Beruflich Dokumente
Kultur Dokumente
Sodium Thiosulfate Assignment Help
Hochgeladen von
YudhistiraOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Sodium Thiosulfate Assignment Help
Hochgeladen von
YudhistiraCopyright:
Verfügbare Formate
9/11/13
Sodium Thiosulfate, Preparation And Properties Of Sodium Thiosulfate, Uses Of Sodium Thiosulfate, Inorganic Chemistry, Homework Assignment Help
Home +1.617.933.5480 Login +1.866.649.0192
Search Your Question Here... or submit a new assignment
Home Assignment Help Chemistry Assignment Help S and P Block Elements Sodium Thiosulfate
Search
11 Science/Math experts Online
Sodium Thiosulfate
Homework Help Related Questions Comments
Like 4.6k 1k
Sodium Thiosulfate Assignment Help
Sodium thiosulfate
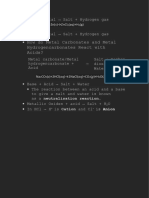
Na2S2O3.52O: It is manufactured by saturating a solution of sodium carbonate with SO2 which gives a solution of sodium sulfite, Na2CO3 + SO2 + H2O Na2SO3 + CO2 + H2O 373K The resulting solution is boiled with powdered sulfur as, Na2SO3 + S > Na2S2O3 The solution is then cooled to get crystals of sodium thiosulfate. Physical properties : (i) Sodium thiosulfate is a colorless crystalline solid. In the hydrated form, it is called hypo. (ii) It melts at 320 K and loses its water molecules of crystallization on heating to 490K. Chemical properties (i) Action with halogens : It reacts with halogens as, (a) Chlorine water oxidizes sodium thiosulphate to sodium sulphate and sulphur is precipitated, Na 2S2O3 + Cl2 + H2O > 2HCl + Na2SO4 +S This property enables it to act as an antichlor in bleaching i.e. it destroys the unreacted chlorine in the process of bleaching. (b) Bromine water also oxidizes sodium thiosulfate to sodium sulfate and sulfur, Na 2S2O3 + Br2 + H2O > Na2SO4 + 2HBr + S (c) With iodine it forms a soluble compound called sodium tetrathionate, 2Na 2S2O3 + l2 > Na2S4O6 + 2NaI Sod. tetrathionate Therefore, hypo is commonly used to remove iodine stains from the clothes. (ii) Action of heat : Upon heating, sodium thiosulfate decomposes to form sodium sulfate and sodium pentasulfide, Heat 4Na 2S2O3 > 3Na2SO4 + Na2S5 Chat now Sodium pentasulphate (iii) Action with acids : Sodium thiosulphate reacts with dilute hydrochloric acid or Sulfuric acid forming sulfur dioxide and sulfur. The solution turns milky yellow due to sulfur. Na2S2O3 + 2HCl > 2NaCl + SO2 + H2O + S (iv) Action with silver halides : Sodium thiosulfate forms soluble complex when treated with silver chloride or silver bromide, 2Na 2S2O3 + 2AgBr > Na3Ag(S2O3)2 + NaBr Sodium disthiosulphate argentate (I)complex
Questions Asked
1,034,490
2,172
Experts
Questions Answered
190,968
Ask Your Question Now!
Related Topics
Action of Acids Alkalies on Boron Family Alkali Metals Alkalies Bleaching Action on Halogens Allotropy Allotropy In Sulfur Alum Anomalous Behavior of Boron Anomalous Behavior of Carbon Anomalous Behavior of Fluorine
Free Accounting Cheat Sheet
Download our free Accounting Cheat Sheet and stay updated with the latest questions & answers. Email So far 10,000+ downloads Go
This property of hypo is made use in photography. Uses of sodium thiosulfate (i) It is largely used in photography as a fixing agent. (ii) It is used as a preservative for fruit products such as jams and squashes. (iii) It is used as an antichlor in bleaching. (iv) It is used as a volumetric agent for the estimation of iodine. (v) It is used in medicine.
