Beruflich Dokumente
Kultur Dokumente
A Convenient Method For Chlorination in Allylic Position
Hochgeladen von
chidambaramrOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
A Convenient Method For Chlorination in Allylic Position
Hochgeladen von
chidambaramrCopyright:
Verfügbare Formate
CHEM . RES . CHIN ESE U .
2005, 21( 2) , 169 171
A Convenient Method for Chlorination in Allylic Position
WANG Xin-yan, SH I Hong-chang * , CHEN Gang, HONG Xiao y in and XU Shou-yi Dep ar tment of Chemistry , T si nghua Univer sity , Beij ing 100084, P . R . China
Receiv ed M a rch 17, 2004 A conv enient method for the chlor ination in ally lic positio n w as dev elo ped by using the aqueo us solutio n o f so dium hypochlor ite ( 2% _ 5% activ e chlo r ine) and an acid as chlor ination r eag ent in a diphase system . T he method has the advantag e of cheap r eagents, mild reaction conditio ns and g oo d yields. T he quantity and t he feeding r ate o f the chlor inatio n r ea gent can be co nt ro lled easily . T he met ho d is par ticula rly suit able fo r t he chlor inatio n in labo rato r ies. Keywords Chlor ination , A lly lic po sitio n , Sodium hypo chlor ite , Diphase system Articl e ID 10059040( 2005) 0216904
Introduction
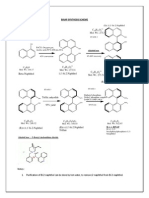
T he chl orinat ion in allylic po sit ion of olefins is an import ant t ransfo rmat io n in or ganic synt hesis and many procedures have been est ablished. F or example, t he chlo rinat ion of dithioazet idino ne ( compound 1 , see Scheme 1, an im po rtant synt het ic int ermediat e of cephalospor in ant ibiot ics) can be co mpl et ed by elect ro -chlorination or by using chemical r eag ent s. No rmally , elect rochlor inat ion[ 1_ 4] has a g ood select ivit y in hig h y ields( 80% _ 90% ) , but it is cost ly . T he most com mon reagent s, such as t -but yl hypochl orit e[ 5, 6] , dichlo[ 7] rine m onoxide and chlo rine gas , run w it h severe disadvantag es f or this purpose . For inst ance , co mpound 1 can be convert ed int o com pound 2 o nly in a 30% y ield by using t-but yl hypo chlorit e . Alt hough dichlo rine monox ide and chlo rine lead g ood selectiv it y and yields , t hey of ten po llute t he environ[ 1_ 4, 8] ment . We now repo rt an environment all y fr iendly m et hod f or t he chlor inat ion in ally lic positio n o f ol ef ins. It uses t he aqueous solution of sodium hy pochlorit e( 2% _ 5% act ive chlorine) t og ether w ith an inorganic acid or an o rganic acid as chlorinat ion reag ent s and is carried out in a diphase sy stem such as CCl4 -H 2O and CH 2Cl2-H 2 O.
branching on the doubl e bonds ( compounds 1 , 3, 5, 8, 10 , 12 in Scheme 1) .
R 1N H N O CO O R 2 1 CH 3 CH 2
N aClO, H3 PO 4 CH 2C l2 H 2 O , - 15_ 25
S _ SO 2 _ A r
N aClO , AcOH C Cl4 -H2 O, 0
R 1N H N O
S _ SO 2 _ A r Cl
C OO R 2 ( 80% _ 90% ) 2 CH 2 Cl C H2 ( 72% ) 4 + Cl H
1
H1
3
NaC lO , H 3 PO 4 CC l4 H2 O, - 15_ 25
2
H1
Cl ( 40% ) 7
H ( 30% ) 6
5
N aClO , H3 PO 4 CC l4 H 2 O , - 15_ 25
8 CH 3 C CH 3 10 CH 3 C CH 3 C H 12 O C CH 3 C CH 3 CH 3
H2 CH 2C l 9
( 70% ) CH 3 CH 3
NaC lO, H 3P O 4 C H2 C l2-H2 O , - 15_ 25
H2C
C Cl CH 3
11
NaClO, H3 PO 4 CC l4 H 2O , - 15_ 25
( 90% )
Results and Discussion
As show n in Scheme 1, a dithioazet idino ne derivat ive( compound 1) w as used f or t he chlo rinatio n f irst ly and satisfacto ry result s w ere obt ained w ith excell ent select ivit y and hig h yields ( 80% _ 90% ) . T he furt her exper im ent al r esult s indicat e t hat t he met hod can be used g enerally for t he chlorinat ion in all ylic position o f m any olef ins w it h
H2C
CH 3 H C C
O C CH 3 ( 70% )
Cl 13
Scheme 1 The chlorination in allylic position of some olef ins by using sodium hypochlorite and an acid as chlorination reagents in a diphase system.
