Beruflich Dokumente
Kultur Dokumente
Lecture 18
Hochgeladen von
alfiatuzCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Lecture 18
Hochgeladen von
alfiatuzCopyright:
Verfügbare Formate
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement The Lecture Contains: EMISSION MEASUREMENT CO and CO2 NDIR Analyzers Flame Ionization Detector (FID) Chemiluminescence Analyzer (CLA) Smokemeters Constant Volume Sampler (CVS) Particulate Emission Measurement Partial Flow Dilution Tunnel Full Flow Dilution Tunnel
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_1.htm[6/15/2012 3:03:15 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement EMISSION MEASUREMENT
The emission regulations specify the type, principle of operation used and generic construction of the exhaust gas analyzers which can be employed for emission certification of vehicles and engines. Table 4.9 gives the type of analyzers used for measurement of different exhaust gas constituents.
Table 4.9 Measurement Principles of Exhaust Gas Analyzers Gas component
CO HC NO x
Measurement Principle
NDIR (Non-dispersive infrared) FID (Flame Ionization detector) CLD (Chemiluminescence detector)
CO and CO 2 NDIR Analyzers
Beer-Lambert's Law is used for operation of NDIR analyzers by measuring the degree of absorption of infrared (IR) radiations when they pass through a column of gas. The fraction of incident radiations absorbed is given by,
(4.1)
where I = Radiation energy absorbed I0 = Incident radiation energy k = characteristic absorption constant for the gas, m2 /gmol c = concentration of the gas, gmol/m 3 d = length of the gas column, m
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_2.htm[6/15/2012 3:03:15 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement contd...
NDIR analyzer is shown schematically in Fig. 4.10. As the absorption of IR radiations is measured only in a narrow range of wavelengths (not the entire range of wavelength of IR radiations) which has specifically a high absorbance for the particular gas, the technique is called as Non-dispersive infra-red'. For example carbon monoxide has a strong absorbance in the wavelength band of 4.5-5 m. The analyzer measures differential in absorption of energy from two columns of gas; (i) the gas to be analyzed in the sample cell' and (ii) a gas of fixed composition like N 2 contained in the reference cell which is free of the gas of interest and relatively non-absorbing in the infrared region. The infrared beam from a single source is usually split into two beams of the same intensity, one each for the sample and reference cells. The detector is divided in two compartments separated by a flexible diaphragm; one section receives transmitted IR energy from the sample cell and the other from the reference cell. The detector is filled with the gas of interest, so that the energy transmitted to the detector is fully absorbed. The flexible diaphragm of the detector senses the differential pressure between the two sections of the detector caused by the difference in the amount of transmitted IR energy absorbed. The deflection in the diaphragm is used to generate an electrical signal that determines the concentration of the gaseous species of interest. A rotating interrupter in the path of IR beam is put to generate AC signal output that can be amplified.
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_3.htm[6/15/2012 3:03:15 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement contd....
NDIR analyzers enable accurate measurements of CO and CO 2 in the exhaust gases.
Schematic of an NDIR analyzer for measurement of Figure 4.10 CO and CO concentration. 2
NDIR instruments are seldom used for measurement of hydrocarbons except in the garage type analyzers as the IR absorbance to different hydrocarbons varies substantially. The unsaturated hydrocarbons are primarily responsible for photochemical smog but they do not have an adequate absorption in the IR wavelength range that is specific to the saturated hydrocarbons and vice versa. Sensitivity and response of NDIR to the exhaust HC is typically only half of the probable true value. NO absorbs only weekly in the infrared region. Moreover, CO, CO 2 and water vapours interfere seriously; hence NDIR analyzers are also not used for NO measurement.