Email Based Homework Assignment Help in Sodium Thiosulfate
Transtutors is the best place to get answers to all your doubts regarding sodium thiosulfate, preparation and properties of sodium thiosulfate, uses of sodium thiosulfate with examples . You can submit your school, college or university level homework or assignment to us and we will make sure that you get the answers you need which are timely and also cost effective. Our tutors are available round the clock to help you out in any way with chemistry.
Live Online Tutor Help for Sodium Thiosulfate
Transtutors has a vast panel of experienced chemistry tutors who specialize in sodium thiosulfate and can explain the different concepts to you effectively. You can also interact directly with our chemistry tutors for a one to one session and get
www.transtutors.com/chemistry-homework-help/s-and-p-block-elements/sodium-thiosulfate.aspx
1/2
Das könnte Ihnen auch gefallen
- Inorganic Chemistry Lab 3Dokument8 SeitenInorganic Chemistry Lab 3LinhNguyeNoch keine Bewertungen
- Salts PDFDokument7 SeitenSalts PDFpiyushNoch keine Bewertungen
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastVon EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNoch keine Bewertungen
- 10 SC Chem AcidBaseSaltDokument9 Seiten10 SC Chem AcidBaseSaltAnwarYousafzaiNoch keine Bewertungen
- Baking Soda Solutions: Economical, Eco-Friendly Ideas for Your House, Your Yard and YouVon EverandBaking Soda Solutions: Economical, Eco-Friendly Ideas for Your House, Your Yard and YouBewertung: 5 von 5 Sternen5/5 (2)
- Ch-2 Part-3Dokument4 SeitenCh-2 Part-3Kartik BhardwajNoch keine Bewertungen
- Topic 3 Acids Bases Salts SolutionDokument13 SeitenTopic 3 Acids Bases Salts Solutionindira.seebachanNoch keine Bewertungen
- SaltsDokument22 SeitenSaltssaanvi katyalNoch keine Bewertungen
- Sodium Thiosulfate PDFDokument7 SeitenSodium Thiosulfate PDFjcoppala4476Noch keine Bewertungen
- Exp 3 (Prep - of Na2S2O3.5H2O) & 4 (Excercise)Dokument19 SeitenExp 3 (Prep - of Na2S2O3.5H2O) & 4 (Excercise)KarzanNoch keine Bewertungen
- Chapter 10. Sulphuric Acid: Short QuestionsDokument14 SeitenChapter 10. Sulphuric Acid: Short QuestionsAbhay VishwakarmaNoch keine Bewertungen
- 2 - Acids, Bases and Salts (1)Dokument6 Seiten2 - Acids, Bases and Salts (1)HONEY YOYONoch keine Bewertungen
- 3 Sodium ThiosalphateDokument8 Seiten3 Sodium ThiosalphateRohan GohilNoch keine Bewertungen
- Shortcut ch2Dokument5 SeitenShortcut ch2vemayis701Noch keine Bewertungen
- Exp 3 (Prep - of Na2S2O3.5H2O) & 4 (Excercise)Dokument12 SeitenExp 3 (Prep - of Na2S2O3.5H2O) & 4 (Excercise)photocopy photocopyNoch keine Bewertungen
- 10 Chemistry ABS 6Dokument2 Seiten10 Chemistry ABS 6Aryan GuptaNoch keine Bewertungen
- Acid Base - Synthesis of Nitrocellulose - Chemistry Stack ExchaDokument3 SeitenAcid Base - Synthesis of Nitrocellulose - Chemistry Stack ExchapriyankaNoch keine Bewertungen
- Unit 23 SolutionDokument9 SeitenUnit 23 Solutionapi-3704862Noch keine Bewertungen
- Acids, Bases and Salts Notes - Part 4Dokument7 SeitenAcids, Bases and Salts Notes - Part 4Dhyan ShahNoch keine Bewertungen
- CH 2 Topic - Salts 2Dokument5 SeitenCH 2 Topic - Salts 2siratNoch keine Bewertungen
