch gefallen
- Lab 3 KineticsDokument6 SeitenLab 3 KineticsMichelle SamayoaNoch keine Bewertungen
- Experiment 2: Kinetics of The Reaction Between Permanganate and Oxalic AcidDokument4 SeitenExperiment 2: Kinetics of The Reaction Between Permanganate and Oxalic AcidMaryNicoleDatlanginNoch keine Bewertungen
- Iodination of Acetone KineticsDokument4 SeitenIodination of Acetone Kineticsinstrutech0% (2)
- Reaction KineticsDokument7 SeitenReaction Kineticsjathan160% (1)
- E3: Kinetics of The Hydrogen Peroxide/Iodide ReactionDokument5 SeitenE3: Kinetics of The Hydrogen Peroxide/Iodide ReactionAlfian HadiwijayaNoch keine Bewertungen
- Heat of Neutralization f10Dokument9 SeitenHeat of Neutralization f10Nishat AhmedNoch keine Bewertungen
- Vapor Liquid Equilibria: Experiment No: 1Dokument8 SeitenVapor Liquid Equilibria: Experiment No: 1Harsh DuttaNoch keine Bewertungen
- Kinetics of The Acid Catalysed Reaction Between Iodine and Propanone Final LolDokument23 SeitenKinetics of The Acid Catalysed Reaction Between Iodine and Propanone Final LolYann Perusset90% (10)
- Lab #10: Determine Rate Law and Activation EnergyDokument7 SeitenLab #10: Determine Rate Law and Activation EnergyVenus PondevidaNoch keine Bewertungen
- Kinetics Expt 4-2011Dokument7 SeitenKinetics Expt 4-2011Wilo JaraNoch keine Bewertungen
- Liquid Phase Chemical Reactor FinalDokument38 SeitenLiquid Phase Chemical Reactor FinalToMemNoch keine Bewertungen
- 112 Experiment 3Dokument3 Seiten112 Experiment 3Amal ..Noch keine Bewertungen
- 7 LAB Sp14KineticsDokument10 Seiten7 LAB Sp14KineticsLaura RiceNoch keine Bewertungen
- AP Chemistry - Finding The Ratio of Moles of Reactants in A Chemical ReactionDokument4 SeitenAP Chemistry - Finding The Ratio of Moles of Reactants in A Chemical ReactionJonathan Chen88% (8)
- Factors Affecting Enzyme ActivityDokument6 SeitenFactors Affecting Enzyme ActivityDrewNoch keine Bewertungen
- Chem A 13 Comp EnthalpyDokument5 SeitenChem A 13 Comp EnthalpyKrystela Cariza Ramos MercadoNoch keine Bewertungen
- Method - Calculations ChemistryDokument3 SeitenMethod - Calculations ChemistrygamebozNoch keine Bewertungen
- Kinetics But Yl ChlorideDokument8 SeitenKinetics But Yl ChlorideNicole HuertaNoch keine Bewertungen
- Kinetics of a Reaction Rate LawDokument15 SeitenKinetics of a Reaction Rate LawSebatHian Santiago60% (5)
- Gizmo Collision TheoryDokument6 SeitenGizmo Collision TheoryDanitza RojasNoch keine Bewertungen
- Effect of Temp & pH on EnzymeDokument3 SeitenEffect of Temp & pH on Enzymejuliakang03Noch keine Bewertungen
- Iodine Clock A2 CourseworkDokument7 SeitenIodine Clock A2 Courseworkafjwsbgkjdhkwz100% (2)
- Determining Enthalpy of Chemical ReactionDokument5 SeitenDetermining Enthalpy of Chemical ReactionCristian AlamosNoch keine Bewertungen
- Lab Report Exp 1Dokument15 SeitenLab Report Exp 1Justine Camille CastilloNoch keine Bewertungen
- Chap11 StoichiometryDokument34 SeitenChap11 StoichiometryAnonymous Ajd486NNoch keine Bewertungen