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_4.htm[6/15/2012 3:03:15 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement Flame Ionization Detector (FID)
Pure hydrogen-air flames are practically ion-free but on introduction of even little amount of hydrocarbons the flame causes considerable ionization and becomes electrically conducting. The ionization current is proportional to the number of carbon atoms present in the hydrocarbon molecules. Thus, FID is effectively a carbon atom counter e.g., one molecule of propane generates three times the response generated by one molecule of methane. The measurement of HC by FID is expressed as parts per million of methane i.e. as ppmC 1 i.e., ppm of hydrocarbon containing equivalent of one carbon atom. The HC concentration is commonly written as ppmC. HC concentration measured as ppm propane (C3 ) is to be multiplied by a factor of 3 to convert it to ppmC. All classes of hydrocarbons i.e., paraffin, olefins, aromatics, etc. show practically the same response to FID. Oxygenates, e.g. aldehydes and alcohols however, have a somewhat lower response. FID essentially consists of a hydrogen-air burner and an ion collector assembly as shown in Fig. 4.11. Sample gas is introduced with hydrogen in the burner assembly and the mixture is burned in a diffusion flame. An electric potential is applied between the collector plates that makes the ionization current to flow and generate signal proportional to HC concentration in the sample gas. This current is amplified and the output signal is measured. A well-designed burner will generate ionization current that is linearly proportion to hydrocarbon content over a dynamic range of almost 1 to 10 6 . The commercial FID analyzers have the most sensitive range set at about 0-50 ppmC and the maximum range reaching 0-100,000 ppmC. Hydrogen is mixed with helium in ratio of 40:60 to decrease flame temperature that increases flame stability. The FID analyzer is calibrated with propane or methane mixtures in nitrogen. For the measurement of hydrocarbons in diesel exhaust, sampling line and FID are heated to a temperature of 191 11C to minimize condensation of heavy hydrocarbons present in the diesel exhaust in the sampling system.
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_5.htm[6/15/2012 3:03:16 PM]
Objectives_template
Figure 4.11
FID for HC measurement.
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_5.htm[6/15/2012 3:03:16 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement contd...
Measurement OF Non-Methane Hydrocarbons (NMHC) Presently, the emission standards are specified in terms of non-methane hydrocarbons. Methane content of HC emissions is determined by one of the following methods: Gas chromatographic (GC) method or Non-methane cutter (NMC) method In the GC method, sample is injected into GC column which separates the sample into two parts:
(i) CH 4 -air-CO, and
(ii) NMHCCO 2 H 2 O. A molecular sieve column separates methane from air and CO before passing it to FID. Thus methane content is measured that is deducted from the total hydrocarbon content. In the NMC method, all hydrocarbons except CH4 are oxidized to CO 2 and water on a catalyst, so that when the gas sample is passed through NMC only CH4 is detected by HFID. The NMC cutter is calibrated for catalytic effect on CH4 and higher hydrocarbon (ethane) mixtures in presence of water vapours with values typical of exhaust gas at or above 600 K. The sample can be alternatively passed through NMC or bypasses the NMC. In this manner, the total HC and methane alone present in the exhaust gas sample are determined.
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_6.htm[6/15/2012 3:03:16 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement Chemiluminescence Analyzer (CLA)
When NO and ozone (O 3 ) react a small fraction (about 10% at 26.7 C) of excited NO 2 * molecules is produced as per the following reactions:
(4.2) (4.3)
As the excited molecules of NO 2 * decay to ground state, light in the wavelength region 0.6-3.0 m is emitted. The quantity of excited NO 2 produced is fixed at a given reaction temperature and the intensity of light produced during decay of excited NO 2 is proportional to the concentration of NO in the sample.
Figure 4.12
Schematic and flow diagram of a c hemiluminescence NO x analyzer.