- Shuledirect: Non Metals and Their CompoundsDokument11 SeitenShuledirect: Non Metals and Their Compoundsvirtual ClassNoch keine Bewertungen
- Study of Components Sulphuric AcidDokument4 SeitenStudy of Components Sulphuric AcidKavya YadavNoch keine Bewertungen
- Shuledirect: Non Metals and Their CompoundsDokument11 SeitenShuledirect: Non Metals and Their Compoundsvirtual ClassNoch keine Bewertungen
- Acids Bases and SaltsDokument4 SeitenAcids Bases and SaltsMandeep SinghNoch keine Bewertungen
- Estimation of Cu (II) Using Sodium Thiosulphate Solution (Iodometrically)Dokument11 SeitenEstimation of Cu (II) Using Sodium Thiosulphate Solution (Iodometrically)Gayatri Govind NairNoch keine Bewertungen
- Important Question ICSE 2010 Class 10th Sulphur Dioxide Sulphuric Acid and Hydrogen SulphideDokument8 SeitenImportant Question ICSE 2010 Class 10th Sulphur Dioxide Sulphuric Acid and Hydrogen SulphideYash KapoorNoch keine Bewertungen
- Chemistry Project: Name: Azad Abdullahi Class: Ss3S Teacher: Mr. AdekunleDokument14 SeitenChemistry Project: Name: Azad Abdullahi Class: Ss3S Teacher: Mr. Adekunleapi-383198550% (2)
- Daligdig & Dibartun - Salt and Miscellaneous Compounds PDFDokument66 SeitenDaligdig & Dibartun - Salt and Miscellaneous Compounds PDFsidick dibaratunNoch keine Bewertungen
- SaltsDokument14 SeitenSaltsKDZ S M O O T H 亗Noch keine Bewertungen
- Lecture 20 Sodium ThiosulfateDokument5 SeitenLecture 20 Sodium ThiosulfateVarsha Kankani100% (1)
- Lecture 20 Sodium Thiosulfate PDFDokument5 SeitenLecture 20 Sodium Thiosulfate PDFPutri AzzahraNoch keine Bewertungen
- Chem 10Dokument11 SeitenChem 10Everton KingNoch keine Bewertungen
- IntroductionDokument10 SeitenIntroductionAmith Singh J100% (1)
- TitrationDokument8 SeitenTitrationsam50% (4)
- Sulphur and Its CompoundsDokument19 SeitenSulphur and Its Compoundspaqurette3Noch keine Bewertungen
- Important Question ICSE 2010 Class 10th Acids Bases Salts ADokument7 SeitenImportant Question ICSE 2010 Class 10th Acids Bases Salts AYash KapoorNoch keine Bewertungen
- Topic7-Making Salts - L3Dokument25 SeitenTopic7-Making Salts - L3haotongxu14Noch keine Bewertungen
- Acid Bases and SaltsDokument7 SeitenAcid Bases and SaltsSubhash suhasariaNoch keine Bewertungen
- Notes - Acids Bases and SaltsDokument2 SeitenNotes - Acids Bases and SaltsantonyNoch keine Bewertungen
- Chemistry Manufactured Substances in IndustryDokument81 SeitenChemistry Manufactured Substances in Industrynabiellahuda88% (8)
- Expt6 - Det-of-the-Dissolved-Oxygen-Content-of-Water - (DO)Dokument5 SeitenExpt6 - Det-of-the-Dissolved-Oxygen-Content-of-Water - (DO)Rex BayonaNoch keine Bewertungen
- Wiki Sodium HydroxideDokument15 SeitenWiki Sodium HydroxidesaiNoch keine Bewertungen
- Sodium - Sulfate From Wikipedia PDFDokument7 SeitenSodium - Sulfate From Wikipedia PDFWage KarsanaNoch keine Bewertungen
- 7.8.1 Sulphur Chemistry NotesDokument6 Seiten7.8.1 Sulphur Chemistry NotesbhartiyaanujNoch keine Bewertungen
- Nitric Acid Semester 2Dokument11 SeitenNitric Acid Semester 2Aditya M GuptaNoch keine Bewertungen
- Important Question ICSE 2010 Class 10th Acids Bases Salts BDokument8 SeitenImportant Question ICSE 2010 Class 10th Acids Bases Salts BYash KapoorNoch keine Bewertungen
