Beruflich Dokumente
Kultur Dokumente
Pathophysiology Exam 4 - Review
Hochgeladen von
Waqas GillCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Pathophysiology Exam 4 - Review
Hochgeladen von
Waqas GillCopyright:
Verfügbare Formate
Waqas A. Gill Pathophysiology Exam 4 - Review Ch.
15 Brain & Spinal Cord Injury II Acute spinal cord injury: o Prognosis: Focuses on the degree of brain/spinal function, strength of reflexes, life expectancy, risks for other conditions (pneumonia, thrombosis, urinary disorders, etc), and mortality rates. Spinal shock:
Lesions and signs associated with severing of spinal cord o Sympathetic: Lesions along the spine commonly lead to very high BP, severe headaches, skin irritation above the area of spinal injury, and severe convulsions. o Parasympathetic: Slow heart rate, pale skin, and perfuse sweating. Autonomic dysreflexia: o Manifestations: 1-12 weeks after Spinal injury at/above T-7 A potentially fatal complication that occurs in response to stimulation of internal organs (i.e. expanded bladder) or of the skin, after the shock of a spinal injury has worn off. o Reasons for Clinical Manifestations: Spinal lesions usually lead to afferent impulses becoming blocked (no sensory input to the brain), and so in the example of urinary bladder distension, reflexes are activated below the lesion to increase sympathetic activity below the lesion Increased BP.
As this happens, cardiac baroreceptors detect this BP shift and compensates by lowering heart-rate and dilating blood-vessels above the lesion. Since the brain still cannot send signals to regulate activity below the lesion, overall, BP is not regulated (up to 300mm Hg).
o Treatment and Management: Elevation of bed to 45o to help decrease BP, removal of noxious stimuli by providing bladder/bowel relief & removal of tight clothing, and through ganglionic blockers. Phantom pain o Pain experienced despite the loss/realignment of sensory/synaptic pathways, release of excitatory or accessory pain pathways, & dendritic sprouting in the area of injury as tissues heals.
Ch. 16 Autonomic Nervous System Understand the divisions of the nervous system particularly ANS o Nervous system CNS & PNS Sensory & Motor Somatic & ANS o ANS Sympathetic, Parasympathetic, and Enteric (digestive tract smooth muscle contractions, glandular secretions, & detect physical/ionic changes in lumen content). Somatic: Skeletal muscle, Conscious & unconscious (step on tac) movement, Skeletal muscle contracts, 1-synapse mechanism, ACh, Nicotinic receptors, and myelinated axons. Autonomic: Smooth/Cardiac muscle & glands, unconscious regulation, stimulation/inhibition, 2synapse mechanism, ACh (pre-ganglionic) + ACh/Nor-Epineph (post-ganglionic), Myelinated axons (pre-ganglionic) & Unmyelinated axons (post-ganglionic). Sympathetic and parasympathetic system: Organizational and functional differences o Sympathetic & parasympathetic divisions often supply the same organs but differ in # of features and will often exhibit opposing actions. o Sympathetic Chain-Ganglion: Preganglionic cell bodies in lateral horns of spinal vertebrae T1-L2. ( Thoracolumbar division ) o Parasympathetic Division: Preganglionic cell bodies in nuclei of brainstem or lateral parts of spinal cord gray matter from S2-S4 (Craniosacral division). o Preganglionic axons Cranial III, VII, IX, and X Pelvic splanchnic nerves o Terminal Ganglia: Located near the innervated organ or embedded in the organ-wall. Routes of sympathetic axons (4) o Spinal nerves, Sympathetic nerves, Splanchnic nerves, & Innervation to adrenal gland.
ANS receptors and neurotransmitters
o Parasympathetic only uses ACh for both pre and post-ganglionic binding Cholinergic receptors Nicotinic (always excitatory) & Muscarinic (Excitatory/Inhibitory) cholinergic receptors. o Sympathetic system only uses ACh for pre & either ACh/NE for post Adrenergic receptors (NE) Alpha (1 & 2) and Beta (1 & 2) adrenergic receptors (Excitatory/Inhibitory).
Influence of brain on ANS functions
ANS regulation o Autonomic reflexes control most of activity of visceral organs, glands, and blood vessels, and is: o Influenced by the Hypothalamus (overall ANS control) & higher brain-centers. o Brainstem: Survival functions (especially related to Glossopharyngeal and Vagus nerve, but others too).
Visceral reflex arc and various autonomic reflexes o Visceral reflex: Always have polysynaptic pathways & afferent fibers are found in spinal/autonomic nerves. o Parasympathetic reflex: Vagus lowers heart rate. o Sympathetic reflex: Cardiac accelerator nerves cause heart rate to increase. o Baroreceptors in the carotid sinus and aortic arch monitor blood pressure. o If BP inc., APs are conducted by Glossopharyngeal and Vagus to cardioregulatory & vasomotor centers in the medulla to initiate homeostatic mechanisms. o Cushings response (Hypertension & Bradycardia) happens because of a disconnect b/w the brainstem and middle of spine, which allows opposing reflexes to activate, simultaneously. Functional generalization of ANS o Dual innervation: To most organs with sympathetic and parasympathetic having the opposite effects. o Coordination: Either division alone or both working together can coordinate activities of different structures. o Sympathetic prepares body for physical activity: or flight-or-fight response. At rest, the sympathetic system is responsible for maintaining BP. Exercise: Inc. heart-rate, skeletal/cardiac vessel + airway dilation, inc. glycolysis + -Oxid., body-temp. inc., sweat-gland secretions inc., & dec. in non-essential organ activities. o Parasympathetic - Resting state: SLUDD: salivation, lacrimation, urination, digestion, defecation Various types of receptors in detail o Cholinergic: Bind Acetylcholine (ACh) 2 types, which are named after drugs that mimic ACh. i. Nicotinic: Found on somatic targets (post-synaptic), all ganglionic neurons of Sym/Parasym divisions, & the hormone-producing cells of the adrenal medulla. (Always stimulatory). Works by opening Na+-gated channels on post-synaptic membranes. ii. Muscarinic: Found on all effector cells stimulated by postganglionic cholinergic fibers. Induces indirect effects through G-proteins. (Stimulatory or Inhibitory). Works by activating G-protein coupled receptors. o Adrenergic: Bind Epinephrine & Norepinephrine (NE) 2 types, all mediated through G-proteins i. Alpha: Generally stimulatory: 1 Releases intracellular Ca2+ from the E.R. (Excitatory Vasoconstriction) 2 Decreases cAMP levels (Inhibitory Arterial dilation with venous constriction) Mechanism: Endothelial cells make up blood vessels, which are surrounded by smooth muscle cells. When you have a sympathetic (alpha) response, these smooth muscle cells contract, thus constricting the blood vessels and increasing the blood pressure to increase O2 transport. It will usually lead to vasoconstriction of peripheral blood vessels, thus increasing the blood flow to essential skeletal muscles and the heart, and dec. blood flow to extremities. ii. Beta: Generally inhibitory, except for the heart, where -receptors are stimulatory. This exception explains why -blockers (antagonists) reduce ones heart-rate. 1 Increases cAMP levels (Inc. metabolism, heart-rate, and force of contraction) 2 Decreases cAMP levels (Relax respiratory smooth muscle to ease breaths 3 Increases cAMP levels (Activation of lipolysis in Adipose [fat] tissue).