- The Kinetic Study of The IodinationDokument6 SeitenThe Kinetic Study of The IodinationsamNoch keine Bewertungen
- Exp 4 Kinetics Order of ReactionDokument8 SeitenExp 4 Kinetics Order of ReactionNur Fadhilah0% (1)
- LE 005 007 General Chemistry 1 Continuation .Updated FinalDokument26 SeitenLE 005 007 General Chemistry 1 Continuation .Updated FinalShaman KingNoch keine Bewertungen
- The Indian SchoolDokument21 SeitenThe Indian SchoolSabreena BasheerNoch keine Bewertungen
- Verification of the Lever Rule Using Liquid-Liquid EquilibriaDokument1 SeiteVerification of the Lever Rule Using Liquid-Liquid EquilibriaJanoIgnacioNoch keine Bewertungen
- Detailed Notes Topic 5 Formulae Equations and Amounts of Substance Edexcel Chemistry A LevelDokument7 SeitenDetailed Notes Topic 5 Formulae Equations and Amounts of Substance Edexcel Chemistry A LevelttjjjNoch keine Bewertungen
- Lab #4 - FinalDokument8 SeitenLab #4 - FinalEmmaNoch keine Bewertungen
- 1.2 TechDokument4 Seiten1.2 TechĐinh Văn BắcNoch keine Bewertungen
- Exothermic and Endothermic ReactionsDokument2 SeitenExothermic and Endothermic ReactionsHoward KacheNoch keine Bewertungen
- Ox Alic Acid KineticsDokument10 SeitenOx Alic Acid Kineticsmkhurram79Noch keine Bewertungen
- 4 UVVis TroubleshootingDokument2 Seiten4 UVVis TroubleshootingYoya LoyaNoch keine Bewertungen
- VCE Chemistry Unit 3 SAC 2: Chemical Equilibrium ExperimentsDokument5 SeitenVCE Chemistry Unit 3 SAC 2: Chemical Equilibrium ExperimentsJefferyNoch keine Bewertungen
- Last Class: Classifying Process TypesDokument43 SeitenLast Class: Classifying Process TypesStevenNoch keine Bewertungen
- Experiment 2 Chemical Kinetics: Rate of Disappearance of CV Rate of Appearance of CVOHDokument8 SeitenExperiment 2 Chemical Kinetics: Rate of Disappearance of CV Rate of Appearance of CVOHAbd El-Fattah Mohamed OufNoch keine Bewertungen
- Shayma Chem II Lab Manual.... Petrochemical Engineering DepartmentDokument55 SeitenShayma Chem II Lab Manual.... Petrochemical Engineering DepartmentMUHAMMAD AKRAM100% (1)
- Additivity of Heats of Reaction: Hess's LawDokument5 SeitenAdditivity of Heats of Reaction: Hess's Lawnipuna920% (1)
- Chemistry Lab Research PaperDokument4 SeitenChemistry Lab Research Paperxvszcorif100% (1)
- Liquid-Liquid Equilibria: Verification of The Lever Rule: A. D. JordanDokument1 SeiteLiquid-Liquid Equilibria: Verification of The Lever Rule: A. D. JordanblastingawayNoch keine Bewertungen
- Colorimeter 1Dokument2 SeitenColorimeter 1Eng CirroNoch keine Bewertungen
- Determine Order of ReactionDokument10 SeitenDetermine Order of ReactionHusna Insyirah Bt SamadNoch keine Bewertungen
- Producing Exactly 2.00 Grams of A Compound Lab MSDSDokument2 SeitenProducing Exactly 2.00 Grams of A Compound Lab MSDSMichael Kevin YangNoch keine Bewertungen
- Lab Manual Phys 1Dokument8 SeitenLab Manual Phys 1Sammy Njenga KhanNoch keine Bewertungen
- Exp 1,2,3Dokument13 SeitenExp 1,2,3JWAN RA YA3QOBNoch keine Bewertungen
- AP Chemistry - Kinetics of A Reaction LabDokument8 SeitenAP Chemistry - Kinetics of A Reaction LabJonathan Chen50% (2)