T he real chlorinat ing r eag ent is considered t o be mo lecul ar st at e Cl 2, w hich is pro duced by t he
* * T o w ho m cor respondence should be addr essed. E-mail: shihc@ mail. t sing hua. edu. cn
170
CHEM . R ES . CHIN ESE U .
V ol . 21
react ion o f sodium hypochlor it e w ith acids in t he aqueo us phase show n as f ollow ing : + NaClO+ Cl + 2H Cl2+ Na + H 2O T hen Cl2 go es int o t he or ganic phase im mediately and the chlorination of olef ins o ccurs in t he org anic phase( see Fig . 1) . T he chl orinat ion carried o n by Cl2 in no npolar [ 9_ 12] so lvent s has been st udied by P outsma and ionic
or r adical pat hw ays were pr opo sed. T he o lef ins w ith branches( especially alkyl groups) on t he doubl e bonds go t hrough m ainly an ionic pat h , w hich pr oduces a new olefin chlorinat ed in the allylic posit ion . T herefo re , t he chlorinat ion react ions in Scheme 1 should be in pro gress along an ionic pat h and t he m echanism of t he react ion is described in Fig . 1.
Fig. 1 The mechanism of the chlorination in allylic position by using sodium hypochlorite and an acid as chlorination reagents in a diphase system.
Since m olecular HCl is produced in t he r eactio n, t he aqueo us phase is very import ant due t o t hat it can absorb HCl and t hen t he side react ion caused by HCl can be reduced significant ly. Inor ganic acids, such as phosphoric acid, sulf ur ic acid or hy drochloric acid can all be used in t his procedure and phosphoric acid is t he most eff ect iv e one, fo llow ed by sulf uric acid. It w as fo und t hat t he chlorination could be carr ied o ut w hen w at er solubl e o rganic acids w ere used. Amo ng t hem , acet ic acid w as t he best . How ever, t he react ion co uld hardly occur w hen a w ater insoluble acid such as pent ano ic acid or hex anoic acid w as used. T he sit uat ion may be caused by t he very st ro ng af finit y existing bet w een t he large alkyl groups of t he acids and t he allylic + gr oups of olefins. T he af finity can inhibit Cl t o react w it h t he double bo nd on the ally lic group . T he acids t hat react w it h chlor ine or olef ins canno t be used in this chlorination. F or ex ampl e, fo rmic acid is f reely soluble in wat er , how ever , no t suit able f or t he chlo rinat ion because it could r educe chlor ine into chlo rine anion: 2H + + 2Cl- + CO 2 HCOOH + Cl 2 T he pr oduct s , co mpo unds 6, 7, 9, 11 and 13, are so act iv e t hat t he chemical changes can o ccur during the process of flash chro mato graphy . Ho w ev er , t heir st ruct ures and t he yields can be det er -
m ined fro m the 1H NMR spectr a of t heir crude product s. Am ong those product s, compound 13 is t he m ost active and t he chlo ride w oul d deco mpose m ore t han half o f it w hen it w as kept at ro om t emper at ure for 2 h, which show s that ev en very act ive ally lic chlorides could be pr epared by t he chlo rinat ion m et hod. Com po unds 6, 7 and 9 are substit utes of cyclohex ene or cyclo hex ane w it h 3 _ 4 m et hylenes. T he NM R peaks of hydrog en at oms o n methyl enes are very clo se , even crow ded . T heref ore , w hen t he pro duct s are not pure , it is hard to det er mine t he chemical shift of each m et hylenehydrogen. Ho w ever, the chemical shift s of hy drog en at om s o n double bonds and so me gro ups( such as met hyl and chlor o-met hy l) can be det erm ined. T he chemical shif t s of hydrog en at o ms on double bonds of chlorides 4, 6, 7, 9 can be est i[ 13] m at ed w it h empirical fo rmula : 5. 25+ Z iso + Z cis + Z trans H w here Z iso , Zc is and Z trans are empir ical parameters . T he resul ts mat ch w ell w it h t he m easured result s ( see T able 1) , w hich indicat es that t he o lef ins ( compounds 3, 5 , 8 , 11 ) have changed int o new o lef ins chlorinated in al lylic posit ions. T he chemical shift s o f t he chloride( compound 13) could not be estim at ed f or lack of em pirical paramet ers, but
N o. 2
WA N G X in y an et al .
1
171
t he new chemical shift peaks of hydr ogen ato ms on t he doubl e bo nd appear at 5. 16 and 5. 27, w hich show s t he f ormat ion o f a new do uble bond ( see Scheme 1) .
