Beruflich Dokumente
Kultur Dokumente
2.2.7 Entropy
Hochgeladen von
May LeeOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
2.2.7 Entropy
Hochgeladen von
May LeeCopyright:
Verfügbare Formate
2.2.
7 Entropy
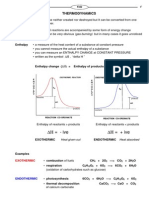
There are two thermodynamic concepts that are considered in chemical reactions; enthalpy and entropy. Entropy is often described as a measure of disorder. All substances possess some degree of disorder because particles are always moving, so S is always a __________ __________. Entropy (S)
Nature tends to move from order to disorder in isolated systems e.g. gas molecules spread out over time to fill a space, increasing their entropy. Entropy explains why some things happen naturally:
Entropy increases during the changes in state that give more randomness:
Entropy increases when a solid lattice dissolves Entropy increases in a reaction where a gas is evolved
Entropy Change in Chemical Reactions, S Entropy calculations using standard entropy values use the following equation:
For Example: Formation of ammonia from nitrogen and hydrogen
Key Definitions Standard entropy change of reaction -
Gibbs Free Energy Change in Chemical Reactions, G A combination of two factors, enthalpy and entropy, govern the feasibility of reactions. Exothermic processes tend to happen quite often but, equally endothermic processes which lead to an increase in entropy also happen quite often. The sometimes conflicting demands of enthalpy and entropy are brought together in the relationship:
This combines the influence of both enthalpy change and entropy change. With H negative (exothermic) and S positive (increase in entropy) then G will be ___________ If G for a reaction is negative (or zero) then the reaction is feasible; if it is positive then the reaction is not feasible. Note 1: If H is positive (endothermic) then, providing TS is __________ positive then the reaction will be feasible. Note 2: The term TS is temperature dependent meaning that some reactions may be feasible at one temperature but not at another. Note 3: A reaction with G negative means it can go. It does not necessarily mean that it will go. There is the kinetics of a reaction to take into account as well as the thermodynamics. For example, a feasible reaction ( thermodynamically unstable) may have a very high _________ preventing it from proceeding to any significant extent (kinetically stable). Example: Calculate the standard free-energy change for the combustion of graphite at 298K. C(s) + O2(g) CO2(g) (H = -394 kJ mol-1 S = +3.3 J K-1 mol-1) Answer:
Note: Because S is so small, G will not vary much with temperature, though in practice the reaction is extremely slow at 298K due to high activation energy. Example: Calculate the standard free-energy change for the rusting of iron at 298K 2Fe(s) + 1.5O2(g) Fe2O3(s) (H = -825 kJ mol-1 S = -272 J K-1 mol-1) Answer:
Note: G is very negative so the rusting of iron is highly feasible at room temperature even though S is negative. The reaction would become less feasible at higher temperatures as TS gets closer in value to H. Example: Calculate the standard free-energy change for the decomposition of 1 mol sodium hydrogencarbonate at 298K. 2NaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g) (H = +130 kJ mol-1 S = +335 J K-1 mol-1) Answer:
Note: At 298K G is positive so the reaction is not feasible. However, G is not very positive so raising the temperature will make TS more positive and at a certain temperature the reaction will become feasible. Effect of Temperature on Feasibility Reaction H negative (exothermic) S positive (entropy gain) Feasible at all T S negative (entropy loss)
H positive (endothermic)
Not feasible at any T.
Calculating the Temperature at which a reaction becomes Feasible A reaction that is not feasible at one temperature may become feasible if the temperature is changed. The temperature at which feasibility is just achieved is the temperature at which there is no tendency for the reaction to go one way or the other. At this temperature G = 0 so:
Example: Calculate the temperature at which the thermal decomposition of sodium hydrogencarbonate becomes feasible. 2NaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g) (H = +130 kJ mol-1 S = +335 J K-1 mol-1) Answer:
Note: The feasibility temperature gives the break-even point for the reaction. That is the temperature at which the concentrations of reactants and products become roughly equal. In this example, the reaction becomes more feasible as the temperature is increased above this point and product formation will be increasingly favoured. Example: Calculate the temperature above which limestone will spontaneously decompose to quicklime and carbon dioxide. CaCO3(s) CaO(s) + CO2(g) (H = +178 kJ mol-1 S = +161 J K-1 mol-1) Answer:
