Beruflich Dokumente
Kultur Dokumente
Monthly Test
Hochgeladen von
Khondokar TarakkyOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Monthly Test
Hochgeladen von
Khondokar TarakkyCopyright:
Verfügbare Formate
The City School International Subject: Chemistry Name: Class: Senior I Marks: 30 1 Which statement is correct about sulphur,
atomic number 16? 1 A sulphur can form the ion S2-. B sulphur dissolves in water to form sulphuric acid. C sulphur forms ionic oxides. D sulphur will react with metals to produce S6+ ions. 2 The atoms P and S have the same 1 A nucleon number B number of electrons C number of neutrons D number of protons 3 An element X has 2 isotopes, which may be represented by 238X and 235X. How does 238X differ from 235X? A it has 3 more protons and 3 more electrons 1 B it has 3 more protons but no more electrons C it has 3 more neutrons and 3 more electrons D it has 3 more neutrons but no more electrons 4 Which ion has the most shells that contain electrons? 1 3+ 2+ 32A Al B Be C N D S 5 How does a magnesium atom form a bond with an oxygen atom? 1 A by giving one pair of electrons to the oxygen atom B by sharing one pair of electrons, both provided by the magnesium atom C by sharing 2 pairs of electrons, both pairs provided by the oxygen atom D by sharing 2 pairs of electrons each atom donating one pair of electrons 6 In which of the following pairs do the elements form a compound by sharing electrons? 1 A carbon and chlorine B lithium and iodine C neon and oxygen D potassium and bromine 7 Which electron arrangement is that of a metallic element? 1 A 2,1 B 2,4 C 2,5 D 2,7 8 Which molecules have the same number of protons? 1 A O2 and N2 B Cl2 and Br2 C CO2 and SO2 D CH4 and NH3 9 An ion X+ has 23 mass number and 10 electrons. What does the nucleus of the ion X+ contain? 1 proton
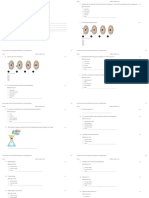
A B C D 12 11 10 9
neutron
11 12 13 14
10 In which set do the three particles each have the same total number of electrons? A ClBrIB FNe Na+ + 2+ C K Ca BrD Li+ Na+ K+
The City School International Subject: Chemistry Name: Class: Senior I 11 Give some examples of atom, molecule and compound.
Marks: 30 3
12 Fluorine can form either covalent or ionic bonds. Draw a dot-and-cross diagram to show the bonding in i) sodium fluoride, NaF ii) fluorine, F2 Your diagrams must show all the electrons. 13. The diagram below shows the outer shell electrons of an atom of element X. 4 4
a) To which group of the Periodic table does X belong? b) Draw a dot and cross diagram to show the bonding in the compound of X with carbon. Your diagram should show outer electrons only. c) (i) Write the formula for the compound of element X with sodium. (ii) Predict the type of bonding present in the compound of X with sodium. 14. Magnesium reacts with chlorine to form the ionic compound magnesium chloride. 4 a) Draw a dot and cross diagram to show the bonding in magnesium chloride. You only need to draw the outer (valence) electrons of magnesium and of chlorine. b) The physical properties of a compound are related to its structure and bonding. Magnesium chloride has an ionic lattice structure. Suggest two physical properties of magnesium chloride. 15. Tritium is an isotope of hydrogen. An ion of tritium has the following structure. 5
a) Complete the following table to show the names and charges of the particles in this tritium ion. symbol name neutron charge +1 -1
b) Using the symbol T to represent tritium, give the formulae of i) the ion shown above ii) the compound formed between tritium and sodium c) Would you expect the oxide of tritium to be a solid, a liquid or a gas? Explain your reasoning.