Function of sympathetic and parasympathetic nerves on target organs
o Radial muscle radiates (spreads): Contraction leads to pupillary dilation Sympathetic system Rich in Alpha-1 receptors Small pupil to large pupil (Mydriasis) o Sphincter muscle: Contraction leads to pupillary contraction Parasympathetic system Big pupil to small pupil (Miosis) o Ciliary muscle: -2 activation leads to relaxation and lens flattening Helps with far-sight. Nightshade (flower) leads to pupillary dilation (but also super-hypertension), it was originally given to female entertainers by the Romans to make their eyes more beautiful.
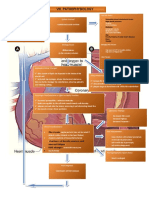
o The picture on the right shows the movement of SA-impulses as they travel from the SA-node AV-node Interventricular septum Left/Right Bundle branches Purkinje fibers
o For all vessels/glands/tissues, sympathetic activation commonly leads to constriction, while parasympathetic activation usually leads to dilation.
o Bronchial relaxation helps us breathe faster and more: Relaxation is usually paired with less secretion, while Constriction is usually paired with more secretion.
o Sympathetic activation of the Liver leads to all functions that would increase glucose; vice-versa Effects of drugs: o Atropine Blocks parasympathetic effects, and so helpful for pupillary dilation. o Neostigmine Inhibits acetylcholinesterase & used to treat Myasthenia-Gravis (muscle weakness) Influence Cholinergic receptor-activity o Tricyclic Antidepressants Prolong the activity of Norepinephrine on postsynaptic membranes. Influence Adrenergic receptor-activity o OTC drugs for Colds, Allergies, and Nasal Congestion Stimulate -Adrenergic receptors o Beta-blockers attach mainly to 1 receptors and reduce heart rate and prevent arrhythmias ANS Disorders o Raynauds Disease: Constriction of blood vessels in the periphery of the body, especially the fingers. Symptoms: Pale cold hands prone to ulcerations & gangrene (dec.blood) Causes: High sensitivity of blood vessels to sympathetic innervation. o Hyperhidrosis: excessive sweating, exaggerated sympathetic innervation of the sweat glands o Achalasia: Difficulty swallowing/controlling contractions of esophagus where it enter the stomach and therefore interrupting normal peristaltic contractions of esophagus (bottom pic severe constriction in central region). o Hirschsprung Megacolon Disease: Functional obstruction of lower colon and rectum. Ineffective parasympathetic function and a predominance of sympathetic stimulation of colon inhibit peristaltic action.
Ch. 17 Integration of Nervous System Sensation:
i.
Senses and Sensory Receptors: Detect stimuli inside/outside the body and converts them into APs General receptors: All over body and generate APs (generator potentials) from primary receptors and send them to the brain. a) Somatic: Body & environment (touch, pressure, temp., proprioception, pain). b) Visceral: Internal organs (pain, pressure) Special senses: Smell, taste, sight, hearing, & balance. These receptors produce potentials but also release neurotransmitters to secondary receptors that travel to the brain. Types of receptors: Based on stimuli a) Mechanoreceptors: Compression, bending, & stretching of cells. o Touch, pressure, proprioception, hearing, and balance b) Chemoreceptors: Chemicals attach to receptors on their membranes (Smell & taste) c) Thermoreceptors: Respond to changes in temperature d) Photoreceptors: Respond to light (vision) e) Nociceptors: Extreme mechanical, chemical, or thermal stimuli. (Pain-perception) o Any of the above receptors, under extreme conditions, can act as a nociceptor. Types of receptors: Based on location a) Exteroreceptors: Associated with skin b) Visceroreceptors: Associated with organs c) Proprioceptors: Associated with joints/tendons Responses to senses: a) Accommodation (adaptation): Decreased sensitivity to a continued stimulus. b) Proprioception: Provide info about the precise position/rate of movement of various body parts, the weight of an object being held in hand, & joints range of movement. o Slowly adapting (Tonic) receptors: Accommodate very slowly. Ex. Know where little finger is without looking o Rapidly adapting (Phasic) receptors: Accommodate rapidly. Ex. You know where hand is as it moves Ascending (Sensory) Nerve Tracts: Carry the APs from the receptors to the CNS & ganglions Nomenclature: 1st half of word (origin) & 2nd half (termination) Ex. SpinoCerebellar tract Spino-Thalamic Tract: 3-neuron system: i. Primary: Periphery posterior horn of spine ii. Secondary: Decussate, enter Spinothalamic tract Ascend to Thalamus, & iii. Tertiary: Thalamus Somatic sensory cortex This tract can detect pain, temp, touch, pressure, tickle, and itch.
ii.
iii.