- Assignment For Sessional: Environmental Chemistry - (CHEM-431)Dokument25 SeitenAssignment For Sessional: Environmental Chemistry - (CHEM-431)sawaira ikramNoch keine Bewertungen
- Exp 3Dokument3 SeitenExp 3Crystal VangelineNoch keine Bewertungen
- Lab Practice #1Dokument5 SeitenLab Practice #1Lucero AguilarNoch keine Bewertungen
- University of Northeastern Philippines (UNEP) Iriga CityDokument9 SeitenUniversity of Northeastern Philippines (UNEP) Iriga CityNica GumbaNoch keine Bewertungen
- Rate of A Chemical Reaction Using Colorimetry Oxidation of Propan2olDokument4 SeitenRate of A Chemical Reaction Using Colorimetry Oxidation of Propan2olArham AdnanNoch keine Bewertungen
- Enzyme Action - Testing Catalase ActivityDokument7 SeitenEnzyme Action - Testing Catalase ActivitytahamidNoch keine Bewertungen
- Lab Chem - Rate of ReactionDokument7 SeitenLab Chem - Rate of Reactionapi-351094730Noch keine Bewertungen
- Experiment 6-Molar Mass of A GasDokument7 SeitenExperiment 6-Molar Mass of A GasSoso AnoosNoch keine Bewertungen
- Thermometric Titrimetry: International Series of Monographs in Analytical ChemistryVon EverandThermometric Titrimetry: International Series of Monographs in Analytical ChemistryNoch keine Bewertungen
- O Level Biology Practice Questions And Answers EnzymesVon EverandO Level Biology Practice Questions And Answers EnzymesBewertung: 5 von 5 Sternen5/5 (1)
- Thermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeVon EverandThermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeNoch keine Bewertungen
- Resumen: Karen, Fernanda y BrandonDokument4 SeitenResumen: Karen, Fernanda y BrandonIvan Alberto NinaNoch keine Bewertungen
- Cta 1 SecDokument22 SeitenCta 1 SecIvan Alberto NinaNoch keine Bewertungen
- 1237 Expt1 - Qual2008Dokument26 Seiten1237 Expt1 - Qual2008Reborn TayNoch keine Bewertungen
- 综合练习题及答案 第4套 PDFDokument7 Seiten综合练习题及答案 第4套 PDFIvan Alberto NinaNoch keine Bewertungen
- 2425 Chapt 13Dokument10 Seiten2425 Chapt 13Ivan Alberto NinaNoch keine Bewertungen
- PPTT HuaynacapacDokument4 SeitenPPTT HuaynacapacIvan Alberto NinaNoch keine Bewertungen
- GUI2005 Biodiversity in Impact Assessment - IAIADokument4 SeitenGUI2005 Biodiversity in Impact Assessment - IAIAsusCitiesNoch keine Bewertungen
- 66kv Earthing System R-1 (For Print Out)Dokument8 Seiten66kv Earthing System R-1 (For Print Out)g_nraja100% (1)
- Manufacturing of Turbo GenratorsDokument27 SeitenManufacturing of Turbo Genratorspavan6754Noch keine Bewertungen
- A Critical Analysis of The Erasmus Bridge:: Christopher J. HewettDokument10 SeitenA Critical Analysis of The Erasmus Bridge:: Christopher J. HewettAndik Mardiyanto MellodyNoch keine Bewertungen
- Greenwich Apparent Sidereal Time: Function Gast1Dokument3 SeitenGreenwich Apparent Sidereal Time: Function Gast1GERMANCRICKETNoch keine Bewertungen
- 5 Longitudinal and Transverse VibrationsDokument4 Seiten5 Longitudinal and Transverse Vibrationsenggsantu0% (1)