A schematic diagram of the chemiluminescence NO x analyzer is shown in Fig. 4.12. The sample containing NO flows to a reactor where it reacts with ozone produced from oxygen in ozonator' .In the reactor NO is converted to NO 2 . A photomultiplier tube detects the light emitted by the excited NO 2 . The signal is then amplified and fed to recorder or indicating equipment. For the measurement of nitrogen oxides (NOx ), NO 2 in the sample is first converted to NO by heating in a NO 2 - to-NO converter prior to its introduction into the reactor. At 315 C, about 90 percent of NO 2 is converted to NO 2 . The total concentration of NO x in the sample is thus, measured as NO. When the sample is introduced in the reactor bypassing the NO 2 - to- NO converter, concentration of NO alone is determined. The difference between the two measurements provides the concentration of NO 2 in the sample. The response of the instrument is linear with NO concentration. The technique is very sensitive and can detect up to 10 -3 ppm of NO x . The output signal is proportional to the product of sample flow rate and NO concentration. As the
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_7.htm[6/15/2012 3:03:16 PM]
Objectives_template
method is flow sensitive an accurate flow control is necessary. The calibration and operation are done at the same flow rate and reactor temperature.
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_7.htm[6/15/2012 3:03:16 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement Smokemeters
In the filtration type smokemeters like Bosch smokemeter a fixed volume of the exhaust gas is drawn through a white filter paper of specified quality. The density of smoke stain obtained on the filter paper is evaluated using a reflectance meter which gives the measure of smoke density of diesel exhaust gas. Now, mostly light extinction/absorption smokemeters based on Beer-Lambert Law are used. The light extinction type smokemeters are more commonly called as opacimeters' as these provide a more realistic measurement of the visible smoke emissions from diesel engines. . Both the sampling type and full flow type opacimeters are in use. The construction requirements, installation and operational details of opacimeters are described in the relevant international standards. A sampling type smokemeter is shown schematically in Fig. 4.13.
Figure 4.13
Schematic of a sampling type light extinction smokemeter.
An incandescent lamp with a colour temperature in the range of 2 800 K to 3 250 K or a green light emitting diode (LED) with a spectral peak between 550 nm and 570 nm is used as light source. The transmitted light is received on a photocell or a photo diode (with filter if necessary). When the light source is an incandescent lamp, the receiver should have maximum response in the range 550 nm to 570 nm wavelength as is for the human eye. When light from a source is transmitted through a certain path length of the exhaust gas, smoke opacity is the fraction of light that is absorbed in the exhaust gas column and does not reach the light detector of smoke meter. The absolute smoke density is given by the absorption coefficient, k s which has units of m -1 and is given by:
(4.4)
where L is length of smoke column in meter through which light from the source is made to pass, I0 is the intensity of incident light, I is the transmitted light falling on the smokemeter receiver.
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_8.htm[6/15/2012 3:03:16 PM]
Objectives_template
In the full flow type smokemetersm, the light source and detector are placed directly across the exhaust gas stream usually at the end of exhaust pipe. In this case, path length of smoke measurement varies with the cross sectional size of the exhaust gas stream or tail pipe. Hence, conversion charts of the measured value to the absolute smoke density, k s for different exhaust pipe diameter or path lengths are made available for the full flow smoke meters.
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_8.htm[6/15/2012 3:03:16 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement Constant Volume Sampler (CVS)
When emissions are to be measured from a vehicle being run on a driving cycle sampling of the representative gas is very critical. Constant Volume Sampling (CVS) is used in European, US and other tests to make it possible that a representative sample of the exhaust gas is withdrawn for measurement of the gaseous emissions.
Figure 4.14
C onstant volume sampling (CVS) unit using critical flow venturi (CFV-CVS ) for measurement of mass of the exhaust emission.
A Constant Volume Sampling (CVS) system is shown schematically in Fig. 4.14 . In the CVS system; The entire exhaust gas from the vehicle is diluted with the filtered room air. An air to exhaust gas dilution ratio of about 10:1 is used. The dilution with air lowers partial pressure of unburned hydrocarbons and water, and prevents their condensation in the sampling line. The diluted exhaust gas is drawn by a constant volume pump system employing either a positive displacement pump (PDP) or a critical flow venturi (CFV) and a blower. A PDP capacity of about 10 to 12 m3 /min of air flow provides sufficient dilution for most passenger cars The volume flow rate of the diluted exhaust (exhaust gas + air) is maintained constant throughout the test . Before the diluted exhaust gas enters the CFV or PDP, its temperature is controlled within the 5 C of the average gas temperature during the test by a heat exchanger. From the diluted gas a small sample is continuously withdrawn and collected in evacuated Teflon bags. This process integrates the concentration of the pollutants over the entire driving schedule. A small part of the dilution air is sampled simultaneously and collected in a separate
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_9.htm[6/15/2012 3:03:16 PM]
Objectives_template
bag to correct for any background concentration of pollutant present in the dilution air. The sample bags are analyzed after the test is completed. The mass of individual pollutants is determined from its measured concentration in the sample bag, its density and the total volume flow rate of the diluted exhaust during the test through CVS.