- Making Copper SulphateDokument10 SeitenMaking Copper SulphateNamoNoch keine Bewertungen
- Lecture 23 Sodium SulfiteDokument5 SeitenLecture 23 Sodium Sulfiteselvaraj5natesanNoch keine Bewertungen
- Class Slides - Physical and Chemical Changes Grade 7Dokument56 SeitenClass Slides - Physical and Chemical Changes Grade 7Theia JacobNoch keine Bewertungen
- Folio Chemistry: Sulphuric AcidDokument6 SeitenFolio Chemistry: Sulphuric AcidmissyunnaNoch keine Bewertungen
- 6-Iodometric Determination of CopperDokument4 Seiten6-Iodometric Determination of CopperBen Chr100% (1)
- Introduction: The Iodine (Triiodide) - Iodide Redox System, I + 2 e 3 IDokument5 SeitenIntroduction: The Iodine (Triiodide) - Iodide Redox System, I + 2 e 3 IHarshavarthini AnanthasayananNoch keine Bewertungen
- DR Mike Thompson: Winchester College, UKDokument4 SeitenDR Mike Thompson: Winchester College, UKazilatulNoch keine Bewertungen
- Salt AnalysisDokument6 SeitenSalt Analysisashraf_mphilNoch keine Bewertungen
- 1.11 CHEM FINAL Chapter 11 Sulfuric AcidDokument21 Seiten1.11 CHEM FINAL Chapter 11 Sulfuric AcidSudhanshuNoch keine Bewertungen
- Microscale Chemistry in A Plastic Petri Dish: Preparation and Chemical Properties of Chlorine GasDokument6 SeitenMicroscale Chemistry in A Plastic Petri Dish: Preparation and Chemical Properties of Chlorine GasPaul SchumannNoch keine Bewertungen
- Estimation of Iodine in Iodized Common SaltDokument10 SeitenEstimation of Iodine in Iodized Common SaltRajesh ChoudharyNoch keine Bewertungen
- Iodometry (1) - Read-OnlyDokument9 SeitenIodometry (1) - Read-Onlyananyapandey6582Noch keine Bewertungen
- ITunes Plus SiteDokument1 SeiteITunes Plus SiteYudhistiraNoch keine Bewertungen
- COPPER (II) SULFATE - National Library of Medicine HSDB Database PDFDokument12 SeitenCOPPER (II) SULFATE - National Library of Medicine HSDB Database PDFYudhistiraNoch keine Bewertungen
- Yeast Fermentation and The Making of BeerDokument5 SeitenYeast Fermentation and The Making of BeerYudhistiraNoch keine Bewertungen
- Group Policy Preferences OverviewDokument6 SeitenGroup Policy Preferences OverviewadycristiNoch keine Bewertungen
- Multi Field Coupling Dynamic Modeling and Simula 2014 Simulation Modelling PDokument24 SeitenMulti Field Coupling Dynamic Modeling and Simula 2014 Simulation Modelling P刘文Noch keine Bewertungen
- Example 9.2 1Dokument3 SeitenExample 9.2 1jthaaschNoch keine Bewertungen
- Team 2, Lab 1 - Determination of The Concentration of Ethanoic Acid in Commercial VinegarDokument24 SeitenTeam 2, Lab 1 - Determination of The Concentration of Ethanoic Acid in Commercial VinegarAlondra Fernández AcadémicoNoch keine Bewertungen
- FDA - ICH M7 (R1) - Control of Mutagenic Impurities in Pharmaceuticals 03.2018Dokument131 SeitenFDA - ICH M7 (R1) - Control of Mutagenic Impurities in Pharmaceuticals 03.2018Catrinescu OanaNoch keine Bewertungen
- Investigating The Effect of Temperature On Cell MembranesDokument3 SeitenInvestigating The Effect of Temperature On Cell MembranesbeccatannerrrNoch keine Bewertungen
- Assignment Booklet: BTME ProgrammeDokument20 SeitenAssignment Booklet: BTME ProgrammeSarvanKumarNoch keine Bewertungen