Sensory Areas of the Cerebral Cortex: Translate the APs into perception of pain. Sensory Primary somatic sensory cortex (general sensory area): Posterior to the central sulcus. General sensory input: Pain, pressure, & temperature Taste area: Inferior end of postcentral gyrus Olfactory cortex: Inferior surface of frontal lobe Primary auditory cortex: Superior part of temporal lobe Sensory speech: Wernickes Area senses speech APs and transfers them to the Motor speech: Brocas area to translate those APs into motor functions. Association Areas Somatic sensory: posterior to primary somatic sensory cortex Visual association: anterior to visual cortex: present visual information compared to past information.
o Somatic Sensory Cortex: Homunculus The relative size of each region correlates to the # of sensory receptors in that area of the body. Projection: The processing of info from the point of origin of the stimuli to _____
Receptors:
o Muscle spindles: 3-10 specialized muscle cells providing info about muscle-length & involved in the stretch-reflex. o Golgi Tendon Organ: Proprioceptors associated w/ tendons that respond to increased tension. o Referred Pain: Sensation in one region of body that is not source of stimulus. Organ pain usually referred to the skin. Both the organ and that region of the skin input to the same spinal segment and converge on the same ascending neurons. o Phantom Pain: Occurs in people who have appendages amputated or structures removed (tooth). Gate-Control Theory: In an uninjured limb, pressure & touch sensation can inhibit pain (i.e. massage). This explains why amputees can experience phantom pain, since the lack of pressure & touch sensation also leads to the lack of pain-inhibition. Control of Skeletal Muscles: o Motor Areas of the Cerebral Cortex: Motor system: Maintains posture & balance, moves limbs, trunk, head, eyes, facial expressions, & conducts speech. Contains 3 motor areas: i. Precentral gyrus (primary motor cortex/area): 30% of upper motor neurons, another 30% in premotor area, and the rest in the somatic sensory cortex. ii. Premotor area: Anterior to primary motor cortex. Motor functions organized before initiation iii. Prefrontal area: Motivation, foresight to plan and initiate movements, emotional behavior, & mood. Reflexes: Movements that occur without conscious thought Voluntary movements: Consciously activated to achieve a specific goal in 3 steps: i. Initiation in the premotor areas of the cerebral cortex and results in the stimulation of upper motor neurons. ii. The axons of the upper motor neurons form the descending nerve tracts. They stimulate lower motor neurons which stimulate skeletal muscles to contract. iii. The cerebral cortex interacts with the basal nuclei and cerebellum in the planning, coordination and execution of movements. o Motor Nerve Tracts: Direct pathways: Control muscle tone and conscious fine, skilled movements in the face and distal limbs through the direct synapse of upper motor-neurons of the cerebral cortex w/ lower motor-neurons in the brainstem or spinal cord. i. Corticospinal Tract: Direct control of movements below the head. ii. Corticobulbar Tract: Direct control of movements in the head and neck.
Indirect pathways: Control (un)conscious muscle movements in trunk & proximal limbs through indirect synapse in intermediate nucleus rather than directly w/ lower motor neurons. i. Rubrospinal: Upper neurons synapse in red nucleus. Similar to comparator function of cerebellum. Regulates fine motor control of muscles in distal parts of upper limbs. ii. Vestibulospinal: Influence neurons innervating extensor muscles in trunk and proximal portions of lower limbs; help maintain upright posture. iii. Reticulospinal: Maintenance of posture.
Cerebellum: o Helps maintain muscle tone in postural muscles, controls balance during movement, and coordinates eye movements. o Purkinje cells help control fine motor movements through: Comparative Function: The cerebellum compares the bodys actual movements to the motor signals being sent from the brain and adjusts accordingly. Parkinsons disease: The experiencing of tremors at rest due to damaged basal nuclei Cerebellum damage (comparative function damage), leads to an intended tremor, meaning not at rest, but when performing simple tasks, they become exaggerated. Brainstem Functions: Gives way to all ascending/descending pathways. o Cranial nuclei 2-12, and many important survival reflexes are located here: Heart rate, blood pressure, respiration, sleep, swallowing, vomiting, coughing, and sneezing o Reticular activating system (RAS): Controls sleep/wake cycle RAS receives input from cranial nerves II (Optic), VIII (Vestibulocochlear), ascending tactile sensory pathways, & descending neurons from the cerebral cortex. Wakefulness is maintained by info coming in from the eyes, ears, and because of info coming in from the cerebral cortex. Other Brain Functions: o Speech: Area normally in left cerebral cortex, since the right side isnt as developed: Wernicke's area: Sensory Understanding what is heard & thinking of what one will say. Broca's area: Motor Sending messages to the appropriate muscles to make the sounds. Sound is heard 1st in the 1o association area, then info travels to Wernicke's area. Neuronal connections exist b/w Wernicke's area (sensory) and Broca's area (motor). Aphasia: Absent or defective speech or language comprehension. Caused by a lesion somewhere in the auditory/speech pathway.
Pic 1: Occipital lobe activation (looking before speaking Visual cortex) Pic 2: Brocas area is activated as you speak your words Pic 3: Cerebral cortex activation leading to more fine-tuned speech. Pic 4: Wernickes area is activated as you think about what youre going to say
o Cerebral Cortex: Outermost layered structure of the Cerebrum that is split into 2 cortices: Left cortex (controls right side of body): Math & Speech Right cortex (controls left side of body): 3-D/Spatial perception, know faces, musical ability Both sides of info, however, is shared through commissures (Corpus Callosum). Brain Waves and Sleep: o Electroencephalogram (EEG): Record of brains electrical activity, through the summation of all of the APs occurring at a particular moment sensed by electrodes placed on the scalp. Patterns include: Alpha: Wakeful, resting state with eyes closed. Beta: During intense mental activity (Test-taking) Theta: Occur in children, in adults experiencing frustration, or in brain disorders (Stress) Delta: Occur in deep sleep, infancy, and severe brain disorders (large deflecting waves) Note the pattern of nREM and REM sleep (Nystagmus).
Memory: o Sensory: Very short-term retention of sensory input o Short-term: Information retained for few seconds to minutes o Long-term: Explicit or declarative (Retention of facts) Accessed by hippocampus (actual memory) and amygdaloid nucleus (emotional) o Implicit (procedural; reflexive) memory: Development of skills (Riding a bicycle).
