Beruflich Dokumente
Kultur Dokumente
Refrigeration: From Wikipedia, The Free Encyclopedia
Hochgeladen von
Carlito PantalunanOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Refrigeration: From Wikipedia, The Free Encyclopedia
Hochgeladen von
Carlito PantalunanCopyright:
Verfügbare Formate
Refrigeration
From Wikipedia, the free encyclopedia
Jump to: navigation, search
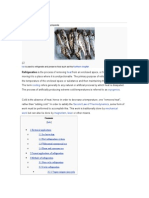
Ice is used to refrigerate and preserve food such as this Northern kingfish Refrigeration is the process of removing heat from an enclosed space, or from a substance, and moving it to a place where it is unobjectionable. The primary purpose of refrigeration is lowering the temperature of the enclosed space or substance and then maintaining that lower temperature. The term cooling refers generally to any natural or artificial process by which heat is dissipated. The process of artificially producing extreme cold temperatures is referred to as cryogenics.
Cold is the absence of heat, hence in order to decrease a temperature, one "removes heat", rather than "adding cold." In order to satisfy the Second Law of Thermodynamics, some form of work must be performed to accomplish this. This work is traditionally done by mechanical work but can also be done by magnetism, laser or other means.
Contents
[hide]
1 Historical applications o 1.1 Ice harvesting o 1.2 First refrigeration systems o 1.3 Widespread commercial use o 1.4 Home and consumer use 2 Current applications of refrigeration 3 Methods of refrigeration o 3.1 Non-cyclic refrigeration o 3.2 Cyclic refrigeration 3.2.1 Vapor-compression cycle 3.2.2 Vapor absorption cycle 3.2.3 Gas cycle o 3.3 Thermoelectric refrigeration
3.4 Magnetic refrigeration 3.5 Other methods 4 Unit of refrigeration 5 See also 6 References 7 Additional reading 8 External links
o o
[edit] Historical applications
Main article: Timeline of low-temperature technology
[edit] Ice harvesting
The use of ice to refrigerate and thus preserve food goes back to prehistoric times.[1][2] Through the ages, the seasonal harvesting of snow and ice was a regular practice of most of the ancient cultures: Chinese, Hebrews, Greeks, Romans, Persians. Ice and snow were stored in caves or dugouts lined with straw or other insulating materials. The Persians stored ice in pits called yakhchals. Rationing of the ice allowed the preservation of foods over the warm periods. This practice worked well down through the centuries, with icehouses remaining in use into the twentieth century. In the 16th century, the discovery of chemical refrigeration was one of the first steps toward artificial means of refrigeration. Sodium nitrate or potassium nitrate, when added to water, lowered the water temperature and created a sort of refrigeration bath for cooling substances. In Italy, such a solution was used to chill wine.[3] During the first half of the 19th century, ice harvesting became big business in America. New Englander Frederic Tudor, who became known as the "Ice King", worked on developing better insulation products for the long distance shipment of ice, especially to the tropics.
[edit] First refrigeration systems
Further information: Timeline of low-temperature technology The first known method of artificial refrigeration was demonstrated by William Cullen at the University of Glasgow in Scotland in 1756. Cullen used a pump to create a partial vacuum over a container of diethyl ether, which then boiled, absorbing heat from the surrounding air. The experiment even created a small amount of ice, but had no practical application at that time. In 1758, Benjamin Franklin and John Hadley, professor of chemistry at Cambridge University, conducted an experiment to explore the principle of evaporation as a means to rapidly cool an object. Franklin and Hadley confirmed that evaporation of highly volatile liquids such as alcohol and ether, could be used to drive down the temperature of an object past the freezing point of water. They conducted their experiment with the bulb of a mercury thermometer as their object and with a bellows
used to "quicken" the evaporation; they lowered the temperature of the thermometer bulb down to 7F while the ambient temperature was 65F. Franklin noted that soon after they passed the freezing point of water (32F) a thin film of ice formed on the surface of the thermometer's bulb and that the ice mass was about a quarter inch thick when they stopped the experiment upon reaching 7F. Franklin concluded, "From this experiment, one may see the possibility of freezing a man to death on a warm summer's day".[4]
In 1805, American inventor Oliver Evans designed but never built a refrigeration system based on the vapor-compression refrigeration cycle rather than chemical solutions or volatile liquids such as ethyl ether. In 1820, the British scientist Michael Faraday liquefied ammonia and other gases by using high pressures and low temperatures. An American living in Great Britain, Jacob Perkins, obtained the first patent for a vapor-compression refrigeration system in 1834. Perkins built a prototype system and it actually worked, although it did not succeed commercially.[5] In 1842, an American physician, John Gorrie, designed the first system for refrigerating water to produce ice. He also conceived the idea of using his refrigeration system to cool the air for comfort in homes and hospitals (i.e., airconditioning). His system compressed air, then partially cooled the hot compressed air with water before allowing it to expand while doing part of the work required to drive the air compressor. That isentropic expansion cooled the air to a temperature low enough to freeze water and produce ice, or to flow "through a pipe for effecting refrigeration otherwise" as stated in his patent granted by the U.S. Patent Office in 1851.[6] Gorrie built a working prototype, but his system was a commercial failure. Alexander Twining began experimenting with vapor-compression refrigeration in 1848 and obtained patents in 1850 and 1853. He is credited with having initiated commercial refrigeration in the United States by 1856.