Entropy in Physical Changes In many physical changes there is an increase or decrease in disorder (and hence entropy). 1. Melting (Fusion) At 00C (273K) and 100 kPa a mixture of ice and water has no spontaneous tendency to either solidify or liquefy. The mixture is at equilibrium and G = 0 H2O(s) = H2O(l) H = +6 kJ mol-1 The entropy change S for melting of ice at 273K can be determined as follows:
2..Freezing (Solidification) H2O(l) = H2O(s) H = -6 kJ mol-1 The entropy change S for freezing of water at 273K can be determined as follows:
3. Boiling (Vaporisation) H2O(l) = H2O(g) H = +44 kJ mol-1 The entropy change S for boiling of water at 373K can be determined as follows:
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Determine Specific Heat of Metal (PHY400Dokument5 SeitenDetermine Specific Heat of Metal (PHY400May LeeNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Potentiometric Titration of a Weak AcidDokument14 SeitenPotentiometric Titration of a Weak AcidMay LeeNoch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Analysis of Aspirin in Commercial Apc Tablet Using Ftir SpectrosDokument5 SeitenAnalysis of Aspirin in Commercial Apc Tablet Using Ftir SpectrosMay Lee100% (1)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (894)
- Questions CHM 520 Exp 8Dokument2 SeitenQuestions CHM 520 Exp 8May Lee100% (1)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- SM Case StudyDokument13 SeitenSM Case StudyMay LeeNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Fid1 Rettime Width (Min) HeightDokument1 SeiteFid1 Rettime Width (Min) HeightMay LeeNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Strategic Vision and MissionDokument33 SeitenStrategic Vision and MissionMay LeeNoch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Find Sum and Reverse 50 NumbersDokument7 SeitenFind Sum and Reverse 50 NumbersMay LeeNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Exp 3 - Determination of Avogadro's NumberDokument3 SeitenExp 3 - Determination of Avogadro's NumberMay LeeNoch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- UTM Arau Prepares 4-BromobenzophenoneDokument7 SeitenUTM Arau Prepares 4-BromobenzophenoneMay Lee100% (1)
- An Introduction To FluorescenceDokument36 SeitenAn Introduction To FluorescenceFrank LaporteNoch keine Bewertungen
- Introduction MGTDokument3 SeitenIntroduction MGTMay LeeNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- ANALYSIS OF HYDROCARBONS IN COMMON FUELSDokument1 SeiteANALYSIS OF HYDROCARBONS IN COMMON FUELSMay LeeNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- C:\Data File Peak AnalysisDokument1 SeiteC:\Data File Peak AnalysisMay LeeNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Img 0009Dokument1 SeiteImg 0009May LeeNoch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Img 0010Dokument1 SeiteImg 0010May LeeNoch keine Bewertungen
- Img 0008Dokument1 SeiteImg 0008May LeeNoch keine Bewertungen
- Fid1 Rettime Height: X X X X X X X X X BV VB BV VV VP VV PV 0 - 61Dokument1 SeiteFid1 Rettime Height: X X X X X X X X X BV VB BV VV VP VV PV 0 - 61May LeeNoch keine Bewertungen
- ' Injection I0/10/2AI3 Location: Inj: Inj:: Operator DieselDokument1 Seite' Injection I0/10/2AI3 Location: Inj: Inj:: Operator DieselMay LeeNoch keine Bewertungen
- Data File Peak AnalysisDokument1 SeiteData File Peak AnalysisMay LeeNoch keine Bewertungen
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Fid1 Rettime Width (Min) Height: BV VV VV VV VV VV VV - VV VV VV VV VV VV VV VVDokument1 SeiteFid1 Rettime Width (Min) Height: BV VV VV VV VV VV VV - VV VV VV VV VV VV VV VVMay LeeNoch keine Bewertungen
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- Diesel fuel analysis by gas chromatographyDokument1 SeiteDiesel fuel analysis by gas chromatographyMay LeeNoch keine Bewertungen
- Result Exp 5 6 chm510Dokument1 SeiteResult Exp 5 6 chm510May LeeNoch keine Bewertungen
- Manually Method: C: /Hpchem/1/Methods/Jeff.M AMDokument1 SeiteManually Method: C: /Hpchem/1/Methods/Jeff.M AMMay LeeNoch keine Bewertungen
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Img 0001 PDFDokument1 SeiteImg 0001 PDFMay LeeNoch keine Bewertungen
- Result Exp 5 6 chm510Dokument1 SeiteResult Exp 5 6 chm510May LeeNoch keine Bewertungen
- Enhanced imaging results reportDokument1 SeiteEnhanced imaging results reportMay LeeNoch keine Bewertungen
- Exp 5 & 6 DataDokument1 SeiteExp 5 & 6 DataMay LeeNoch keine Bewertungen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Fid1 Rettime Width (Min) HeightDokument1 SeiteFid1 Rettime Width (Min) HeightMay LeeNoch keine Bewertungen
- Img 0001 PDFDokument1 SeiteImg 0001 PDFMay LeeNoch keine Bewertungen