Effects of Aging on the N.S. o Gradual decline in sensory/motor function, slower reflexes, brain-size/weight dec., dec. short-term memory, and changes in sleep patterns. (Long-term memory unaffected or improved).
Ch. 18 Pain
Understand the conceptual basis of the following: o Pain: Unpleasant sensory & emotional experience associated with actual or potential tissue damage Whatever the experiencing person says it is, existing whenever he says it does. o Pain theory: Specificity theory: Amount of pain is related to the amount of tissue injury. Accounts for many types of injuries, but does not explain psychological contributions Gate control theory: Developed to explain the complexities of the pain phenomenon o Neuroanatomy: Nociception: Perception of pain Nociceptors: Bare nerve endings in skin, muscle, joints, arteries, & viscera that respond to chemical, mechanical, and thermal stimuli (A fibers, Unmyelinated C polymodal fibers).
Various Pathways of Nociception
o Neuromodulation of pain: This is achieved by neurotransmitters that are released by neurons into synaptic terminals, but not reabsorbed or metabolized and end up diffusing into the CSF, where they modulate the activity of different neuronal pathways. Neuromodulators: Serotonin, Acetylcholine, etc. Function: May be segmental inhibition of periphery sensory axons, spinal interneurons, or Top-down control pathways on spinal dorsal-horn regions. Direct excitation: Threshold depolarization from direct stimuli. Indirect excitation: Threshold depolarization from inflammatory mediators post-injury. Endorphin Response: o Clinical Description: i. Pain threshold: Point at which a stimulus is perceived as pain ii. Perceptual dominance: Pain at one location may cause an increase in the threshold in another location. iii. Pain tolerance: Duration of time or the intensity of pain that a person will endure before initiation of pain responses o Pain types: Nociceptive Pain Receptor-mediated pain (Pain from normal injury). Somatic & Visceral Non-Nociceptive Pain Neuropathic pain (Ex. Trigeminal Neuralgia) Peripheral pain: Linked to PNS Central pain: Linked to CNS Psychogenic Pain Psyalgia Pain induced by mental/emotional state, but not induced by physical injury o Ex. Heart-brokenness o Manifestations: Acute Pain: Triggers Sympathetic system (fear, anxiety) Leading to the symptoms below: Protective mechanism: o Alerts an individual to conditions/experience that immediately harms the body Symptoms: Tachycardia, hypertension, fever, diaphoresis, dilated pupils, outward pain behaviors, elevated blood sugar levels, decreased gastric acid secretion and intestinal motility, and a general decrease in blood flow. i. Somatic: Very high in # (Connective tissue, muscle, bone, skin) o A fibers Pain is sharp and well localized o C fibers Dull, aching, and poorly localized ii. Visceral: Not very high in # (Internal organs) o Poorly localized due to the lesser # of nociceptors. iii. Referred Pain: Pain that is present in an area removed/distant from its point of origin. Ex. Myocardial infarction pain that radiates down the left arm.
Chronic Pain: A situation/state that may develop suddenly or slowly and last for at least 3 months w/ varying response patterns, producing significant behavioral/psychological changes Usually leads patients into Depression. Common types include: i. Myofascial pain syndromes: Injury to the muscle & fascia (connective-tissue that covers muscle) Spasm, tenderness, and stiffness. ii. Chronic postoperative pain: Immediately after a surgery (nerve damage from cutting flesh) iii. Cancer pain: Due to multiple reasons: Cancer mass pressure, cancer treatment sideeffects, & diasthesia/paresthesia (pins-n-needles sensation). Neuropathic Pain: Mostly results from nerve-trauma/disease (Postherpetic-Neuralgia) i. Diabetic: Painful neuropathy Leads to damage of retinal ganglia/neurons. ii. Deafferentation pain: Unnecessary activation of receptors (w/out stimuli) Usually due to receptor damage/disease. iii. Sympathetically maintained pain: iv. Complex regional pain syndromes (CRPS): Plexus damage Ex. Brachial plexus damage from sleeping on ones arms too much. v. Central pain: vi. Phantom limb pain: Pain experienced despite the loss/realignment of a limb. o Pediatric pain: Pathways/chemicals assoc. w/ pain are functional in preterm & newborns. Nociceptor system is functional by 24 weeks (end of 2nd trimester) Expressions: Crying, facial expressions, & body language o Aging pain: i. Increase in pain threshold: Peripheral neuropathies & Skin thickness changes ii. Decrease in pain tolerance: More sensitive to pain iii. Alteration in metabolism of drugs and metabolites in patients: Require a higher dosage of medication to remove pain because of increased metabolic enzymes. o Drug therapy for pain: Non-steroidal anti-inflammatory drugs (NSAIDs): The NSAIDs inhibit the biosynthesis of hyperalgesic and proinflammatory PGs. Pharmacologically, they exhibit anti-inflammatory, antipyretic, + analgesic activity. Adverse reactions: Gastric-bleeding/ulceration & reduced platelet aggregation. Mechanism: Tissue damage Activates phospholipase-A Which cleaves lipidbilayers & releases arachidonic acid Leaving the acid susceptible to attack by: o COX-1: Produces Prostaglandins & Thromboxane (causes clots) Aspirin: Acts as a Thromboxane-inhibitor (dissolves clots) o COX-2: Synthesized in inflammatory cells (neutrophils, mast-cells) following bacterial endotoxin/cytokine exposure, and works to generate only Prostaglandins at the site of inflammation/tissue injury Ex. Tumor Necrosis Factor (TNF), Interluken-1 Most NSAIDs show little or no selectivity as inhibitors of the two COX isoforms, w/ the exception of Meloxicam (Mobic), which is a relatively selective inhibitor of COX-2 and combines anti-inflammatory & antiociceptive activity in animals. NSAIDs inhibit COX-1 & COX-2 Inhibit the production of prostaglandins.