Das könnte Ihnen auch gefallen
- Quiz On COvalentDokument2 SeitenQuiz On COvalentAllan RoyNoch keine Bewertungen
- ExamView - CHP 3 QuizDokument5 SeitenExamView - CHP 3 Quizkylev100% (1)
- Covennatone Line Notation PDFDokument5 SeitenCovennatone Line Notation PDFifiokNoch keine Bewertungen
- Ionic and Covalent BondsDokument5 SeitenIonic and Covalent Bondsapi-233981890Noch keine Bewertungen
- MCW (Chemistry) HURDCO International School Grade VII A: Name: . Total Marks: 10 Duration: 40 Mins. 1Dokument2 SeitenMCW (Chemistry) HURDCO International School Grade VII A: Name: . Total Marks: 10 Duration: 40 Mins. 1Khondokar TarakkyNoch keine Bewertungen
- Quiz LetDokument5 SeitenQuiz LetFarihah FazimNoch keine Bewertungen
- Mass Spectrometry ADokument39 SeitenMass Spectrometry AToader GeorgianNoch keine Bewertungen
- LSM Grade 3 CLED 1st Trim Exam SY 2009-2010 Answer KeyDokument6 SeitenLSM Grade 3 CLED 1st Trim Exam SY 2009-2010 Answer KeyMauie FloresNoch keine Bewertungen
- BiomoleculesDokument12 SeitenBiomoleculesHarshita Singh TanwarNoch keine Bewertungen
- Catcher in The Rye All Blog QuestionsDokument7 SeitenCatcher in The Rye All Blog Questionsapi-281167422Noch keine Bewertungen
- Ad II (Ae2303) (Univ QP, Nov2010)Dokument3 SeitenAd II (Ae2303) (Univ QP, Nov2010)aeroacademicNoch keine Bewertungen
- Quiz 1Dokument3 SeitenQuiz 1Greg LoncaricNoch keine Bewertungen
- Tutorial 2Dokument4 SeitenTutorial 2GowriprasadHanumanNoch keine Bewertungen
- Assignment 1Dokument2 SeitenAssignment 1Greg LoncaricNoch keine Bewertungen
- Aerodynamics-2 Two Mark QuestionsDokument15 SeitenAerodynamics-2 Two Mark QuestionsArun Raja K KNoch keine Bewertungen
- EntropyDokument2 SeitenEntropyFaisal ARNoch keine Bewertungen
- Exercise I.: Field of Experience Field of ExperienceDokument3 SeitenExercise I.: Field of Experience Field of ExperiencePrecious FernandezNoch keine Bewertungen
- Acids and BasesDokument8 SeitenAcids and BasesJoric MagusaraNoch keine Bewertungen
- AMSOIL Material Safety Data Sheet: Nfpa & Hmis RatingDokument4 SeitenAMSOIL Material Safety Data Sheet: Nfpa & Hmis Ratingapi-19921780Noch keine Bewertungen
- Chapter 2 Lesson 1 Self CheckDokument2 SeitenChapter 2 Lesson 1 Self CheckAndrew BondadNoch keine Bewertungen
- Following Are A Few Basic Points One Must Keep in MindDokument1 SeiteFollowing Are A Few Basic Points One Must Keep in MindKarthik BoopathyNoch keine Bewertungen
- 2012-05-06 07.03.45 CrashDokument14 Seiten2012-05-06 07.03.45 Crashelln1806Noch keine Bewertungen
- ExamDokument4 SeitenExamMichael RojoNoch keine Bewertungen
- Summary of Bernard Leach.Dokument3 SeitenSummary of Bernard Leach.Sewella BarnesNoch keine Bewertungen
- Double and Triple Bond (Covalent Bond) No.27Dokument3 SeitenDouble and Triple Bond (Covalent Bond) No.27ONG TEIK MING -Noch keine Bewertungen
- 2012 June Exam Y10 Chemistry BDF New CourseDokument6 Seiten2012 June Exam Y10 Chemistry BDF New CourseLeilaNoch keine Bewertungen
- Focus 3 Chemical BondingDokument10 SeitenFocus 3 Chemical BondingHengLow100% (1)
- Chem F4 Mid ExamDokument10 SeitenChem F4 Mid ExamYong SiewkuanNoch keine Bewertungen
- Adii Quest BankDokument13 SeitenAdii Quest Bankvinod kumarNoch keine Bewertungen
- Chemistry: Writing Ionic Formulas For CompoundsDokument5 SeitenChemistry: Writing Ionic Formulas For CompoundsTiffany GallinaNoch keine Bewertungen