Table 1 The chemical shifts of the hydrogen atoms on the double bonds f rom NMR detection and estimation by empirical f ormula
Chemical sh if t Compound
1H
H1 NMR Es t imat ion 5. 45 4. 84 5. 56 5. 77 5. 08 4. 85
1H
H2 NM R Est imat ion 5. 57 4. 90 5. 55 4. 97
4 6 7 9 11
5. 44 4. 79 5. 59 5. 81 5. 05
4. 99
Experimental
T he H NM R spect ra were recorded on a Br uker AC 300M NM R spect ro meter and r ef er enced t o M e 4Si. T he concent rat ion of act ive chlo ride in sodium hypo chlorit e solution w as detected by iodomet ry . 1 Chlorination Usi ng Sodium Hypochl orite To gether with an Organic Acid ( such as AcOH ) as Chl orinating Agent ( Compound 1 as the Substrate) Wit h w ell st irring , t he aqueous so lut ion of so dium hypochlo rite ( 5 m L , 5. 0% act ive chl orine ) w as added dropw ise t o a sol ut ion o f co mpound 1 ( 3. 0 g , about 5 m mol ) in a t et rachl oride ( 100 mL ) and acet ate acid( 10 m L ) at - 5 . One hour lat er , t he solid w as f ilt er ed of f and w ashed w it h met hy lene chlo ride( 20 m L ) . T he org anic lay er w as separ at ed and w ashed w ith the sat urat ed aqueous so lut ion o f NaCl and dried w it h so dium sulfat e anhy dride. T he so lvent w as moved t o give about 3. 1 g of t he crude pro duct , w hich w as purif ied by Fl ash chrom at o graphy [ silica gel, V ( benzene ) V ( et hyl acetat e) = 21] t o give 2. 8 g of com pound 2( 88% ) as a yellow ish no ncryst all ine solid. 2 Chlorination Usi ng Sodium Hypochl orite To gether with an Inorganic Acid( such as Phosphoric Acid ) as Chlorinating Agent ( Compound 3 as a Substrate ) Wit h w ell st irring, t he aqueous so lut ion of so dium hypo chlorit e ( 7. 0 mL , 5. 0% active chlo rine ) w as added dr opw ise to a mixt ure of com pound 3 ( 1 m L , about 7. 6 mm ol ) in methyl ene dichlo ride( 40 mL ) , 8 m L of a 25% o f sodium chlo ride so lut ion and phosphoric acid ( 2. 0 m L , 85% ) at - 20 . T hen the fo llow ing pro cedures w ere sim ilar t o t ho se described abo ve t o g ive 0. 84 g ( 72% ) of compound 4 as a colorless o il.
1
3 H NMR Spectra and IR Spectra 1 Com po und 2: H NM R ( 300 M Hz, 25 , CDCl 3) , : 7. 79( d, J = 7. 8 Hz, 2H ) , 7. 58 ( d, J = 7. 2 Hz , 3H ) , 7. 48( d , J = 7. 8, 2H ) , 7. 28( m , 7H ) , 6. 89( d , J = 8. 5 Hz , 2H ) , 6. 18( d , J = 8. 9 Hz , 1 H ) , 5. 85( d , J = 4. 9 Hz , 1H ) , 5. 16_ 5. 01 ( m, 5H ) , 4. 65 ( s, 1H ) , 4. 08( dd, J 1 = 47 Hz, J 2= 12. 5 Hz, 2H) , 3. 81( s, 3H) , 3. 57( s, 2H ) . - 1 IR( KBr ) , ~/ cm : 3400( NH) , 1780, 1745, 1673 ( C O) . 1 Com po und 4: H NM R ( 300 MHz, 25 , CDCl 3) , : 4. 47( s, 2H) , 5. 45( s, 1H ) , 5. 56( s, 1H ) , 7. 26_ 7. 20( m , 3H ) , 7. 47( d , J = 7. 2 Hz , 2H ) . 1 Com po und 6: H NM R ( 300 M Hz , 25 , CDCl 3) , : 4. 52 ( dd, J = 10. 5, 4. 4 Hz, 1H ) , 4. 65( s, 1H) , 4. 80( s, 1H) . 1 Com po und 7: H NM R ( 300 M Hz, 25 , CDCl 3) , : 1. 79( s, 3H) , 4. 42( s, 1H ) , 5. 59( s, 1H) . Com po und 9: 1H NM R ( 300 M Hz, 25 , CDCl 3) , : 3. 98( s, 2H ) , 5. 81( s, 1H) . 1 Com po und 11 : H NMR ( 300 M Hz, 25 , CDCl 3 ) , : 1. 74( s , 6H ) , 1. 95( s, 3H ) , 4. 85( s , 1H) , 5. 05( s, 1H ) . 1 Com po und 13 : H NMR ( 300 M Hz, 25 , CDCl 3 ) , : 1. 78( s, 3H ) , 2. 27( s, 3H) , 4. 85( s, 1H ) , 5. 16( s , 1H ) , 5. 27( s, 1H ) . Ref erences