Other uses of NSAIDs: i. Pain of cancer metastases and some types of headache, which are also associated with an inflammatory reaction. ii. Dysmenorrhea, which results from increased uterine PG formation. NSAIDs are generally given orally and the analgesic activity is likely to persist for approximately 6-8 hrs, although the effect of some NSAIDs such as Piroxicam and Phenylbutazone (withdrawn several years ago) can last much longer (12 hours -to- several days). o Analgesics: Are drugs designed to provide relief from pain (painkillers) by affecting the PNS/CNS Unlike opioids or anesthetics in the sense that they do not numb the patient from sensation. o Opioids: Interact w/ specific opioid-receptors to produce pharmacologic effects. 3 main classes: i. Mu functions: Analgesia, sedation, inhibit respiration Slow GI (addiction-potential). ii. Kappa functions: Analgesia, modulation of hormone & neurotransmitter release iii. Delta functions: Analgesia and affective behavior Slow GI Drug Interactions: Full or Partial agonists, mixed agonists (Full & Partial), + Antagonists. Adverse Effects: Respiratory depression, constipation, which leads to nausea + vomiting, pupillary constriction, rapid development of tolerance, & physical dependence (addiction). Ex: Morphine, codeine, heroin, methadone, meperidine, papaveretum, etorpine, fentanyl Very useful for neuropathic pain: like Trigeminal Neuralgia & Phantom Limb-pain. o Pain sensitive and insensitive structures: Sensitive: Skin, scalp, muscles, arteries, skull periosteum (layer along bone), cranial sinuses (headache), intracranial venous sinuses, parts of dura and base of brain, & arteries w/in dura. Insensitive: Brain parenchyma (no pain receptors), meningeal tissue, & abdominal viscera. Ch. 19 Thermoregulation Understand the mechanism of o Thermoregulation: The ability of an organism to maintain internal body temperature, where the main variables to consider are location, activity, environment, circadian (bio-clock) rhythm, & gender. Humans are considered Homeotherms, because of their ability to maintain body-temp, where our core-temp (highest in rectal region) is about 0.5-1oF warmer than our peripheries. The lowest body-temp is recorded at night, particularly around 2am. Gender differentiates regions during different hormonal activity, such as body-temp inc. during ovulation. This balance is mediated by: Hypothalamus & Neural/Hormonal mechanisms. o Means of heat Production: Metabolism, muscle-contraction, chemical thermogenesis (mediated by brown-fat), & vasoconstriction. Mechanism: Hypothalamus releases TSH-releasing hormone Makes the Anterior pituitary gland release TSH Causes Thyroid to secrete Thyroxin Makes Adrenal-medulla release Epinephrine Which stimulates Glycolysis, inc. metabolic rate, & vasoconstriction ( heat) o Means of heat Loss: Radiation, conduction, convection, vasodilation (inc. permeability), dec. muscle-tone (post-exercise), evaporation, inc. respiration (goes both ways depending on airs temp.), adaptation to warmer climates (usually takes around 2 weeks to adapt). o Thermoregulation in pediatric and aging population: Pediatrics: Produce sufficient heat, but cannot conserve (high body surface-to-weight ratio), small body, and thin subcutaneous layer (bad insulation). Aging: Slower blood circulation, vasoconstrictive response, & metabolic rate, coupled w/ decreased sweating and perception of heat/cold (sensation dec.) as receptors are lost/dec. Ex. Our average body-temp decreases as we get older (Adult: 96.8 99.5oF)
o Neural axis for thermoregulation: Temp. Sensors (Thermoreceptors) in skin, abdominal organs, spinal cord, & hypothalamus. Sensory Nerves carry impulses to the neurons of the Preoptic nucleus (hypothalamic thermostat) in the anterior hypothalamus. Autonomic nerves mediate vasoconstriction/vasodilation, sweating, piloerection, & short-term increases in basal metabolic rate. Motor nerves initiate voluntary/involuntary muscle movement (Ex. shivering). o Role of hypothalamus: It detects the bodys temp. & makes adjustments to maintain it: Hypothalamic Thermostat (Preoptic nucleus): Controls the set-point for body temp. Manifestations: Shivering (makes heat), vasoconstriction (reroutes blood to core to warm) Fever: Pyrogenic hyperthermia that causes an elevation in body-temperature through pyrogen activation. o 4 Stages: i. Prodrome: Period where patient feels cold & heat-activation mechanisms predominate. ii. Chills: Heat activation mechanisms are even more pronounced and shivering is present. iii. Flushing: Vasodilation occurs to enhance heat-loss (removal of the pyrogenic stimulus), & iv. Defervescence breaking of fever: Heat loss accelerated by evaporative cooling (sweating). Febrile seizures: Pediatric condition where fever is >102.5 and lead to seizures.
(feel cold) | (feel hot) o Types: Etiology Exogenous pyrogens include bacterial toxins & cell wall components, while Endogenous pyrogens include many cytokines produced during inflammatory responses. o Production: Exogenous pyrogens (endotoxins) are phagocytized Causing the phagocytes to release cytokines (TNF, IL) Which act on the preoptic nucleus Which releases Prostaglandins Causing the set-point to shift higher So body adjusts temp. & releases more cytokines, which provide negative feedback in efforts to help the body eventually get back to its normal body-temp. o Treatment: Should not be given too quickly, or else the pathogenic microorganisms causing this fever will continue to proliferate. Intervention is ideally aimed at the underlying cause. o Prognosis: Fevers induce mechanisms that help kill microorganisms (beneficial), dec. serum levels of zinc, iron, & copper, lead to lysosomal breakdown, lymphocytic transformation, & phagocytosis.