- Facts About Insects and BugsDokument18 SeitenFacts About Insects and BugsNikita YadavNoch keine Bewertungen
- Chemical Bond QuizDokument5 SeitenChemical Bond QuizBrielle Kyle O. SERRANO0% (1)
- 1to100MCQssolved100correct PDFDokument17 Seiten1to100MCQssolved100correct PDFAmjadNoch keine Bewertungen
- The Catcher in The Rye - ReviewDokument2 SeitenThe Catcher in The Rye - ReviewIoana MironNoch keine Bewertungen
- Covalent Bonding Lewis Structure WebquestDokument16 SeitenCovalent Bonding Lewis Structure WebquestDean JezerNoch keine Bewertungen
- Anna UniversityDokument4 SeitenAnna UniversityAnonymous RJfsy8PtNoch keine Bewertungen
- Quiz FinallDokument12 SeitenQuiz FinallHông HuángNoch keine Bewertungen
- Quiz 5Dokument1 SeiteQuiz 5Luis de leon100% (2)
- Pericyclic ReactionDokument6 SeitenPericyclic ReactionUrugonda VenumadhavNoch keine Bewertungen
- Final Test Chemistry 10Dokument5 SeitenFinal Test Chemistry 10rohmatul aziziNoch keine Bewertungen
- Chemistry Quiz 4Dokument2 SeitenChemistry Quiz 4NoniaqAmadNoch keine Bewertungen
- Exam # 2 Chemistry 208, Organic Chemistry I Spring 2016: Your Name: - Laboratory SectionDokument6 SeitenExam # 2 Chemistry 208, Organic Chemistry I Spring 2016: Your Name: - Laboratory SectionHieyeNoch keine Bewertungen
- Introduction To FLIP FLOPSDokument16 SeitenIntroduction To FLIP FLOPSOliver Barrina JaguinesNoch keine Bewertungen
- Arcjet RocketDokument1 SeiteArcjet RocketBinod Da Perfectsoul0% (1)
- Chapter 5 QuestionsDokument68 SeitenChapter 5 Questions06-087Noch keine Bewertungen
- Chapter 21 Further Aspects of EquilibriaDokument6 SeitenChapter 21 Further Aspects of EquilibriaAndrea MelissaNoch keine Bewertungen
- Nmat Biology Mock Simulations 2 (1116) DIRECTIONS: Select The Best Answer To Each of TheDokument4 SeitenNmat Biology Mock Simulations 2 (1116) DIRECTIONS: Select The Best Answer To Each of TheHanny CapsNoch keine Bewertungen
- 7.3 Oxidation Pond Question Answer BaruDokument2 Seiten7.3 Oxidation Pond Question Answer BaruIbrahim MuhamadNoch keine Bewertungen
- Catcher&Rye QuestionsDokument2 SeitenCatcher&Rye Questionsojhadog1Noch keine Bewertungen
- CM011 ReviewerDokument5 SeitenCM011 ReviewerSofia Isabelle GarciaNoch keine Bewertungen
- General Chemistry Quiz - PrelimDokument5 SeitenGeneral Chemistry Quiz - PrelimRochelle Anne Abad BandaNoch keine Bewertungen
- Ch.6 PracticeQuestionsDokument19 SeitenCh.6 PracticeQuestionsLiew Dong YeeNoch keine Bewertungen
- Comsats University Islamabad, Abbottabad Campus Afghan Students "Zero Semester" Biology Quiz Mcqs Name - Reg# - Time: 40 MinDokument3 SeitenComsats University Islamabad, Abbottabad Campus Afghan Students "Zero Semester" Biology Quiz Mcqs Name - Reg# - Time: 40 Minanon_572243106Noch keine Bewertungen
- APEF Electrochem MC Ans PDFDokument2 SeitenAPEF Electrochem MC Ans PDFMuhammad UsmanNoch keine Bewertungen
- IGCSE CHEMISTRY (Katryne)Dokument7 SeitenIGCSE CHEMISTRY (Katryne)PriyantoBudiLaksonoNoch keine Bewertungen
- TT2.1 - Ionic and Covalent BondDokument9 SeitenTT2.1 - Ionic and Covalent BondDaniel VictoriaNoch keine Bewertungen
- Sample Quiz For LACAS 9THDokument4 SeitenSample Quiz For LACAS 9THShehbaz YaseenNoch keine Bewertungen
- U04 Notes Part2 Shapes PolarityDokument49 SeitenU04 Notes Part2 Shapes PolarityKhondokar Tarakky100% (1)
- Writing Ionic FormulaeDokument6 SeitenWriting Ionic FormulaeKhondokar TarakkyNoch keine Bewertungen