file:///C|/...%20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_9.htm[6/15/2012 3:03:16 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement Particulate Emission Measurement
For measurement of particulate emissions, the gas is diluted with air in a dilution tunnel and, a sample is continuously collected from the diluted gas and filtered to collect particulate matter. The mass of the collected PM is measured to determine specific PM emissions in terms of g/km or g/kWh. The dilution tunnels are of two types Partial flow, and Full flow dilution tunnel
Partial Flow Dilution Tunnel
In the partial-flow system, only a small part of the exhaust stream is diluted. To withdraw a true representative of the exhaust gas the following systems have been developed; Isokinetic sampling systems Flow controlled systems with concentration measurement, and Flow controlled systems with flow measurement In an isokinetic system, the gas velocity in the sampling tube which leads the sampled exhaust gas to dilution tunnel is kept same in magnitude as the velocity of the bulk exhaust gas stream. In this way, an undisturbed and uniform exhaust gas sample flow at the inlet of sampling probe is obtained. An isokinetic system is shown schematically in Fig. 4.15. Raw exhaust gas is transferred from the exhaust pipe to the dilution tunnel (DT) through isokinetic sampling probe (ISP) and the transfer tube. The differential pressure of the exhaust gas between exhaust pipe and inlet to the probe is measured with a pressure transducer. The signal is fed to a flow controller that controls the suction blower and, a differential pressure of zero at the tip of probe is maintained. Under these conditions, exhaust gas velocities in the exhaust pipe and probe are identical and the flow through isokinetic probe is a constant fraction of the exhaust flow. The sampling rate can be obtained by the ratio of cross sectional areas of probe and the exhaust pipe. The dilution airflow rate is measured with the flow meter. The dilution ratio is calculated from the dilution air flow rate and exhaust sample to total exhaust flow ratio.
file:///C|/...20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_10.htm[6/15/2012 3:03:16 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement Lecture18:Emission Measurement
Figure 4.15 Full Flow Dilution Tunnel
Partial flow dilution systems with isokinetic sampling probe.
In the full flow system, entire exhaust of the engine/vehicle is diluted with the filtered room air. The full flow system is quite large in size and expensive. A full-flow double dilution tunnel is shown schematically in Fig. 4.16 . For small engines/vehicles only the primary dilution tunnel is used. For the large engines to provide the desired dilution ratio, the gas is diluted again in the secondary dilution tunnel. The sample is withdrawn for measurements from the secondary dilution tunnel in the large engines. The dilution ratio is maintained around 10:1. The temperature of the diluted exhaust gases at the primary filter is maintained at 325 K or less by a heat exchanger. The flow rate of diluted gas is kept constant during the test by a CVS system. After thorough mixing of exhaust and air in the dilution tunnel, a constant flow rate sample is extracted that is filtered through a Teflon coated glass fibre filter. The mass of particulate is determined by weighing the particulate mass collected on the filter. A reference filter is used to determine the particulate mass in the dilution air for correction of PM measurement for the background PM. The filter papers are conditioned before and after filtration to prevent condensation of any moisture or deposition of foreign particulate matter from atmosphere. With the full flow dilution tunnel and CVS system, gaseous pollutants are also measured simultaneously with particulates
file:///C|/...20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_11.htm[6/15/2012 3:03:17 PM]