Dunedin, the first commercially successful refrigerated ship. Meanwhile in Australia, James Harrison began operation of a mechanical ice-making machine in 1851 on the banks of the Barwon River at Rocky Point in Geelong, Victoria. His first commercial ice-making machine followed in 1854 and his patent for an ether liquid-vapour compression refrigeration system was granted in 1855.
Harrison introduced commercial vapor-compression refrigeration to breweries and meat packing houses, and by 1861 a dozen of his systems were in operation. Australian, Argentine, and American concerns experimented with refrigerated shipping in the mid 1870s, the first commercial success coming when William Soltau Davidson fitted a compression refrigeration unit to the New Zealand vessel Dunedin in 1882, leading to a meat and dairy boom in Australasia and South America. J & E Hall of Dartford, England outfitted the 'SS Selembria' with a vapor compression system bring 30,000 carcasses of mutton from the Falkland Islands in 1886.[7][8] The first gas absorption refrigeration system using gaseous ammonia dissolved in water (referred to as "aqua ammonia") was developed by Ferdinand Carr of France in 1859 and patented in 1860. Due to the toxicity of ammonia, such systems were not developed for use in homes, but were used to manufacture ice for sale. In the United States, the consumer public at that time still used the ice box with ice brought in from commercial suppliers, many of whom were still harvesting ice and storing it in an icehouse. Thaddeus Lowe, an American balloonist from the Civil War, had experimented over the years with the properties of gases. One of his mainstay enterprises was the highvolume production of hydrogen gas. He also held several patents on ice making machines. His "Compression Ice Machine" would revolutionize the cold storage industry. In 1869 he and other investors purchased an old steamship onto which they loaded one of Lowes refrigeration units and began shipping fresh fruit from New York to the Gulf Coast area, and fresh meat from Galveston, Texas back to New York. Because of Lowes lack of knowledge about shipping, the business was a costly failure, and it was difficult for the public to get used to the idea of being able to consume meat that had been so long out of the packing house. Domestic mechanical refrigerators became available in the United States around 1911.[9][dead link]
[edit] Widespread commercial use
Loading blocks of factory-made ice from a truck to an "ice depot" boat in the fishing harbor of Zhuhai, China By the 1870s breweries had become the largest users of commercial refrigeration units, though some still relied on harvested ice. Though the ice-harvesting industry had grown immensely by the turn of the 20th century, pollution and sewage had
begun to creep into natural ice making it a problem in the metropolitan suburbs. Eventually breweries began to complain of tainted ice. This raised demand for more modern and consumer-ready refrigeration and ice-making machines. In 1895, German engineer Carl von Linde set up a large-scale process for the production of liquid air and eventually liquid oxygen for use in safe household refrigerators. Refrigerated railroad cars were introduced in the US in the 1840s for the short-run transportation of dairy products. In 1867 J.B. Sutherland of Detroit, Michigan patented the refrigerator car designed with ice tanks at either end of the car and ventilator flaps near the floor which would create a gravity draft of cold air through the car. By 1900 the meat packing houses of Chicago had adopted ammonia-cycle commercial refrigeration. By 1914 almost every location used artificial refrigeration. The big meat packers, Armour, Swift, and Wilson, had purchased the most expensive units which they installed on train cars and in branch houses and storage facilities in the more remote distribution areas. It was not until the middle of the 20th century that refrigeration units were designed for installation on tractor-trailer rigs (trucks or lorries). Refrigerated vehicles are used to transport perishable goods, such as frozen foods, fruit and vegetables, and temperature-sensitive chemicals. Most modern refrigerators keep the temperature between -40 and +20 C and have a maximum payload of around 24 000 kg. gross weight (in Europe).
[edit] Home and consumer use
With the invention of synthetic refrigerants based mostly on a chlorofluorocarbon (CFC) chemical, safer refrigerators were possible for home and consumer use. Freon is a trademark of the Dupont Corporation and refers to these CFC, and later hydrochlorofluorocarbon (HCFC) and hydrofluorocarbon (HFC), refrigerants developed in the late 1920s. These refrigerants were considered at the time to be less harmful than the commonly used refrigerants of the time, including methyl formate, ammonia, methyl chloride, and sulfur dioxide. The intent was to provide refrigeration equipment for home use without danger: these CFC refrigerants answered that need. However, in the 1970s the compounds were found to be reacting with atmospheric ozone, an important protection against solar ultraviolet radiation, and their use as a refrigerant worldwide was curtailed in the Montreal Protocol of 1987.