- U04 Notes Part4 Intermolecular ForcesDokument66 SeitenU04 Notes Part4 Intermolecular ForcesKhondokar TarakkyNoch keine Bewertungen
- U04 Notes Part5 Metals Physical PropertiesDokument43 SeitenU04 Notes Part5 Metals Physical PropertiesKhondokar TarakkyNoch keine Bewertungen
- U04 Notes Part3 Sp3d2 DelocalizationDokument54 SeitenU04 Notes Part3 Sp3d2 DelocalizationKhondokar TarakkyNoch keine Bewertungen
- Hybridization TarakkyDokument36 SeitenHybridization TarakkyKhondokar TarakkyNoch keine Bewertungen
- Air and WaterDokument12 SeitenAir and WatermirnaNoch keine Bewertungen
- U05 Notes Part3 Energy CyclesDokument29 SeitenU05 Notes Part3 Energy CyclesKhondokar TarakkyNoch keine Bewertungen
- U04 Notes Part1 Ionic CovalentDokument52 SeitenU04 Notes Part1 Ionic CovalentKhondokar TarakkyNoch keine Bewertungen
- U05 Notes Part1 Heat CalorimDokument32 SeitenU05 Notes Part1 Heat CalorimKhondokar TarakkyNoch keine Bewertungen
- U05 Notes Part2 Bond Enthalpy HessDokument17 SeitenU05 Notes Part2 Bond Enthalpy HessKhondokar TarakkyNoch keine Bewertungen
- Q NmrH1highresDokument5 SeitenQ NmrH1highresKhondokar TarakkyNoch keine Bewertungen
- U05 Notes Part4 Entropy SpontaneityDokument47 SeitenU05 Notes Part4 Entropy SpontaneityKhondokar TarakkyNoch keine Bewertungen
- 9701 m17 QP 12Dokument16 Seiten9701 m17 QP 12Khondokar TarakkyNoch keine Bewertungen
- 7038 02BangladeshStudiesDokument8 Seiten7038 02BangladeshStudiesKhondokar TarakkyNoch keine Bewertungen
- Drying Agent and Dehydrating AgentDokument1 SeiteDrying Agent and Dehydrating AgentKhondokar TarakkyNoch keine Bewertungen
- All A2 Level Terms and DefinationsDokument0 SeitenAll A2 Level Terms and DefinationsHussain MustafaNoch keine Bewertungen
- CT On at STR For VII SeptDokument4 SeitenCT On at STR For VII SeptKhondokar TarakkyNoch keine Bewertungen
- Naming WorksheetsDokument9 SeitenNaming WorksheetsKhondokar TarakkyNoch keine Bewertungen
- Naming WorksheetsDokument9 SeitenNaming WorksheetsKhondokar TarakkyNoch keine Bewertungen
- Q RateexptsDokument3 SeitenQ RateexptsKhondokar TarakkyNoch keine Bewertungen
- H-1 NMR: Low Resolution: Chemical ShiftsDokument1 SeiteH-1 NMR: Low Resolution: Chemical ShiftsKhondokar TarakkyNoch keine Bewertungen
- H-1 NMR: Introduction: Kms TarakkyDokument2 SeitenH-1 NMR: Introduction: Kms TarakkyKhondokar TarakkyNoch keine Bewertungen
- Chapter 1 Kinetic Theory and DiffusionDokument4 SeitenChapter 1 Kinetic Theory and DiffusionKhondokar TarakkyNoch keine Bewertungen
- Answer All The Questions in This Section in The Spaces Provided. The Total Mark For This Section Is 45Dokument20 SeitenAnswer All The Questions in This Section in The Spaces Provided. The Total Mark For This Section Is 45Khondokar TarakkyNoch keine Bewertungen
- The Mass Spectrometer: Kms TarakkyDokument2 SeitenThe Mass Spectrometer: Kms TarakkyKhondokar TarakkyNoch keine Bewertungen
- Q MsmplusDokument1 SeiteQ MsmplusKhondokar TarakkyNoch keine Bewertungen
- Finding Orders of Reaction Experimentally: Chemguide - AnswersDokument2 SeitenFinding Orders of Reaction Experimentally: Chemguide - AnswersKhondokar TarakkyNoch keine Bewertungen
- Fragmentation Patterns: Kms TarakkyDokument2 SeitenFragmentation Patterns: Kms TarakkyKhondokar TarakkyNoch keine Bewertungen
- Mass Spectra of Elements: Kms TarakkyDokument1 SeiteMass Spectra of Elements: Kms TarakkyKhondokar TarakkyNoch keine Bewertungen