Hyperthermia: Not mediated by pyrogens o Symptoms: Convulsions (105.8oF), Death (109.4oF) High internal heat lead to protein coagulation. Heat cramps: Severe spasmodic cramps in the abdomen/extremities after prolonged sweating and associated sodium loss. This is common in individuals not accustomed to heat or those performing strenuous work in warm climates (fever, rapid pulse, and inc. BP). Heat exhaustion: Collapse due to prolonged high core/environmental temp. from prolonged vasodilation & profuse sweating (dehydration, depressed plasma, hypotension, dec. cardiac output, & tachycardia Dizziness, weakness, nausea, and syncope. Heat stroke: Potentially lethal result of a breakdown in overstressed thermoregulatory center The brain cannot tolerate temperatures >104.9oF (40.5oC), so temp. is maintained by blood flow through the veins in the head/face, because Cardiovascular & thermoregulatory centers may shut down if the temp. gets too high. o Rapid peripheral cooling will cause peripheral vasoconstriction & limit core-cooling, meaning that cooling should be done gradually and not just on extremities. o Children are more susceptible: More metabolic heat during exercise, greater surface area-to-mass ratio, and less sweating capacity than adults (so less cooling off). Leads to: Cerebral edema, CNS degen., swollen dendrites, & renal tubular necrosis o Treatment: Move patient to a cool area and remove any tight clothing that would restrict heat loss. Malignant Hyperthermia: o Complication of inherited muscular disorder that is precipitated by the administration of volatile anesthetics and neuromuscular blocking agents Increased Ca2+ release or decreased Ca2+ uptake in muscles (sustained contraction) Increased O2 consumption and lactic-acid production. Hypothermia: Body temp. falls below 95oF (35oC) o Symptoms: Vasoconstriction, alterations in microcirculation (inc. blood viscosity & slows circulation), coagulation, & ischemic tissue damage. Ice crystals form inside the cells rupture/die! i. Accidental hypothermia: Sudden immersion in cold water or prolonged exposure to cold. ii. Therapeutic hypothermia: Used to slow metabolism and preserve ischemic tissue during surgery or limb reimplantation. May lead to ventricular fibrillation and cardiac arrest. Trauma-induced Temp. change: o CNS trauma, accidental injuries, hemorrhagic shock, major surgery, & thermal burns Headache: A mixture of nociceptive & neuropathic pain that have primary disorders + secondary effects o Risk factors: Stress, Depression, Sinus infection, Fatigue, Hypertension, Constipation, Caffeine, Hormonal cycles/therapy, Cigarettes, Alcohol, and some foods (cheese/chocolate) o Types: Acute vs. Chronic or Vascular vs. Tension (Medicated w/ OTC analgesics & TCAs) i. Tension: Muscle contraction associated w/ stress or activities (assembly line, sewing, or keyboard work) that may directly deform pain receptors or may compress blood vessels, leading to ischemia (inadequate blood supply). Pain-band around head & down back of neck. ii. Vascular: Some degree of hyperemia (too much blood) within the cerebral and scalp vessels which may or may not be preceded by a period of vasoconstriction. o Pathophysiology: Neural tissue does not have pain-receptors, but there are many receptors in the meninges, skull, & blood vessels and in the muscles of the face, scalp, and neck. o Mechanisms of headache: Distention/displacement/pulling of blood-vessels, contraction of head/neck muscles, stretching of periosteum, upper-spine degeneration, & encephalin deficiency. High risk: Worst headache, vomiting, neck-stiffness, photophobia, & neuralgic deficits. New headache in older persons: Depression, subdural hematoma, & intracranial masses.
Migraine: Prototype headache that can be Classic (with aura) or Common (without aura) o Symptoms: Unilateral, throbbing pain (4-72hrs), nausea, phonophobia & photophobia. 1/8 adults (age:25-34) have them, women <40 are 2-3x as likely to get it, and can be triggered by various things (Ex. Cycling estrogen levels or Neurological dysfunction). o Causes: A trigger (stress, estrogen, lights, alcohol, chocolate) Releases Biogenic Amines (serotonin, NE neurotransmitters) Leads to early Vasoconstriction Reactive hyperemia (as amines are degraded, leading to inflammation of sensory nerves) Vasodilation (in efforts to relieve this excess blood build-up) Migraine (from this pattern of blood excess/release). Trigger phase Begins with the Prodrome/Aura-phase (10-20 mins) & then an acute attack. Visual disturbances (Scotoma, lights, hallucinations) & Parasthesias (vertigo, ataxia). o Treatment: Prevention or Prophylactic Treatment: Beta blockers (Propranolol) Antidepressants, Ca2+ blockers, & anti-seizure medications (Valpronic acid) Methylsergide - Blocks inflammatory/vessel-constricting effects of serotonin. Trigger Management: If recognized, avoid the following triggers. Ex. Certain foods, Sunlight, glare, Aspartame, MSG, caffeine, stress Attack Aborting Medications: May be administered IM, oral, rectal, SC, or inhaled. General Pain Management: Norcotic, non-norcotic analgesics, & NSAIDS Cluster headaches: Excruciating daily-pain for weeks, followed by periods of remission (Toxic Vascular). o Symptoms: Rare unilateral pain over eye/temporal region, ptosis & nasal congestion on affected side Sudden onset of burning/boring pain (lasts 30-45mins on average), usually in men. o Causes: Unknown causes, 2-3hrs after sleeping (REM), and associated w/ inflamed conjunctiva, lacrimation, blocked nostril, alcohol, stress, barometric pressure changes, weather, & hay-fever. o Treatment: Resistant to analgesic painkillers because these drugs take effect too slowly. Pure oxygen inhalation: Most effective night-treatment. Ergotamine tartrate: Suppository, tablet, injection or aerosol Sumatriptan (Imitrex): Proven effective in subcutaneous injections when attack begins. Local anesthetics: Cocaine, Lidocaine, & nasal infiltration.