[edit] Current applications of refrigeration
Probably the most widely-used current applications of refrigeration are for the airconditioning of private homes and public buildings, and the refrigeration of foodstuffs in homes, restaurants and large storage warehouses. The use of refrigerators in kitchens for the storage of fruits and vegetables has permitted the addition of fresh salads to the modern diet year round, and to store fish and meats safely for long periods.
In commerce and manufacturing, there are many uses for refrigeration. Refrigeration is used to liquify gases like oxygen, nitrogen, propane and methane for example. In compressed air purification, it is used to condense water vapor from compressed air to reduce its moisture content. In oil refineries, chemical plants, and petrochemical plants, refrigeration is used to maintain certain processes at their required low temperatures (for example, in the alkylation of butenes and butane to produce a high octane gasoline component). Metal workers use refrigeration to temper steel and cutlery. In transporting temperature-sensitive foodstuffs and other materials by trucks, trains, airplanes and sea-going vessels, refrigeration is a necessity. Dairy products are constantly in need of refrigeration, and it was only discovered in the past few decades that eggs needed to be refrigerated during shipment rather than waiting to be refrigerated after arrival at the grocery store. Meats, poultry and fish all must be kept in climate-controlled environments before being sold. Refrigeration also helps keep fruits and vegetables edible longer. One of the most influential uses of refrigeration was in the development of the sushi/sashimi industry in Japan. Prior to the discovery of refrigeration, many sushi connoisseurs suffered great morbidity and mortality from diseases such as hepatitis A[citation needed]. However the dangers of unrefrigerated sashimi was not brought to light for decades due to the lack of research and healthcare distribution across rural Japan. Around mid-century, the Zojirushi corporation based in Kyoto made breakthroughs in refrigerator designs making refrigerators cheaper and more accessible for restaurant proprietors and the general public.
[edit] Methods of refrigeration
Methods of refrigeration can be classified as non-cyclic, cyclic and thermoelectric.
[edit] Non-cyclic refrigeration
In these methods, refrigeration can be accomplished by melting ice or by subliming dry ice. These methods are used for small-scale refrigeration such as in laboratories and workshops, or in portable coolers. Ice owes its effectiveness as a cooling agent to its constant melting point of 0 C (32 F). In order to melt, ice must absorb 333.55 kJ/kg (approx. 144 Btu/lb) of heat. Foodstuffs maintained at this temperature or slightly above have an increased storage life. Solid carbon dioxide, known as dry ice, is used also as a refrigerant. Having no liquid phase at normal atmospheric pressure, it sublimes directly from the solid to vapor phase at a temperature of -78.5 C (-109.3 F). Dry ice is effective for maintaining products at low temperatures during the period of sublimation.
[edit] Cyclic refrigeration
Main article: Heat pump and refrigeration cycle This consists of a refrigeration cycle, where heat is removed from a low-temperature space or source and rejected to a high-temperature sink with the help of external work,
and its inverse, the thermodynamic power cycle. In the power cycle, heat is supplied from a high-temperature source to the engine, part of the heat being used to produce work and the rest being rejected to a low-temperature sink. This satisfies the second law of thermodynamics. A refrigeration cycle describes the changes that take place in the refrigerant as it alternately absorbs and rejects heat as it circulates through a refrigerator. It is also applied to HVACR work, when describing the "process" of refrigerant flow through an HVACR unit, whether it is a packaged or split system. Heat naturally flows from hot to cold. Work is applied to cool a living space or storage volume by pumping heat from a lower temperature heat source into a higher temperature heat sink. Insulation is used to reduce the work and energy required to achieve and maintain a lower temperature in the cooled space. The operating principle of the refrigeration cycle was described mathematically by Sadi Carnot in 1824 as a heat engine. The most common types of refrigeration systems use the reverse-Rankine vaporcompression refrigeration cycle although absorption heat pumps are used in a minority of applications. Cyclic refrigeration can be classified as: 1. Vapor cycle, and 2. Gas cycle Vapor cycle refrigeration can further be classified as: 1. Vapor-compression refrigeration 2. Vapor-absorption refrigeration [edit] Vapor-compression cycle (See Heat pump and refrigeration cycle and Vapor-compression refrigeration for more details) The vapor-compression cycle is used in most household refrigerators as well as in many large commercial and industrial refrigeration systems. Figure 1 provides a schematic diagram of the components of a typical vapor-compression refrigeration system.