[ 1 ] T orii S. , U n eyama K . , N acai T. , e t al . , T etr ahedr on L et t. , 1981, 22, 2291 [ 2 ] T orii S. , U n eyama K . , N acai T. , e t al . , T etr ahedr on L et t. , 1981, 22, 3193 [ 3 ] T orii S. , T an ak a H. , Sait oh N . , et al. , Tet rahed ron L et t. , 1982, 23, 2187 [ 4 ] T orii S. , Tanaka H. , Sait oh N . , et al. , Chem . L et t. , 1982, 11, 1829 [ 5 ] Cooper R . D. G. , T etrahe dron L ett . , 1980, 21, 781 [ 6 ] T an iguch i M . , M isaw a T. , T orii S . , et al . , Bul l . Chem . S oc . Jp n, 1995, 8, 577 [ 7 ] K en N . , Shinj iro S. , O s amu S. , 0500081, 1992 et al . , EP P ate nt
[ 8 ] Y osh iok a M . , T su ji T . , U yeo S . , T etrahe dron L et t. , 1980, 21, 351 [ 9 ] Pout sm a M . [ 10] Pout sm a M . [ 11] Pout sm a M . [ 12] Pout sm a M . L. , S ci ence, L . , J . A m. L. , J . A m . L. , J . A m . 1967, 157, 997 Chem . S oc. , 1963, 85, 3511 Chem . S oc. , 1965, 87, 2161 Chem . S oc. , 1965, 87, 2172
[ 13] N in g Y . C. , S tr uctur al Id entif i cation of Or ganic Comp ounds and Or gani c Sp ect roscopy , Science Pub lish ing Comp any, Beijing , 2000, 28
Das könnte Ihnen auch gefallen
- Cast Resin TransformerDokument29 SeitenCast Resin Transformerkrmurali2000Noch keine Bewertungen
- CAPE Unit 2 LabsDokument4 SeitenCAPE Unit 2 LabsAlex Clarke50% (6)
- Welding of Duplex Stainless SteelDokument7 SeitenWelding of Duplex Stainless Steelel_sharkawy2011Noch keine Bewertungen
- Who Says Akbar Was GreatDokument222 SeitenWho Says Akbar Was GreatArya Veer0% (1)
- TDS-Dow SPECFIL FT630 & SPECFIL FE100-EN - 20181226Dokument2 SeitenTDS-Dow SPECFIL FT630 & SPECFIL FE100-EN - 20181226Mallampati RamakrishnaNoch keine Bewertungen
- CHAPTER 7 Solid Waste ManagementDokument28 SeitenCHAPTER 7 Solid Waste Managementdaabgchi67% (3)
- U585507Dokument201 SeitenU585507chidambaramrNoch keine Bewertungen
- Hydroformylation of PropyleneDokument4 SeitenHydroformylation of Propylenemichelle_tang_9Noch keine Bewertungen
- Syn Aspartame PDFDokument3 SeitenSyn Aspartame PDFAlexiaaaa12Noch keine Bewertungen
- Harada 1984Dokument5 SeitenHarada 1984julianpellegrini860Noch keine Bewertungen
- Oxomolybdenum Chemistry: An ExperimentDokument3 SeitenOxomolybdenum Chemistry: An ExperimentHector LopezNoch keine Bewertungen
- Esterification Process To Synthesize Isopropyl Chloroacetate Catalyzed by Lanthanum Dodecyl SulfateDokument6 SeitenEsterification Process To Synthesize Isopropyl Chloroacetate Catalyzed by Lanthanum Dodecyl SulfateVinay JainNoch keine Bewertungen
- Transesterification Kinetics of Phenyl Salicylate 2Dokument20 SeitenTransesterification Kinetics of Phenyl Salicylate 2Lucas de Lima e SousaNoch keine Bewertungen
- O-Hydroxyethylation of 1 1-DihydroperfluDokument10 SeitenO-Hydroxyethylation of 1 1-DihydroperfluDeepak CharanNoch keine Bewertungen
- An Improved Synthetic Method of SaquinavirDokument5 SeitenAn Improved Synthetic Method of Saquinavirnetelsrt1298Noch keine Bewertungen
- Reaction Testing of Phenol Hydroxylation and Cyclohexane Oxidation by Gas Chromatography: Influence of Residual Hydrogen PeroxideDokument10 SeitenReaction Testing of Phenol Hydroxylation and Cyclohexane Oxidation by Gas Chromatography: Influence of Residual Hydrogen PeroxideAmino BowwowNoch keine Bewertungen
- Socl 2Dokument12 SeitenSocl 2Romano AlbertNoch keine Bewertungen
- Joc 1980,45,1035Dokument7 SeitenJoc 1980,45,1035rrgodboleNoch keine Bewertungen
- A Stereoselective Remote Homochiral Boronate Ester-Mediated Aldol reaction6-1537KP Published MainmanuscriptDokument9 SeitenA Stereoselective Remote Homochiral Boronate Ester-Mediated Aldol reaction6-1537KP Published MainmanuscriptFaroikDahmchiNoch keine Bewertungen