- 00s6. UI SS Lecture 6 Clastic Classification 1002Dokument33 Seiten00s6. UI SS Lecture 6 Clastic Classification 1002MuzzammilAlMackyNoch keine Bewertungen
- Gamma Monitoring Systems EngDokument73 SeitenGamma Monitoring Systems Engnmtan17Noch keine Bewertungen
- Biotech Fourth QuarterDokument4 SeitenBiotech Fourth QuarterRodge BuereNoch keine Bewertungen
- What Is A Performance CurveDokument8 SeitenWhat Is A Performance CurveRanjit PaulNoch keine Bewertungen
- Urea & Melamine Formaldehyde ResinsDokument33 SeitenUrea & Melamine Formaldehyde ResinsAkash Yadav50% (2)
- Batch Distillation PDFDokument26 SeitenBatch Distillation PDFwaqaskhanNoch keine Bewertungen
- Was The Driver Drunk? An Instrumental Methods Experiment For The Determination of Blood Alcohol ContentDokument3 SeitenWas The Driver Drunk? An Instrumental Methods Experiment For The Determination of Blood Alcohol ContentRashitaAsfdakldjNoch keine Bewertungen
- Hand Written NotesDokument12 SeitenHand Written NotesOne phase 23Noch keine Bewertungen
- Science Magazine April 07 2006 PDFDokument144 SeitenScience Magazine April 07 2006 PDFAndrés FrankowNoch keine Bewertungen
- Galaxy Surfactants - Product Range BrochureDokument15 SeitenGalaxy Surfactants - Product Range BrochureTo Ra100% (4)
- Chapter-2 - IS MATTER AROUND US PUREDokument25 SeitenChapter-2 - IS MATTER AROUND US PURESATYAM RATHOURNoch keine Bewertungen
- Manual FM200 PDFDokument44 SeitenManual FM200 PDFDIOGO FRANCO PUREZANoch keine Bewertungen
- Engine Lubrication SystemsDokument8 SeitenEngine Lubrication SystemsMentsnot GetuNoch keine Bewertungen
- Class X - Holiday HWDokument6 SeitenClass X - Holiday HWSaritamaheshNoch keine Bewertungen
- TLE Masonry: Principles in Maintenance of Masonry Tools and EquipmentDokument25 SeitenTLE Masonry: Principles in Maintenance of Masonry Tools and EquipmentChristopher PilotinNoch keine Bewertungen
- PCAB List of Licensed Contractors For CFY 2017-2018 As of 18 Oct 2017 - WebDokument667 SeitenPCAB List of Licensed Contractors For CFY 2017-2018 As of 18 Oct 2017 - WebAlfredo Miguel M. BuenaventuraNoch keine Bewertungen
- Retreatment Efficacy of Endodontic Bioceramic Sealers: A Review of The LiteratureDokument12 SeitenRetreatment Efficacy of Endodontic Bioceramic Sealers: A Review of The LiteratureEndriyana NovitasariNoch keine Bewertungen
- 2 SonicScope SchlumbergerDokument19 Seiten2 SonicScope SchlumbergersudiptodattaNoch keine Bewertungen
- Chapter 3Dokument3 SeitenChapter 3Quennie Dhea AdanzaNoch keine Bewertungen
- DNA Structure + Function 12-1Dokument31 SeitenDNA Structure + Function 12-1Jalajarani AridassNoch keine Bewertungen
- JC-1 Dye For Mitochondrial Membrane Potential - Thermo Fisher ScientificDokument4 SeitenJC-1 Dye For Mitochondrial Membrane Potential - Thermo Fisher Scientificankitsaneja1Noch keine Bewertungen
- Centrifugal Pump Piping Design LayoutDokument19 SeitenCentrifugal Pump Piping Design Layoutvijayakumar2015Noch keine Bewertungen
- Lab 3Dokument4 SeitenLab 3Teresa YanNoch keine Bewertungen
- A Continuous Model For C7+ Fraction CharacterizationDokument9 SeitenA Continuous Model For C7+ Fraction CharacterizationargirotopNoch keine Bewertungen