- Static Pressure Concept 1Dokument8 SeitenStatic Pressure Concept 1bharath477Noch keine Bewertungen
- A - 1 - Product Overview - EN - 20190725 - W Botones AutonicsDokument10 SeitenA - 1 - Product Overview - EN - 20190725 - W Botones Autonicsjcflores.mayaNoch keine Bewertungen
- Infinity Other Solar Systems: TrinityDokument1 SeiteInfinity Other Solar Systems: TrinityP Eng Suraj SinghNoch keine Bewertungen
- PHYSICS (9702) Rules.Dokument5 SeitenPHYSICS (9702) Rules.Sheraz Ahmed - 65640/Laboratory Assistant/BBECNoch keine Bewertungen
- Introduction to Structural AnalysisDokument2 SeitenIntroduction to Structural AnalysisMelvin EsguerraNoch keine Bewertungen
- PreviewpdfDokument63 SeitenPreviewpdf20BM002 AKSHAYANoch keine Bewertungen
- Abaqus Tutorial2Dokument40 SeitenAbaqus Tutorial2asimbuyukNoch keine Bewertungen
- Physics 4 O LevelDokument3 SeitenPhysics 4 O LevelChrispin Msofe100% (1)
- Pumps and Systems MagazineDokument68 SeitenPumps and Systems MagazineMohamed Abdel BasitNoch keine Bewertungen
- Overview of 7FB Turbines PDFDokument22 SeitenOverview of 7FB Turbines PDFNikhil MalhotraNoch keine Bewertungen
- CP395RG 3-9x50mm Owner'S Manual: WarningDokument2 SeitenCP395RG 3-9x50mm Owner'S Manual: WarningFrédéric NannNoch keine Bewertungen
- Reliability Based DesignDokument11 SeitenReliability Based DesigntohemaNoch keine Bewertungen
- C Vm2 Protection From Fire Amendment 3Dokument70 SeitenC Vm2 Protection From Fire Amendment 3yunying21100% (1)
- Pcord - Exemple Envirenemment AnalysisDokument288 SeitenPcord - Exemple Envirenemment Analysisقادة قناية100% (1)
- Cha 2Dokument52 SeitenCha 2yaredNoch keine Bewertungen
- 2003, 94665723-New-energy-technologies-Issue-12 PDFDokument81 Seiten2003, 94665723-New-energy-technologies-Issue-12 PDFJosefina SilveyraNoch keine Bewertungen
- 805 Model Answer Paper Summer 2018 PDFDokument26 Seiten805 Model Answer Paper Summer 2018 PDFDipu Kumar50% (2)
- Finite Element Simulation of Non LinearDokument11 SeitenFinite Element Simulation of Non LinearmostafaNoch keine Bewertungen
- Heating Systems in Buildings - Method For Calculation of System Energy Requirements and System EfficienciesDokument22 SeitenHeating Systems in Buildings - Method For Calculation of System Energy Requirements and System EfficienciesDžana Kadrić100% (1)
- Nonlinear Equations Reduction of Order-When y or X Are MissingDokument3 SeitenNonlinear Equations Reduction of Order-When y or X Are Missingakabaka123Noch keine Bewertungen
- AssignmentDokument3 SeitenAssignmentPrakhar0112Noch keine Bewertungen
- Strength Characteristics of Fly Ash Mixed With Lime Stabilized SoilDokument4 SeitenStrength Characteristics of Fly Ash Mixed With Lime Stabilized SoilCosminNoch keine Bewertungen
- CST Math 2015 - Day 10 - Situational ProblemsDokument20 SeitenCST Math 2015 - Day 10 - Situational Problemsapi-245317729Noch keine Bewertungen
- Chapter 1 Introduction To Physics StudentDokument18 SeitenChapter 1 Introduction To Physics StudentAizat ArifNoch keine Bewertungen
- 31-XX29-6Dokument1 Seite31-XX29-6rohitkush100% (1)