- Determination of Fluoride in Water by Reversed-Phase High-Performance Liquid Chromatography Using F-La3+-Alizarin Complexone Ternary ComplexDokument3 SeitenDetermination of Fluoride in Water by Reversed-Phase High-Performance Liquid Chromatography Using F-La3+-Alizarin Complexone Ternary ComplexpvsnmalleshNoch keine Bewertungen
- FormaldehitDokument7 SeitenFormaldehitNesrin KozanNoch keine Bewertungen
- Methanolic HCLDokument2 SeitenMethanolic HCLKalim IqbalNoch keine Bewertungen
- Fazaeli, R., (2006) - Canadian Journal of Chemistry, 84 (5), 812-818.Dokument7 SeitenFazaeli, R., (2006) - Canadian Journal of Chemistry, 84 (5), 812-818.manuel querolNoch keine Bewertungen
- Matriculation Chemistry HaloalkaneDokument46 SeitenMatriculation Chemistry HaloalkaneNurul ImanNoch keine Bewertungen
- Dissolution Kinetics: Catalysis by SaltsDokument13 SeitenDissolution Kinetics: Catalysis by SaltsborgiamatriceNoch keine Bewertungen
- IntroductionDokument16 SeitenIntroductionAkhtar RazaNoch keine Bewertungen
- Benzylic Oxide PDFDokument3 SeitenBenzylic Oxide PDFebi1364Noch keine Bewertungen
- Kinetic Investigations On The Esterification of Phthalic Anhydride With N-Heptyl, N-Nonyl or N-Undecyl Alcohol Over Sulfuric Acid CatalystDokument7 SeitenKinetic Investigations On The Esterification of Phthalic Anhydride With N-Heptyl, N-Nonyl or N-Undecyl Alcohol Over Sulfuric Acid CatalystVimal PatelNoch keine Bewertungen
- Chen 2006Dokument5 SeitenChen 2006faisalNoch keine Bewertungen
- An Introduction To Methods For Analyzing Thiols and Disul Des ReactionsDokument12 SeitenAn Introduction To Methods For Analyzing Thiols and Disul Des ReactionsFernanda RigoNoch keine Bewertungen
- Assymetric Claisen RearrangmentDokument11 SeitenAssymetric Claisen RearrangmentAngelo MachadoNoch keine Bewertungen
- Aggregation and Stabilization of Carboxylic Acid Functionalized Halloysite Nanotubes (HNT-COOH)Dokument6 SeitenAggregation and Stabilization of Carboxylic Acid Functionalized Halloysite Nanotubes (HNT-COOH)Vahdat VahedyNoch keine Bewertungen
- Esterification Salicylic AcidDokument3 SeitenEsterification Salicylic AcidBobbyGunarsoNoch keine Bewertungen
- M Vitro: 1 B. C H A N C E, Acta Chem. Scand. 1, 236 (1947)Dokument6 SeitenM Vitro: 1 B. C H A N C E, Acta Chem. Scand. 1, 236 (1947)rajeshNoch keine Bewertungen
- I Io. Chemical Physics Letters: Votr N CDokument5 SeitenI Io. Chemical Physics Letters: Votr N CthucinorNoch keine Bewertungen
- Application of Phosphonium Salts To The Reactions of Various Kinds of AmidesDokument7 SeitenApplication of Phosphonium Salts To The Reactions of Various Kinds of AmidesXin LiuNoch keine Bewertungen
- Alquilacion de Arenos Con AlcoholesDokument5 SeitenAlquilacion de Arenos Con AlcoholesJosé Guadalupe García EstradaNoch keine Bewertungen
- A Facile Synthesis of Alkyl Substituted Maleic Anhydrides Radical ApproachDokument4 SeitenA Facile Synthesis of Alkyl Substituted Maleic Anhydrides Radical ApproachShaik SameerNoch keine Bewertungen
- Demethylation With LiCl-DMF (JMolCatA-Chemical2007)Dokument8 SeitenDemethylation With LiCl-DMF (JMolCatA-Chemical2007)Archawin_mooNoch keine Bewertungen
- B040427 566Dokument3 SeitenB040427 566diskordis133Noch keine Bewertungen
- Bi FunctionalDokument36 SeitenBi FunctionalchidambaramrNoch keine Bewertungen
- tmpF0F1 TMPDokument11 SeitentmpF0F1 TMPFrontiersNoch keine Bewertungen
- Chem. Eur. J. 2011, 17, 10208 - 10212Dokument5 SeitenChem. Eur. J. 2011, 17, 10208 - 10212SBNoch keine Bewertungen
- Scipharm 70 00015Dokument5 SeitenScipharm 70 00015Davi Abreu Carvalho MotheNoch keine Bewertungen