Figure 1: Vapor compression refrigeration The thermodynamics of the cycle can be analyzed on a diagram[10][11] as shown in Figure 2. In this cycle, a circulating refrigerant such as Freon enters the compressor as a vapor. From point 1 to point 2, the vapor is compressed at constant entropy and exits the compressor superheated. From point 2 to point 3 and on to point 4, the superheated vapor travels through the condenser which first cools and removes the superheat and then condenses the vapor into a liquid by removing additional heat at constant pressure and temperature. Between points 4 and 5, the liquid refrigerant goes through the expansion valve (also called a throttle valve) where its pressure abruptly decreases, causing flash evaporation and auto-refrigeration of, typically, less than half of the liquid.
Figure 2: TemperatureEntropy diagram That results in a mixture of liquid and vapor at a lower temperature and pressure as shown at point 5. The cold liquid-vapor mixture then travels through the evaporator
coil or tubes and is completely vaporized by cooling the warm air (from the space being refrigerated) being blown by a fan across the evaporator coil or tubes. The resulting refrigerant vapor returns to the compressor inlet at point 1 to complete the thermodynamic cycle. The above discussion is based on the ideal vapor-compression refrigeration cycle, and does not take into account real-world effects like frictional pressure drop in the system, slight thermodynamic irreversibility during the compression of the refrigerant vapor, or non-ideal gas behavior (if any). More information about the design and performance of vapor-compression refrigeration systems is available in the classic "Perry's Chemical Engineers' Handbook".[12] [edit] Vapor absorption cycle Main article: Absorption refrigerator In the early years of the twentieth century, the vapor absorption cycle using waterammonia systems was popular and widely used. After the development of the vapor compression cycle, the vapor absorption cycle lost much of its importance because of its low coefficient of performance (about one fifth of that of the vapor compression cycle). Today, the vapor absorption cycle is used mainly where fuel for heating is available but electricity is not, such as in recreational vehicles that carry LP gas. It's also used in industrial environments where plentiful waste heat overcomes its inefficiency. The absorption cycle is similar to the compression cycle, except for the method of raising the pressure of the refrigerant vapor. In the absorption system, the compressor is replaced by an absorber which dissolves the refrigerant in a suitable liquid, a liquid pump which raises the pressure and a generator which, on heat addition, drives off the refrigerant vapor from the high-pressure liquid. Some work is required by the liquid pump but, for a given quantity of refrigerant, it is much smaller than needed by the compressor in the vapor compression cycle. In an absorption refrigerator, a suitable combination of refrigerant and absorbent is used. The most common combinations are ammonia (refrigerant) and water (absorbent), and water (refrigerant) and lithium bromide (absorbent). [edit] Gas cycle When the working fluid is a gas that is compressed and expanded but doesn't change phase, the refrigeration cycle is called a gas cycle. Air is most often this working fluid. As there is no condensation and evaporation intended in a gas cycle, components corresponding to the condenser and evaporator in a vapor compression cycle are the hot and cold gas-to-gas heat exchangers in gas cycles. The gas cycle is less efficient than the vapor compression cycle because the gas cycle works on the reverse Brayton cycle instead of the reverse Rankine cycle. As such the working fluid does not receive and reject heat at constant temperature. In the gas cycle, the refrigeration effect is equal to the product of the specific heat of the gas and
the rise in temperature of the gas in the low temperature side. Therefore, for the same cooling load, a gas refrigeration cycle will require a large mass flow rate and would be bulky. Because of their lower efficiency and larger bulk, air cycle coolers are not often used nowadays in terrestrial cooling devices. The air cycle machine is very common, however, on gas turbine-powered jet aircraft because compressed air is readily available from the engines' compressor sections. These jet aircraft's cooling and ventilation units also serve the purpose of pressurizing the aircraft.
[edit] Thermoelectric refrigeration
Thermoelectric cooling uses the Peltier effect to create a heat flux between the junction of two different types of materials. This effect is commonly used in camping and portable coolers and for cooling electronic components and small instruments.
[edit] Magnetic refrigeration
Main article: Magnetic refrigeration Magnetic refrigeration, or adiabatic demagnetization, is a cooling technology based on the magnetocaloric effect, an intrinsic property of magnetic solids. The refrigerant is often a paramagnetic salt, such as cerium magnesium nitrate. The active magnetic dipoles in this case are those of the electron shells of the paramagnetic atoms. A strong magnetic field is applied to the refrigerant, forcing its various magnetic dipoles to align and putting these degrees of freedom of the refrigerant into a state of lowered entropy. A heat sink then absorbs the heat released by the refrigerant due to its loss of entropy. Thermal contact with the heat sink is then broken so that the system is insulated, and the magnetic field is switched off. This increases the heat capacity of the refrigerant, thus decreasing its temperature below the temperature of the heat sink. Because few materials exhibit the required properties at room temperature, applications have so far been limited to cryogenics and research.