- Sustainability 10 00604Dokument11 SeitenSustainability 10 00604Besmir BeqiriNoch keine Bewertungen
- CPP & Cqa PDFDokument71 SeitenCPP & Cqa PDFanon_695264516100% (1)
- Corrosion and It's Control: Course Name: Chemistry Course Code: CHL-101Dokument84 SeitenCorrosion and It's Control: Course Name: Chemistry Course Code: CHL-101054- Siva manoj reddyNoch keine Bewertungen
- PembahasaaannDokument4 SeitenPembahasaaannWahyu AdamNoch keine Bewertungen
- Ficha Tecnica - BS EN 1982 CuSn7Zn4Pb7-C (CC493K)Dokument1 SeiteFicha Tecnica - BS EN 1982 CuSn7Zn4Pb7-C (CC493K)freddy benavidesNoch keine Bewertungen
- Bismuth Crackle W-Notes by Ned G - 2 PDFDokument6 SeitenBismuth Crackle W-Notes by Ned G - 2 PDFPijush SarkarNoch keine Bewertungen
- Absorption in Sieve PlateDokument13 SeitenAbsorption in Sieve PlateDEEPAK KUMARNoch keine Bewertungen
- Verniz OffsetDokument11 SeitenVerniz OffsetCelso Prado da SilvaNoch keine Bewertungen
- Petrochemical Processes 1 Alain Chauvel Handbook PDFDokument420 SeitenPetrochemical Processes 1 Alain Chauvel Handbook PDFZander_83% (12)
- Hubungan Jarak Tempuh Dengan Kadar Sisa Chlor Bebas Dan MPN Coliform Di Pdam Reservoir Medini Kudus Noor Zahrotul M, Nurjazuli, TrijokoDokument8 SeitenHubungan Jarak Tempuh Dengan Kadar Sisa Chlor Bebas Dan MPN Coliform Di Pdam Reservoir Medini Kudus Noor Zahrotul M, Nurjazuli, TrijokoUni_31Noch keine Bewertungen
- Andés, L. (1920) "Animal Fats and Oils", 3rd Edition. D. Van Nostrand Company. New York.Dokument332 SeitenAndés, L. (1920) "Animal Fats and Oils", 3rd Edition. D. Van Nostrand Company. New York.Isabel RinconNoch keine Bewertungen
- Astm D2000 Standard Classificaion For Rubber ProductsDokument5 SeitenAstm D2000 Standard Classificaion For Rubber Productsjmj0% (1)
- Din en 1706 Ac - 71100Dokument1 SeiteDin en 1706 Ac - 71100Anudeep NittalaNoch keine Bewertungen
- Evaporators SugarDokument28 SeitenEvaporators SugarAnkur KoulNoch keine Bewertungen
- Muhammad Athar Mahmood 2018 (S) - MS-AME-17 Research Supervisor: Dr. Ghulam Moeen Uddin 2022Dokument79 SeitenMuhammad Athar Mahmood 2018 (S) - MS-AME-17 Research Supervisor: Dr. Ghulam Moeen Uddin 2022Ghanva KhanNoch keine Bewertungen
- Calculo de PH Agua Cervejeira English IPADokument4 SeitenCalculo de PH Agua Cervejeira English IPAFrederico Luiz de SáNoch keine Bewertungen
- Pharmaceutical Incompatibility: Dr. MurtazaDokument38 SeitenPharmaceutical Incompatibility: Dr. MurtazaAneeza AhmedNoch keine Bewertungen
- DPR Vignesh September-2014Dokument1.423 SeitenDPR Vignesh September-2014alpanakaurNoch keine Bewertungen
- Lab Manual 2012-2013Dokument65 SeitenLab Manual 2012-2013boobooNoch keine Bewertungen
- ColaLube 3404Dokument2 SeitenColaLube 3404mndmattNoch keine Bewertungen
- .Ph-Just A Look Visual Symptoms of Nutrient Deficiencies in Plants and How To Treat ThemDokument8 Seiten.Ph-Just A Look Visual Symptoms of Nutrient Deficiencies in Plants and How To Treat ThemVicente SalanapNoch keine Bewertungen
- High Performance Liquid Chromatography (HPLC)Dokument11 SeitenHigh Performance Liquid Chromatography (HPLC)Benjamin DanielNoch keine Bewertungen
- Flyer TripleCoatingsDokument12 SeitenFlyer TripleCoatingshirafarooq2000Noch keine Bewertungen
- 1A.1 (The Chemistry of Life) - : Difference Between Ionic Substances, Charged Particles and Polar MoleculesDokument2 Seiten1A.1 (The Chemistry of Life) - : Difference Between Ionic Substances, Charged Particles and Polar MoleculesFatimah AfzalNoch keine Bewertungen