Das könnte Ihnen auch gefallen
- Paranoia XP - Gamemaster Screen Booklet - Mandatory Fun Enforcement PackDokument24 SeitenParanoia XP - Gamemaster Screen Booklet - Mandatory Fun Enforcement PackStBash100% (3)
- Relationships, 365 Day Devotional Mylesunroe 377pgDokument377 SeitenRelationships, 365 Day Devotional Mylesunroe 377pgEla100% (7)
- Pathopysiology QuestionsDokument159 SeitenPathopysiology QuestionsMaria EcaterinaNoch keine Bewertungen
- Nursing care process in patients with chronic obstructive pulmonary diseaseVon EverandNursing care process in patients with chronic obstructive pulmonary diseaseNoch keine Bewertungen
- Pathophysiology Applications BookshelfDokument35 SeitenPathophysiology Applications Bookshelfmorgan bigelow100% (1)
- Breast ModuleDokument2 SeitenBreast ModuleDasha Vee100% (1)
- Bates Test Questions The Thorax and LungsDokument14 SeitenBates Test Questions The Thorax and LungsRhoda Mae CubillaNoch keine Bewertungen
- Understanding Pathophysiology CH 1: Study Online atDokument6 SeitenUnderstanding Pathophysiology CH 1: Study Online atChristian del Rosario100% (2)
- Patho Phlash Pathophysiology Flash Cards Download Free (EPUB, PDFDokument4 SeitenPatho Phlash Pathophysiology Flash Cards Download Free (EPUB, PDFHerman Orellana Mellado0% (3)
- Pathophysiology Test Bank CH 18-19 & 21-22Dokument47 SeitenPathophysiology Test Bank CH 18-19 & 21-22Joyy100% (5)
- Book BindingDokument14 SeitenBook Bindingpesticu100% (2)
- Genie GS-1930 Parts ManualDokument194 SeitenGenie GS-1930 Parts ManualNestor Matos GarcíaNoch keine Bewertungen
- Pathophysiology Exam QuestionsDokument11 SeitenPathophysiology Exam Questionsjimmy100% (2)
- Pathophysiology OverviewDokument234 SeitenPathophysiology OverviewKimberly Jaé100% (2)
- NotesDokument10 SeitenNotesVu HauNoch keine Bewertungen
- Pharmacology and The Nursing ProcessDokument28 SeitenPharmacology and The Nursing ProcessEdralyn MatalangNoch keine Bewertungen
- Pathophysiology Test Bank CH 6-9Dokument33 SeitenPathophysiology Test Bank CH 6-9Joyy86% (7)
- The Dry Bulk Management StandardDokument18 SeitenThe Dry Bulk Management Standardamu_more44100% (1)
- Patho Physiology Bible: Over 70 Concept MapsDokument139 SeitenPatho Physiology Bible: Over 70 Concept Mapslauramphs79100% (5)
- Viii. PathophysiologyDokument2 SeitenViii. Pathophysiologymacedon145377Noch keine Bewertungen
- Simple Guide For Basic Nursing InterventionsDokument13 SeitenSimple Guide For Basic Nursing InterventionsMelvin NizelNoch keine Bewertungen
- Diuretics For CardiacDokument6 SeitenDiuretics For CardiacBetsy Brown ByersmithNoch keine Bewertungen
- Fundamentals of Nursing NotesDokument34 SeitenFundamentals of Nursing NotesKate Therese TaronNoch keine Bewertungen
- Pathophysiology - Exam 2 ReviewDokument14 SeitenPathophysiology - Exam 2 ReviewWaqas Gill100% (4)
- GI PathoDokument20 SeitenGI Pathojutah2013Noch keine Bewertungen
- Essentials of PathophysiologyDokument5 SeitenEssentials of PathophysiologyJonathan Enojales0% (1)
- Porth PathoDokument6 SeitenPorth PathoTaylor Natale100% (1)
- TestBank Norris Porths Essentials Pathophysiology 5e 2019Dokument505 SeitenTestBank Norris Porths Essentials Pathophysiology 5e 2019Cindy Perez0% (1)
- This Study Resource Was: Dlugasch - Ch02-Applied Pathophysiology For The Advanced Practice Nurse, 1eDokument4 SeitenThis Study Resource Was: Dlugasch - Ch02-Applied Pathophysiology For The Advanced Practice Nurse, 1eHugs0% (1)
- Introduction Clinical Pharmacology 8th Edmunds Test BankDokument13 SeitenIntroduction Clinical Pharmacology 8th Edmunds Test BanksonyaaaqNoch keine Bewertungen
- 0 Ceces Study Guides Part 2Dokument26 Seiten0 Ceces Study Guides Part 2Carolina OrtizNoch keine Bewertungen
- Chapter 26: Antibacterials Mccuistion: Pharmacology: A Patient-Centered Nursing Process Approach, 10Th EditionDokument16 SeitenChapter 26: Antibacterials Mccuistion: Pharmacology: A Patient-Centered Nursing Process Approach, 10Th Editionmichael pattersonNoch keine Bewertungen
- N5315 Advanced Pathophysiology Syllabus Fall 2014Dokument15 SeitenN5315 Advanced Pathophysiology Syllabus Fall 2014meeeh100% (1)
- Moses ManualDokument455 SeitenMoses ManualDadypeesNoch keine Bewertungen
- Patho Ch. 10-12Dokument13 SeitenPatho Ch. 10-12JoyyNoch keine Bewertungen
- Chapter 13: Alterations in Oxygen Transport Banasik: Pathophysiology, 6th EditionDokument10 SeitenChapter 13: Alterations in Oxygen Transport Banasik: Pathophysiology, 6th Editionqwivy.com100% (1)
- Seidels P.E Chapter 5 Test BankDokument5 SeitenSeidels P.E Chapter 5 Test BankAthena Saturday100% (1)
- C 37Dokument9 SeitenC 37Tammie Gore100% (3)
- Exam 1 Study Guide Summary Advanced Health AssessmentDokument27 SeitenExam 1 Study Guide Summary Advanced Health AssessmentRhoda Mae Cubilla100% (1)
- QSEN Simulation Template Post CABG PatientDokument8 SeitenQSEN Simulation Template Post CABG PatientMissK2216Noch keine Bewertungen
- Pathophysiology - Exam 1 ReviewDokument9 SeitenPathophysiology - Exam 1 ReviewWaqas Gill100% (2)
- Pharmacology For Canadian Health Care Practice, 3rd Canadian Edition: Chapter 03 - Legal and Ethical Considerations Chapter SummaryDokument4 SeitenPharmacology For Canadian Health Care Practice, 3rd Canadian Edition: Chapter 03 - Legal and Ethical Considerations Chapter SummaryNadine MahadeoNoch keine Bewertungen