[edit] Other methods
Other methods of refrigeration include the air cycle machine used in aircraft; the vortex tube used for spot cooling, when compressed air is available; and thermoacoustic refrigeration using sound waves in a pressurized gas to drive heat transfer and heat exchange. Many Stirling cycle heat engines can be run backwards to act as a refrigerator, and therefore these engines have a niche use in cryogenics.
[edit] Unit of refrigeration
Domestic and commercial refrigerators may be rated in kJ/s, or Btu/h of cooling. Commercial refrigerators in North America are mostly rated in tons of refrigeration, but elsewhere in kW. One ton of refrigeration capacity can freeze one short ton of
water at 0 C (32 F) in 24 hours. Based on that: A much less common definition is: 1 tonne of refrigeration is the rate of heat removal required to freeze a metric ton (i.e., 1000 kg) of water at 0 C in 24 hours. Based on the heat of fusion being 333.55 kJ/kg, 1 tonne of refrigeration = 13,898 kJ/h = 3.861 kW. As can be seen, 1 tonne of refrigeration is 10% larger than 1 ton of refrigeration. Most residential air conditioning units range in capacity from about 1 to 5 tons of refrigeration.
Vapor-compression refrigeration
From Wikipedia, the free encyclopedia Jump to: navigation, search
Vapor-compression refrigeration[1][2] is one of the many refrigeration cycles available for use. It has been and is the most widely used method for air-conditioning of large public buildings, private residences, hotels, hospitals, theaters, restaurants and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Refrigeration may be defined as lowering the temperature of an enclosed space by removing heat from that space and transferring it elsewhere. A device that performs this function may also be called a heat pump.
Contents
[hide]
1 Description of the vapor-compression refrigeration system o 1.1 Refrigerants 2 Thermodynamic analysis of the system 3 Types of gas compressors o 3.1 Reciprocating compressors o 3.2 Rotary screw compressors o 3.3 Centrifugal compressors o 3.4 Scroll compressors o 3.5 Others 4 Other features and facts of interest 5 Applications 6 Economic analysis o 6.1 Advantages o 6.2 Disadvantages 7 History
8 See also 9 References 10 External links
[edit] Description of the vapor-compression refrigeration system
Figure 1: Vapor compression refrigeration
The vapor-compression refrigeration system uses a circulating liquid refrigerant as the medium which absorbs and removes heat from the space to be cooled and subsequently rejects that heat elsewhere. Figure 1 depicts a typical, single-stage vapor-compression system. All such systems have four components: a compressor, a condenser, an expansion valve (also called a throttle valve), and an evaporator. Circulating refrigerant enters the compressor in the thermodynamic state known as a saturated vapor[3] and is compressed to a higher pressure, resulting in a higher temperature as well. The hot, compressed vapor is then in the thermodynamic state known as a superheated vapor and it is at a temperature and pressure at which it can be condensed with typically available cooling water or cooling air. That hot vapor is routed through a condenser where it is cooled and condensed into a liquid by flowing through a coil or tubes with cool water or cool air flowing across the coil or tubes. This is where the circulating refrigerant rejects heat from the system and the rejected heat is carried away by either the water or the air (whichever may be the case). The condensed liquid refrigerant, in the thermodynamic state known as a saturated liquid, is next routed through an expansion valve where it undergoes an abrupt reduction in pressure. That pressure reduction results in the adiabatic flash evaporation of a part of the liquid refrigerant. The auto-refrigeration effect of the adiabatic flash evaporation lowers the temperature of the liquid and vapor refrigerant mixture to where it is colder than the temperature of the enclosed space to be refrigerated.
The cold mixture is then routed through the coil or tubes in the evaporator. A fan circulates the warm air in the enclosed space across the coil or tubes carrying the cold refrigerant liquid and vapor mixture. That warm air evaporates the liquid part of the cold refrigerant mixture. At the same time, the circulating air is cooled and thus lowers the temperature of the enclosed space to the desired temperature. The evaporator is where the circulating refrigerant absorbs and removes heat which is subsequently rejected in the condenser and transferred elsewhere by the water or air used in the condenser. To complete the refrigeration cycle, the refrigerant vapor from the evaporator is again a saturated vapor and is routed back into the compressor.
[edit] Refrigerants
"Freon" is a trade name for a family of haloalkane refrigerants manufactured by DuPont and other companies. These refrigerants were commonly used due to their superior stability and safety properties: they were not flammable nor obviously toxic as were the fluids they replaced, such as sulfur dioxide. Unfortunately, these chlorinebearing refrigerants reach the upper atmosphere when they escape. In the stratosphere, CFCs break up due to UV-radiation, releasing their chlorine atoms. These chlorine atoms act as catalysts in the breakdown of ozone, which does severe damage to the ozone layer that shields the Earth's surface from the Sun's strong UV radiation. The chlorine will remain active as a catalyst until and unless it binds with another particle, forming a stable molecule. CFC refrigerants in common but receding usage include R-11 and R-12. Newer refrigerants that have reduced ozone depletion effect include HCFCs (R-22, used in most homes today) and HFCs (R-134a, used in most cars) have replaced most CFC use. HCFCs in turn are being phased out under the Montreal Protocol and replaced by hydrofluorocarbons (HFCs), such as R-410A, which lack chlorine. However, CFCs, HCFCs, and HFCs all have large global warming potential. Newer refrigerants are currently the subject of research, such as supercritical carbon dioxide, known as R-744.[4] These have similar efficiencies[citation needed] compared to existing CFC and HFC based compounds, and have many orders of magnitude lower global warming potential.