- Factors Affecting Captopril Stability in Aqueous SolutionDokument8 SeitenFactors Affecting Captopril Stability in Aqueous SolutionTuấn HoàngNoch keine Bewertungen
- Ilcpa 12 04 17Dokument22 SeitenIlcpa 12 04 17JuanManuelAmaroLuisNoch keine Bewertungen
- JChemEduc 1995 72 (8) 751Dokument3 SeitenJChemEduc 1995 72 (8) 751JGARCIA38Noch keine Bewertungen
- Structure and Reactions of ChlorophyllDokument8 SeitenStructure and Reactions of Chlorophyllسید طاہر عباسNoch keine Bewertungen
- Surface Chemistry: Adsorption From SolutionsDokument27 SeitenSurface Chemistry: Adsorption From SolutionsSrijan GoyalNoch keine Bewertungen
- Polystyrin Suported Al (OTF) 3Dokument3 SeitenPolystyrin Suported Al (OTF) 3shailesh19rajputNoch keine Bewertungen
- 09-4054LR Published MainmanuscriptDokument14 Seiten09-4054LR Published MainmanuscriptsubhashpithaniNoch keine Bewertungen
- Communication: Cuo/Sio: A Simple and Efficient Solid Acid Catalyst For Epoxide Ring OpeningDokument4 SeitenCommunication: Cuo/Sio: A Simple and Efficient Solid Acid Catalyst For Epoxide Ring Openingfedericasantoro81Noch keine Bewertungen
- H Silylation of Arenes With: Rhodium-Catalyzed Intermolecular C High Steric RegiocontrolDokument6 SeitenH Silylation of Arenes With: Rhodium-Catalyzed Intermolecular C High Steric RegiocontrolLerchundi KityNoch keine Bewertungen
- Creation of A Monomeric Ru Species On The Surface of Hydroxyapatite As An Efficient Heterogeneous Catalyst For Aerobic Alcohol OxidationDokument2 SeitenCreation of A Monomeric Ru Species On The Surface of Hydroxyapatite As An Efficient Heterogeneous Catalyst For Aerobic Alcohol OxidationSveti JeronimNoch keine Bewertungen
- Dyes and Pigments 21 (1993) 45-66 PDFDokument22 SeitenDyes and Pigments 21 (1993) 45-66 PDFAmitNoch keine Bewertungen
- A Novel Route To A, U-Telechelic Poly (3-Caprolactone) Diols, Precursors of Biodegradable Polyurethanes, Using Catalysis by Decamolybdate AnionDokument10 SeitenA Novel Route To A, U-Telechelic Poly (3-Caprolactone) Diols, Precursors of Biodegradable Polyurethanes, Using Catalysis by Decamolybdate AnionNgũ Viên Gia CácNoch keine Bewertungen
- Synthesis and Characterization of 2,5-Dihydroxy Substituted Chalcones Using Socl /etoh M.R.Jayapal, K.Sreenivasa Prasad and N.Y.SreedharDokument6 SeitenSynthesis and Characterization of 2,5-Dihydroxy Substituted Chalcones Using Socl /etoh M.R.Jayapal, K.Sreenivasa Prasad and N.Y.SreedharGanesamoorthy ThirunarayananNoch keine Bewertungen
- Synthesis of Quinolizinium SaltsDokument3 SeitenSynthesis of Quinolizinium Saltsspkgchem74670% (1)
- 1 s2.0 0040403996013512 MainDokument4 Seiten1 s2.0 0040403996013512 MainSupriya SomvanshiNoch keine Bewertungen
- (134502991) Determination of Residual ChlorineDokument5 Seiten(134502991) Determination of Residual ChlorineraowaleedahmadNoch keine Bewertungen
- Macdougall 1996Dokument12 SeitenMacdougall 1996jfjd6889Noch keine Bewertungen
- Zeng 1991Dokument10 SeitenZeng 1991mfifen aristideNoch keine Bewertungen
- Synthesis of 3-sulfonyloxypyridines: Oxidative ring expansion of α-furylsulfonamides and N→O sulfonyl transferDokument5 SeitenSynthesis of 3-sulfonyloxypyridines: Oxidative ring expansion of α-furylsulfonamides and N→O sulfonyl transferlapsNoch keine Bewertungen
- Synthesis, Configuration, and Dehydration of Some 1-Alkyl - and Aralkyl-3-Methyl-4-O-Tolylpiperidin-4-Ols - AF Casy MA Iorio - J Chem Soc C 1970, 135 - DOI 10.1039 J39700000135Dokument4 SeitenSynthesis, Configuration, and Dehydration of Some 1-Alkyl - and Aralkyl-3-Methyl-4-O-Tolylpiperidin-4-Ols - AF Casy MA Iorio - J Chem Soc C 1970, 135 - DOI 10.1039 J39700000135Jonathan BerryNoch keine Bewertungen