- 6521 Advanced Pharmacology Final ExamDokument19 Seiten6521 Advanced Pharmacology Final ExamSandra JeffersonNoch keine Bewertungen
- Maternal Child Nursing Care Canada 2nd Perry Test BankDokument8 SeitenMaternal Child Nursing Care Canada 2nd Perry Test Banksonyaaaq0% (1)
- Syllabus NGR 6803 EBPDokument12 SeitenSyllabus NGR 6803 EBPmatthewNoch keine Bewertungen
- Calibration of Force ReductionDokument36 SeitenCalibration of Force Reductionvincenzo_12613735Noch keine Bewertungen
- Pathophysiology 5th Edition Copstead Test BankDokument5 SeitenPathophysiology 5th Edition Copstead Test BankBarbara100% (2)
- Five Star Hotel and ResortDokument9 SeitenFive Star Hotel and ResortAISHNoch keine Bewertungen
- Patho Questions and AnswersDokument8 SeitenPatho Questions and AnswersAbdullah Kadir Hillaluddin100% (3)
- Pathophysiology Exam 3 - ReviewDokument15 SeitenPathophysiology Exam 3 - ReviewWaqas GillNoch keine Bewertungen
- 2011 Understanding Pharmacology Essentials For Medication SafetyDokument1 Seite2011 Understanding Pharmacology Essentials For Medication SafetygloriyaNoch keine Bewertungen
- Patho Exam QuestionsDokument3 SeitenPatho Exam QuestionsAndin GangNoch keine Bewertungen
- PathophysExam1 Review Course HeroDokument7 SeitenPathophysExam1 Review Course HeroTammie Gore100% (1)
- This Study Resource Was: G R A D E S L A B - C O MDokument8 SeitenThis Study Resource Was: G R A D E S L A B - C O MHugsNoch keine Bewertungen
- Interactive Quiz: Deal or No DealDokument7 SeitenInteractive Quiz: Deal or No DealShaira Jean GeminaNoch keine Bewertungen
- Test Bank For Pharmacological Aspects of Nursing Care 7th Edition by BroylesDokument10 SeitenTest Bank For Pharmacological Aspects of Nursing Care 7th Edition by Broylesa759488023Noch keine Bewertungen
- Patho 3Dokument11 SeitenPatho 3Dranreb Berylle MasangkayNoch keine Bewertungen
- Pathophysiology Final OutlineDokument24 SeitenPathophysiology Final OutlineElise HowardNoch keine Bewertungen
- Exam 2 ReviewDokument31 SeitenExam 2 ReviewAhsan Tebha50% (2)
- C 38Dokument10 SeitenC 38Tammie Gore100% (2)
- Things I Learned in Medical SchoolDokument19 SeitenThings I Learned in Medical SchooldosmheslNoch keine Bewertungen
- 351-Discussion Board 2Dokument2 Seiten351-Discussion Board 2api-546070989Noch keine Bewertungen
- IV Therapy Lippincott Manual of Nursing PracticeDokument20 SeitenIV Therapy Lippincott Manual of Nursing PracticeHejia MagangcongNoch keine Bewertungen
- Chapter 0017 MedDokument9 SeitenChapter 0017 Medquizme908100% (1)
- Pathophysiology Notes 1-4Dokument24 SeitenPathophysiology Notes 1-4Scotty Banks100% (3)
- ICU Scoring Systems A Complete Guide - 2020 EditionVon EverandICU Scoring Systems A Complete Guide - 2020 EditionNoch keine Bewertungen
- c083c282a43655ec69532f2704c3993aDokument12 Seitenc083c282a43655ec69532f2704c3993aAneilRandyRamdialNoch keine Bewertungen
- Bhagwati School Strap Report AnalysisDokument60 SeitenBhagwati School Strap Report AnalysisReverse Minded100% (1)
- Bio (RocessDokument14 SeitenBio (RocessVijay SansanwalNoch keine Bewertungen
- PTP S3Dokument8 SeitenPTP S3Yongyin SHENGNoch keine Bewertungen
- Astm A194 2020Dokument12 SeitenAstm A194 2020rolando cuadro blancoNoch keine Bewertungen
- Thermoplastic Tubing: Catalogue 5210/UKDokument15 SeitenThermoplastic Tubing: Catalogue 5210/UKGeo BuzatuNoch keine Bewertungen
- Approved Project 25 StandardsDokument5 SeitenApproved Project 25 StandardsepidavriosNoch keine Bewertungen
- Clinical Case StudyDokument20 SeitenClinical Case Studyapi-252004748Noch keine Bewertungen
- AR BuildingDokument819 SeitenAR BuildingShithin KrishnanNoch keine Bewertungen
- Opex and CapexDokument5 SeitenOpex and CapexATM Shafiq Ul AlamNoch keine Bewertungen
- 20160323014547-16MnCr5 - 16MnCrS5Dokument1 Seite20160323014547-16MnCr5 - 16MnCrS5Chaitanya DattaNoch keine Bewertungen
- Solid Modeling Techniques: Constructive Solid Geometry (CSG)Dokument22 SeitenSolid Modeling Techniques: Constructive Solid Geometry (CSG)amolNoch keine Bewertungen
- 09.tracheostomy Management by Speech Language Pathologists in SwedenDokument12 Seiten09.tracheostomy Management by Speech Language Pathologists in SwedenCarlonchaCáceresNoch keine Bewertungen
- Top Coat-200 - Data PDFDokument4 SeitenTop Coat-200 - Data PDFLiliana GeorgianaNoch keine Bewertungen
- LighthouseDokument4 SeitenLighthousejaneborn5345Noch keine Bewertungen
- Statistics and Probability Module 3Dokument3 SeitenStatistics and Probability Module 3Eftychia LeegleeNoch keine Bewertungen
- ANS: (2.59807m/s2 Horizontal) (1.5m/s2 Vertical) (12.93725 Degree Angle That The Water Surface Makes With The Horizontal)Dokument5 SeitenANS: (2.59807m/s2 Horizontal) (1.5m/s2 Vertical) (12.93725 Degree Angle That The Water Surface Makes With The Horizontal)Lolly UmaliNoch keine Bewertungen
- GLP BmsDokument18 SeitenGLP BmsDr.Subhashish TripathyNoch keine Bewertungen
- Generalized Anxiety DisorderDokument24 SeitenGeneralized Anxiety DisorderEula Angelica OcoNoch keine Bewertungen
- Smoldering Combustion: Guillermo ReinDokument20 SeitenSmoldering Combustion: Guillermo ReinAhmed HussainNoch keine Bewertungen
- Class 12 - Maths - MatricesDokument87 SeitenClass 12 - Maths - MatricesAishwarya MishraNoch keine Bewertungen