[edit] Thermodynamic analysis of the system
Figure 2: TemperatureEntropy diagram
The thermodynamics of the vapor compression cycle can be analyzed on a temperature versus entropy diagram as depicted in Figure 2. At point 1 in the diagram, the circulating refrigerant enters the compressor as a saturated vapor. From point 1 to point 2, the vapor is isentropically compressed (i.e., compressed at constant entropy) and exits the compressor as a superheated vapor. From point 2 to point 3, the superheated vapor travels through part of the condenser which removes the superheat by cooling the vapor. Between point 3 and point 4, the vapor travels through the remainder of the condenser and is condensed into a saturated liquid. The condensation process occurs at essentially constant pressure. Between points 4 and 5, the saturated liquid refrigerant passes through the expansion valve and undergoes an abrupt decrease of pressure. That process results in the adiabatic flash evaporation and auto-refrigeration of a portion of the liquid (typically, less than half of the liquid flashes). The adiabatic flash evaporation process is isenthalpic (i.e., occurs at constant enthalpy). Between points 5 and 1, the cold and partially vaporized refrigerant travels through the coil or tubes in the evaporator where it is totally vaporized by the warm air (from the space being refrigerated) that a fan circulates across the coil or tubes in the evaporator. The evaporator operates at essentially constant pressure. The resulting saturated refrigerant vapor returns to the compressor inlet at point 1 to complete the thermodynamic cycle. It should be noted that the above discussion is based on the ideal vapor-compression refrigeration cycle which does not take into account real world items like frictional pressure drop in the system, slight internal irreversibility during the compression of the refrigerant vapor, or non-ideal gas behavior (if any).
[edit] Types of gas compressors
Main article: Gas compressor
The most common compressors used in chillers are reciprocating, rotary screw, centrifugal, and scroll compressors. Each application prefers one or another due to size, noise, efficiency and pressure issues.
[edit] Reciprocating compressors
Main article: Reciprocating compressor
Reciprocating compressors are piston-style, positive displacement compressors.
[edit] Rotary screw compressors
Main article: Rotary screw compressor
Rotary screw compressors are also positive displacement compressors. Two meshing screw-rotors rotate in opposite directions, trapping refrigerant vapor, and reducing the volume of the refrigerant along the rotors to the discharge point.
[edit] Centrifugal compressors
Main article: Centrifugal compressor
Centrifugal compressors are dynamic compressors. These compressors raise the pressure of the refrigerant by imparting velocity or dynamic energy, using a rotating impeller, and converting it to pressure energy.
[edit] Scroll compressors
Main article: Scroll compressor
Scroll compressors are also positive displacement compressors. The refrigerant is compressed when one spiral orbits around a second stationary spiral, creating smaller and smaller pockets and higher pressures. By the time the refrigerant is discharged, it is fully pressurized.