- Bpo C Chapter 14Dokument64 SeitenBpo C Chapter 14Mutia SafitriNoch keine Bewertungen
- Cytochrome P450 Monooxygenases, Chemistry Of: Advanced ArticleDokument7 SeitenCytochrome P450 Monooxygenases, Chemistry Of: Advanced ArticleazzaassNoch keine Bewertungen
- Transition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesVon EverandTransition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesNoch keine Bewertungen
- STRS 2013 06 12 Rose KetonesDokument4 SeitenSTRS 2013 06 12 Rose KetoneschidambaramrNoch keine Bewertungen
- Gallotanin 1Dokument17 SeitenGallotanin 1chidambaramrNoch keine Bewertungen
- GaloreTx-Peptide Chemistry Capabilities Feb 24 2019Dokument13 SeitenGaloreTx-Peptide Chemistry Capabilities Feb 24 2019chidambaramrNoch keine Bewertungen
- An Environmentally Benign Benzylic Oxidation Catalyzed by Hypervalent Iodine Intermediate in WaterDokument4 SeitenAn Environmentally Benign Benzylic Oxidation Catalyzed by Hypervalent Iodine Intermediate in WaterchidambaramrNoch keine Bewertungen
- The Preparation of Secondary Aliphatic Biazo-Compounds From HydrazonesDokument3 SeitenThe Preparation of Secondary Aliphatic Biazo-Compounds From HydrazoneschidambaramrNoch keine Bewertungen
- Macrocyclization by Ring-Closing Metathesis in The Total Synthesis of Natural Products: Reaction Conditions and LimitationsDokument16 SeitenMacrocyclization by Ring-Closing Metathesis in The Total Synthesis of Natural Products: Reaction Conditions and LimitationschidambaramrNoch keine Bewertungen
- Communications To The EditorDokument3 SeitenCommunications To The EditorchidambaramrNoch keine Bewertungen
- Organocatalytic Asymmetric Epoxidation and Aziridination of Olefins and Their Synthetic ApplicationsDokument58 SeitenOrganocatalytic Asymmetric Epoxidation and Aziridination of Olefins and Their Synthetic ApplicationschidambaramrNoch keine Bewertungen
- Kon 2011Dokument1 SeiteKon 2011chidambaramrNoch keine Bewertungen
- Chapter 24Dokument41 SeitenChapter 24chidambaramrNoch keine Bewertungen
- Case Study: Base Effects in A Challenging Suzuki ReactionDokument1 SeiteCase Study: Base Effects in A Challenging Suzuki ReactionchidambaramrNoch keine Bewertungen
- 10 1021@cr0200116Dokument22 Seiten10 1021@cr0200116chidambaramrNoch keine Bewertungen
- MCT Oil Rev2014 Ver2 2014-03-05Dokument8 SeitenMCT Oil Rev2014 Ver2 2014-03-05chidambaramrNoch keine Bewertungen
- Case Study 4Dokument2 SeitenCase Study 4chidambaramrNoch keine Bewertungen
- Working With Hazardous Chemicals: A Publication of Reliable Methods For The Preparation of Organic CompoundsDokument5 SeitenWorking With Hazardous Chemicals: A Publication of Reliable Methods For The Preparation of Organic CompoundschidambaramrNoch keine Bewertungen
- Natural Biotech Solutions To Flavour & Fragrance IndustryDokument18 SeitenNatural Biotech Solutions To Flavour & Fragrance IndustrychidambaramrNoch keine Bewertungen
- Advances in The Krapcho DecarboxylationDokument8 SeitenAdvances in The Krapcho DecarboxylationchidambaramrNoch keine Bewertungen
- PerfumeryDokument14 SeitenPerfumerychidambaramr100% (2)
- Working With Hazardous Chemicals: A Publication of Reliable Methods For The Preparation of Organic CompoundsDokument4 SeitenWorking With Hazardous Chemicals: A Publication of Reliable Methods For The Preparation of Organic CompoundschidambaramrNoch keine Bewertungen
- TBHPDokument12 SeitenTBHPchidambaramrNoch keine Bewertungen