- Class - 10th Chemistry Chapter 1 Chemical Equations PDFDokument248 SeitenClass - 10th Chemistry Chapter 1 Chemical Equations PDFHarsh Sheokand0% (1)
- Enthalpy ChangesDokument2 SeitenEnthalpy Changesapi-296833859100% (1)
- BioenergeticsDokument30 SeitenBioenergeticsObaiah Jamakala100% (1)
- F&EI Calculation WorkbookDokument58 SeitenF&EI Calculation WorkbookMufti Sinergi SolusiNoch keine Bewertungen
- ChemistryDokument7 SeitenChemistryUttam RajNoch keine Bewertungen
- Thermodynamics: DR Onesmus MunyatiDokument73 SeitenThermodynamics: DR Onesmus MunyatiomunyatiNoch keine Bewertungen
- Phase Changes WorksheetDokument4 SeitenPhase Changes Worksheetsquishy squigyNoch keine Bewertungen
- Energetics QuestionsDokument20 SeitenEnergetics Questionslianchen251110Noch keine Bewertungen
- Enthalpy Changes ExplainedDokument27 SeitenEnthalpy Changes ExplainedM BNoch keine Bewertungen
- Chapter 5 Thermochemistry - b5Dokument70 SeitenChapter 5 Thermochemistry - b5kikianitaNoch keine Bewertungen
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- FIRST LAW OF THERMODYNAMICS EXPLAINEDDokument9 SeitenFIRST LAW OF THERMODYNAMICS EXPLAINEDSunshine Llamas GuzmanNoch keine Bewertungen
- Dairy Lesson Plans on Physical and Chemical ChangesDokument12 SeitenDairy Lesson Plans on Physical and Chemical ChangesVikneswaran Gunahlan NeshNoch keine Bewertungen
- Matriculation Chemistry (Thermochemistry)Dokument54 SeitenMatriculation Chemistry (Thermochemistry)ridwan100% (2)
- Experiment #3 / Unit 6 Calorimetry - Measuring Heat Changes During A Physical or Chemical ChangeDokument2 SeitenExperiment #3 / Unit 6 Calorimetry - Measuring Heat Changes During A Physical or Chemical Changeapi-368121935Noch keine Bewertungen
- Chapter 5 - Properties and Structure of MatterDokument52 SeitenChapter 5 - Properties and Structure of MattermishtinilNoch keine Bewertungen
- Two Bit Circus Middle School 8th Grade Chemistry 5e Lesson Plans Educator 3 Lesson Plans For Chemistry UnitDokument18 SeitenTwo Bit Circus Middle School 8th Grade Chemistry 5e Lesson Plans Educator 3 Lesson Plans For Chemistry Unitami1967Noch keine Bewertungen
- Analyzing nuclear reactions and mass changesDokument1 SeiteAnalyzing nuclear reactions and mass changesHaroon GhaniNoch keine Bewertungen
- Hess Exemplar Lab With CommentsDokument6 SeitenHess Exemplar Lab With CommentsT-girlNoch keine Bewertungen
- Chapter 17 ThermochemistryDokument72 SeitenChapter 17 ThermochemistryUma FadziliaNoch keine Bewertungen
- Chapter 6 Chemical EnergeticsDokument42 SeitenChapter 6 Chemical Energeticsgajenrao100% (1)
- Differential Thermal AnalysisDokument42 SeitenDifferential Thermal AnalysisArooj SajjadNoch keine Bewertungen
- Marking Scheme Paper 1 2 3 SBP Trial SPM 2009Dokument21 SeitenMarking Scheme Paper 1 2 3 SBP Trial SPM 2009Mohd Khairul AnuarNoch keine Bewertungen
- Energy and Chemistry: Camarines Norte State CollegeDokument15 SeitenEnergy and Chemistry: Camarines Norte State CollegeFruitiliciousNoch keine Bewertungen
- 7.01 Toolbox AssignmentcompleteDokument2 Seiten7.01 Toolbox AssignmentcompletealexaNoch keine Bewertungen
- Topic 5 Energy Changes Revision MatDokument4 SeitenTopic 5 Energy Changes Revision MatMireiaNoch keine Bewertungen
- Week 4.1 AdvancedDokument21 SeitenWeek 4.1 AdvancedAaron MostNoch keine Bewertungen
- Ive + Ive: ThermodynamicsDokument10 SeitenIve + Ive: ThermodynamicsAbbas HaiderNoch keine Bewertungen
- Thermochemistry KPADDokument16 SeitenThermochemistry KPADJOANNA MAGDALIN A/P JOSEPH MoeNoch keine Bewertungen
- Energetics Lab - ChemDokument5 SeitenEnergetics Lab - Chemvaibhav100% (1)
- Fire Technology and Arson InvestigatiionDokument4 SeitenFire Technology and Arson InvestigatiionRico T. MusongNoch keine Bewertungen
- Nuclear Energy in the 21st Century: World Nuclear University PressVon EverandNuclear Energy in the 21st Century: World Nuclear University PressBewertung: 4.5 von 5 Sternen4.5/5 (3)
- Stuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldVon EverandStuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldBewertung: 4 von 5 Sternen4/5 (289)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeVon EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeBewertung: 5 von 5 Sternen5/5 (1)
- Piping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationVon EverandPiping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationBewertung: 4 von 5 Sternen4/5 (18)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeVon EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeBewertung: 4.5 von 5 Sternen4.5/5 (3)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsVon EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsBewertung: 4 von 5 Sternen4/5 (146)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsVon EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNoch keine Bewertungen