Das könnte Ihnen auch gefallen
- Project On LPG Refrigerator Mechanical ProjectDokument53 SeitenProject On LPG Refrigerator Mechanical Projectpatel ketan86% (69)
- Final Project Report On Domestic RefrigeratorDokument25 SeitenFinal Project Report On Domestic RefrigeratorAniket Kalore88% (8)
- Automobile Air Conditioning Air Conditioning (Album) ACDokument21 SeitenAutomobile Air Conditioning Air Conditioning (Album) ACantoniodrifterNoch keine Bewertungen
- Thermoelectric Refrigeration SystemDokument39 SeitenThermoelectric Refrigeration SystemVikas ChopraNoch keine Bewertungen
- Hydraulic MachineryDokument13 SeitenHydraulic MachineryCarlito PantalunanNoch keine Bewertungen
- 01 History of Refrigeration PDFDokument34 Seiten01 History of Refrigeration PDFNSS GBPECNoch keine Bewertungen
- History of RefrigerationDokument15 SeitenHistory of Refrigerationattan5tsuiNoch keine Bewertungen
- Cold Chain LogisticsDokument65 SeitenCold Chain LogisticsMukund Goel0% (1)
- Air ConditionerDokument13 SeitenAir Conditionermuthu17473Noch keine Bewertungen
- Absorption Chiller Rev3 (MSB) 20-9Dokument96 SeitenAbsorption Chiller Rev3 (MSB) 20-9norshakeela100% (3)
- Refrigerator Priciples and WorkingDokument16 SeitenRefrigerator Priciples and WorkingSanthan SalaiNoch keine Bewertungen
- Dutch Ovens Chronicled: Their Use in the United StatesVon EverandDutch Ovens Chronicled: Their Use in the United StatesBewertung: 3 von 5 Sternen3/5 (1)
- History of Refrigeration SystemsDokument4 SeitenHistory of Refrigeration SystemsZa YonNoch keine Bewertungen
- Non Conventional Refg. SysDokument26 SeitenNon Conventional Refg. SysJack Admark100% (3)
- Boiler (Steam Generator) : From Wikipedia, The Free EncyclopediaDokument20 SeitenBoiler (Steam Generator) : From Wikipedia, The Free EncyclopediaCarlito PantalunanNoch keine Bewertungen
- End Face Mechanical Seal: Navigation SearchDokument16 SeitenEnd Face Mechanical Seal: Navigation SearchCarlito PantalunanNoch keine Bewertungen
- Compressor-Less: Historical ApplicationsDokument70 SeitenCompressor-Less: Historical Applicationssuryakantshrotriya100% (1)
- RefrigerationDokument8 SeitenRefrigerationkgsatish1979Noch keine Bewertungen
- Refrigeration: From Wikipedia, The Free EncyclopediaDokument14 SeitenRefrigeration: From Wikipedia, The Free Encyclopediaayan1688Noch keine Bewertungen
- R&AC Assign # 1Dokument22 SeitenR&AC Assign # 1Hassan TalhaNoch keine Bewertungen
- History and Application of Refrigeration 1Dokument12 SeitenHistory and Application of Refrigeration 1Yvonne Dela RosaNoch keine Bewertungen
- Thermally Insulated Heat Pump Refrigeration Food Storage Technique Bacteria Spoilage Freezing PointDokument5 SeitenThermally Insulated Heat Pump Refrigeration Food Storage Technique Bacteria Spoilage Freezing Pointabdul ali munderNoch keine Bewertungen
- RefrigerationDokument14 SeitenRefrigerationJay ParkerNoch keine Bewertungen
- Project On RefrigeratorDokument13 SeitenProject On Refrigeratordebi ranjan surNoch keine Bewertungen
- Govt. Polytechnic College: Ganderbal. KashmirDokument12 SeitenGovt. Polytechnic College: Ganderbal. KashmirWajahat100% (1)
- R & Ac 1Dokument38 SeitenR & Ac 1ahsanNoch keine Bewertungen
- A Study On Refrigeration: Poonam DhankharDokument9 SeitenA Study On Refrigeration: Poonam Dhankharpanshul enggNoch keine Bewertungen
- Air Conditioning: HistoryDokument9 SeitenAir Conditioning: HistoryHarish ChandranNoch keine Bewertungen
- A History of Refrigeration and Air ConditioningDokument4 SeitenA History of Refrigeration and Air ConditioningSikander GirgoukarNoch keine Bewertungen
- Introduction To Refrigeration and Air ConditioningDokument7 SeitenIntroduction To Refrigeration and Air ConditioningFurqanNoch keine Bewertungen
- Review Article On RefrigerationDokument11 SeitenReview Article On RefrigerationSURAJ NAGNoch keine Bewertungen
- Compilation in Basic Refrigeration and Air Conditioning (Laboratory and Lecture)Dokument39 SeitenCompilation in Basic Refrigeration and Air Conditioning (Laboratory and Lecture)Jolina AnitNoch keine Bewertungen
- 2004-Refriferants Past, Present and FutureDokument11 Seiten2004-Refriferants Past, Present and FutureKary Nadeshiko100% (1)
- Mee 551Dokument3 SeitenMee 551george josephNoch keine Bewertungen
- Freezer: Cooling Thermally Insulated Heat Pump Food Storage TechniqueDokument24 SeitenFreezer: Cooling Thermally Insulated Heat Pump Food Storage TechniquegowthammuthusamyNoch keine Bewertungen