- Binap Synthesis SchemeDokument2 SeitenBinap Synthesis SchemechidambaramrNoch keine Bewertungen
- Mass Transfer Is The Net Movement of Mass From One LocationDokument2 SeitenMass Transfer Is The Net Movement of Mass From One LocationKishore RamNoch keine Bewertungen
- ES GTU Study Material E-Notes Chapter-8 12012020013605PMDokument15 SeitenES GTU Study Material E-Notes Chapter-8 12012020013605PMRIPNoch keine Bewertungen
- Mark Scheme (Results) Summer 2016Dokument31 SeitenMark Scheme (Results) Summer 2016NaushinNoch keine Bewertungen
- Safety Data Sheet: 1. IdentificationDokument7 SeitenSafety Data Sheet: 1. IdentificationGan Chai SInNoch keine Bewertungen
- Toxicity of Common Laboratory Chemicals To Human Red Blood Cells Laboratory ActivityDokument4 SeitenToxicity of Common Laboratory Chemicals To Human Red Blood Cells Laboratory ActivityKuven Malig-onNoch keine Bewertungen
- BLASTING &PRIMER ProtocolDokument104 SeitenBLASTING &PRIMER ProtocolSulekh GhoshNoch keine Bewertungen
- Agar Desalter Control Sys3 Appl 3Dokument2 SeitenAgar Desalter Control Sys3 Appl 3JADNoch keine Bewertungen
- Presentation: Darwisa G. Hawari Dip-Ems 1ADokument35 SeitenPresentation: Darwisa G. Hawari Dip-Ems 1AZeal Alexis MontellorNoch keine Bewertungen
- The Following Tables Provide An Overview of The Key Advantages and Disadvantages of Different Types of Fertilisers On The Market TodayDokument3 SeitenThe Following Tables Provide An Overview of The Key Advantages and Disadvantages of Different Types of Fertilisers On The Market TodayAbdullaNoch keine Bewertungen
- Certificate of Analysis: Mofs - X C PolymerDokument1 SeiteCertificate of Analysis: Mofs - X C PolymerPranav DubeyNoch keine Bewertungen
- Safety Data Sheet (SDS)Dokument13 SeitenSafety Data Sheet (SDS)Helix SanNoch keine Bewertungen
- Produktblatt Auruna 215 en Screen 20190416Dokument2 SeitenProduktblatt Auruna 215 en Screen 20190416Abdulrahman JradiNoch keine Bewertungen
- High Rate Series: CCB 12HD-475Dokument1 SeiteHigh Rate Series: CCB 12HD-475orunmila123Noch keine Bewertungen
- Cationic and Anionic Redox in Lithium-Ion Based Batteries PDFDokument18 SeitenCationic and Anionic Redox in Lithium-Ion Based Batteries PDFLeilane Natsumi Alves KanbaraNoch keine Bewertungen
- Framo Operation ManualDokument16 SeitenFramo Operation Manualcaptulcc100% (2)
- Nanopartikel AseklofenakDokument9 SeitenNanopartikel AseklofenakReski WahyuNoch keine Bewertungen
- Crack Identification in Reinforced Concrete Beams Using ANSYS SoftwareDokument13 SeitenCrack Identification in Reinforced Concrete Beams Using ANSYS SoftwarethaibinhkxNoch keine Bewertungen
- Stefan Boltzmann Law PDFDokument3 SeitenStefan Boltzmann Law PDFESAKKIMALA SNoch keine Bewertungen
- ECSS Q ST 70 15C (1may2021)Dokument124 SeitenECSS Q ST 70 15C (1may2021)Navamani Prakash100% (1)
- Comprehensive 2022 & Summer Leaflet New 1 PDFDokument14 SeitenComprehensive 2022 & Summer Leaflet New 1 PDFAkshay DhoteNoch keine Bewertungen
- 4 Elements, Mixtures and Compounds: WorksheetDokument3 Seiten4 Elements, Mixtures and Compounds: WorksheetMfanafuthiNoch keine Bewertungen
- Ques Paper 2-XI-ENGDokument8 SeitenQues Paper 2-XI-ENGKrish VermaNoch keine Bewertungen
- Physico-Chemical Properties of Drinking WaterDokument12 SeitenPhysico-Chemical Properties of Drinking WaterEmmanuel WinfulNoch keine Bewertungen
- 100 Questions McqsDokument28 Seiten100 Questions McqsAtif NaeemNoch keine Bewertungen
- A Report On Fire Extinguisher OperationDokument3 SeitenA Report On Fire Extinguisher OperationrajkotbpNoch keine Bewertungen