- Capitulo 8 Libro Oxidacion CataliticaDokument11 SeitenCapitulo 8 Libro Oxidacion CataliticaAldair Orozco UlloaNoch keine Bewertungen
- ASME-Notch-Toughness & Supplementary Essential VariablesDokument16 SeitenASME-Notch-Toughness & Supplementary Essential VariablesSuleyman Halicioglu100% (2)
- DPS 2011 2 6 127 131Dokument5 SeitenDPS 2011 2 6 127 131anuradha.d.bhat9860Noch keine Bewertungen
- Composites IntroductionDokument112 SeitenComposites Introductionsanthosh smartNoch keine Bewertungen
- PowerpointDokument59 SeitenPowerpointapi-377597450% (2)
- Aldehyde and KetoneDokument39 SeitenAldehyde and KetoneCitra Siti PurnamaNoch keine Bewertungen
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeVon EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeBewertung: 5 von 5 Sternen5/5 (4)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincVon EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincBewertung: 3.5 von 5 Sternen3.5/5 (137)
- How to Teach Nature Journaling: Curiosity, Wonder, AttentionVon EverandHow to Teach Nature Journaling: Curiosity, Wonder, AttentionBewertung: 4.5 von 5 Sternen4.5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeVon EverandChemistry for Breakfast: The Amazing Science of Everyday LifeBewertung: 4.5 von 5 Sternen4.5/5 (90)
- A-level Biology Revision: Cheeky Revision ShortcutsVon EverandA-level Biology Revision: Cheeky Revision ShortcutsBewertung: 5 von 5 Sternen5/5 (5)
- Lower Secondary Science Workbook: Stage 8Von EverandLower Secondary Science Workbook: Stage 8Bewertung: 5 von 5 Sternen5/5 (1)
- STEM Labs for Physical Science, Grades 6 - 8Von EverandSTEM Labs for Physical Science, Grades 6 - 8Bewertung: 3.5 von 5 Sternen3.5/5 (6)
- Quantum Physics for Beginners: Simple Illustrated Guide to Discover with Practical Explanations the Paradoxes of the Life and Universe Reconsidering RealityVon EverandQuantum Physics for Beginners: Simple Illustrated Guide to Discover with Practical Explanations the Paradoxes of the Life and Universe Reconsidering RealityBewertung: 2 von 5 Sternen2/5 (1)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeVon EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeBewertung: 4 von 5 Sternen4/5 (1)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsVon EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsBewertung: 4 von 5 Sternen4/5 (146)
- Interactive Science Notebook: The Human Body WorkbookVon EverandInteractive Science Notebook: The Human Body WorkbookBewertung: 4 von 5 Sternen4/5 (2)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactVon EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactBewertung: 5 von 5 Sternen5/5 (5)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolVon EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNoch keine Bewertungen
- How Do Cell Phones Work? Technology Book for Kids | Children's How Things Work BooksVon EverandHow Do Cell Phones Work? Technology Book for Kids | Children's How Things Work BooksNoch keine Bewertungen
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableVon EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableBewertung: 3.5 von 5 Sternen3.5/5 (22)
- Nature-Based Learning for Young Children: Anytime, Anywhere, on Any BudgetVon EverandNature-Based Learning for Young Children: Anytime, Anywhere, on Any BudgetBewertung: 5 von 5 Sternen5/5 (1)