- Refrigerator: "Fridge" and "Freezer" Redirect Here. For Other Uses, See and - For Other Uses, See - See AlsoDokument26 SeitenRefrigerator: "Fridge" and "Freezer" Redirect Here. For Other Uses, See and - For Other Uses, See - See Alsosanjaydubey3Noch keine Bewertungen
- RefrigerationDokument13 SeitenRefrigerationharishnowNoch keine Bewertungen
- Refrigeration Systems & Comparative Study of VCRSDokument12 SeitenRefrigeration Systems & Comparative Study of VCRSShashi Bhushan KumarNoch keine Bewertungen
- Dokumen - Tips - Vtu Cryogenics Notes Unit 1 Introduction To Cryogenic SystemsDokument50 SeitenDokumen - Tips - Vtu Cryogenics Notes Unit 1 Introduction To Cryogenic SystemsJennifer ThomasNoch keine Bewertungen
- Lesson1 and Lesson 2Dokument10 SeitenLesson1 and Lesson 2Gerlan Madrid MingoNoch keine Bewertungen
- CryogenicsDokument169 SeitenCryogenicsVedaj KavilNoch keine Bewertungen
- Boston IceDokument203 SeitenBoston IceSandra MianNoch keine Bewertungen
- Assignment 123Dokument23 SeitenAssignment 123Usama MughalNoch keine Bewertungen
- A History of Comfort Cooling Using IceDokument7 SeitenA History of Comfort Cooling Using IceLuis AlonsoNoch keine Bewertungen
- Hydrocarbon Systems Can Significantly Reduce Energy Consumption Compared To Synthetic Fluids in Commercial ApplicationsDokument8 SeitenHydrocarbon Systems Can Significantly Reduce Energy Consumption Compared To Synthetic Fluids in Commercial ApplicationsHamza ZafarNoch keine Bewertungen
- Refrigeration Is A Process of Moving Heat From One Location To AnotherDokument18 SeitenRefrigeration Is A Process of Moving Heat From One Location To AnotherZerotheoryNoch keine Bewertungen
- ME LabDokument24 SeitenME LabVon A. DamirezNoch keine Bewertungen
- Air Conditioning: Hvac Automobile Air Conditioning Air Conditioning (Album) ACDokument21 SeitenAir Conditioning: Hvac Automobile Air Conditioning Air Conditioning (Album) ACArthur BactongNoch keine Bewertungen
- Air Conditioning: Hvac Automobile Air Conditioning Air Conditioning (Album) ACDokument9 SeitenAir Conditioning: Hvac Automobile Air Conditioning Air Conditioning (Album) ACKSHETRIMAYUM MONIKA DEVINoch keine Bewertungen
- Term Paper: Refrigertaor vs. Heat PumpDokument24 SeitenTerm Paper: Refrigertaor vs. Heat PumpHoney BhatiaNoch keine Bewertungen
- History of AC Unit (Project)Dokument4 SeitenHistory of AC Unit (Project)MOVNoch keine Bewertungen
- Refrigeration: Wiki Loves Monuments: Photograph A Monument, Help Wikipedia and Win!Dokument34 SeitenRefrigeration: Wiki Loves Monuments: Photograph A Monument, Help Wikipedia and Win!Ryan Compala LictagNoch keine Bewertungen
- Khushi 22bba027Dokument8 SeitenKhushi 22bba027devamshah286Noch keine Bewertungen
- Solar Thesis Final FinalDokument48 SeitenSolar Thesis Final FinalHaseeb AminNoch keine Bewertungen
- Sankar SK ProjectDokument4 SeitenSankar SK ProjectSanju SkNoch keine Bewertungen
- 347Dokument19 Seiten347jikurNoch keine Bewertungen
- 2005 Carbon Dioxide - New Uses For An Old RefrigerantDokument9 Seiten2005 Carbon Dioxide - New Uses For An Old RefrigerantIvana MacedoNoch keine Bewertungen
- History and Application of RefrigerationDokument18 SeitenHistory and Application of RefrigerationKanwarnain SinghNoch keine Bewertungen
- Nomenclature:: SymbolsDokument24 SeitenNomenclature:: Symbolsjess calderonNoch keine Bewertungen
- Genius Engineering Inventions: From the Plow to 3D PrintingVon EverandGenius Engineering Inventions: From the Plow to 3D PrintingNoch keine Bewertungen
- Scientific American Supplement, No. 455, September 20, 1884Von EverandScientific American Supplement, No. 455, September 20, 1884Noch keine Bewertungen
- Quantity Surveyor - : Rider Levett Bucknall Philippines, IncDokument3 SeitenQuantity Surveyor - : Rider Levett Bucknall Philippines, IncCarlito PantalunanNoch keine Bewertungen
- All in PlanDokument3 SeitenAll in PlanCarlito PantalunanNoch keine Bewertungen
- SeaServiceForm - E.T.o - Cadet Carlito H. PantalunanDokument138 SeitenSeaServiceForm - E.T.o - Cadet Carlito H. PantalunanCarlito PantalunanNoch keine Bewertungen
- Mechanical Seal Axle Bearing Grooves Screw ThreadsDokument1 SeiteMechanical Seal Axle Bearing Grooves Screw ThreadsCarlito PantalunanNoch keine Bewertungen
- Plumbing Continuing Education Test 5Dokument14 SeitenPlumbing Continuing Education Test 5Carlito PantalunanNoch keine Bewertungen
- Entropy: Navigation SearchDokument20 SeitenEntropy: Navigation SearchCarlito PantalunanNoch keine Bewertungen