Objectives_template
Figure 4.16
Full flow dilution tunnels for measurement of particulate emissions
file:///C|/...20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_11.htm[6/15/2012 3:03:17 PM]
Objectives_template
Module 4: Vehicle Emission Standards and Measurement
Questions
(4.1) Average engine-out emissions before catalytic treatment from a SI engine car operating on stoichiometric gasoline-air mixture are CO = 12 g/km, HC = 1.5 g/km and NOx (as NO) = 1.0 g/km. The fuel consumption of car is 80 g/km. Estimate the average concentration of pollutants CO (as % volume), NO (ppm), HC (ppmc1). Take gasoline as isooctane, universal gas constant = 8314.3 kJ/kmol.K. (4.2) The above car meets the Euro 3 standards CO = 2.3 g/km, HC = 0.2 g/km and NOX = 0.15 g/km. What would be the concentration of each pollutant in the exhaust gas at exit of the tail pipe. (4.3) In the US FTP cycle (Fig 4.3) the first 505 seconds of driving schedule is repeated after 10 minutes of hot engine soak. How would the average emissions of CO, HC and NOX differ during the initial and the last 505 seconds of the driving cycle? (4.4) Discuss why NDIR does not measure the unburned hydrocarbons correctly even though it is calibrated with standard mixtures of n-hexane in nitrogen gas. (4.5) A light extinction type smoke meter has smoke column length, L= 430 mm and it shows smoke density of 60% (60% light is absorbed in the smoke column). Determine absolute smoke density of the exhaust gas in terms of m-1. The full flow type of smoke meters are used by fitting them at the end the exhaust pipe. The smoke column length for the full flow smoke meter may be taken equal to the exit diameter of the exhaust pipe. Make a table for the absolute smoke density varying from 0.25 m-1 to 2.5 m-1 in steps of 0.25 m-1 correlating the smoke opacity readings observed with the sampling smoke meter having L = 430 mm and full flow smoke meter fitted to the exhaust pipe of 100 mm dia. (4.6) Discuss the advantages and disadvantages of the full and partial flow dilution tunnels for measurement of PM emissions. (4.7) To measure emissions under fluctuating and transient operating conditions of a vehicle during the driving cycle operation, the emission analyzers having very fast response are required so that instantaneous concentration measurement for the pollutants can be done and the concentrations are then integrated over the driving cycle to determine emissions in terms of g/km. Such emission analyzers are not yet available and there is also delay in the sampling line from the exhaust to analyzer. Discuss how a constant volume sampling (CVS) system functions and this problem is taken care of. In case, the entire exhaust gas during the driving cycle is collected in a big balloon and then the average pollutant concentration is determined, what may be the disadvantages compared to the CVS system.
file:///C|/...20and%20Settings/iitkrana1/My%20Documents/Google%20Talk%20Received%20Files/engine_combustion/lecture18/18_12.htm[6/15/2012 3:03:17 PM]
Das könnte Ihnen auch gefallen
- How Does The VNT WorksDokument3 SeitenHow Does The VNT WorksAyman Al ShabanahNoch keine Bewertungen
- 9 Work Over 71sDokument73 Seiten9 Work Over 71sSlim.B67% (3)
- Flame Ionization DetectorDokument5 SeitenFlame Ionization DetectorJoy Marie JalotjotNoch keine Bewertungen
- Chapter 4 - Gas AbsorptionDokument95 SeitenChapter 4 - Gas AbsorptionBoyHaha100% (7)
- Introduction to RheologyDokument34 SeitenIntroduction to Rheologysaa6383100% (1)
- Carbon Capture Technologies for Gas-Turbine-Based Power PlantsVon EverandCarbon Capture Technologies for Gas-Turbine-Based Power PlantsNoch keine Bewertungen
- Emission Measurement: Measurement Methods of Exhaust Gas AnalyzersDokument16 SeitenEmission Measurement: Measurement Methods of Exhaust Gas Analyzersbazim uddinNoch keine Bewertungen
- Pressure Drop CalculationsDokument4 SeitenPressure Drop CalculationsOmprakaash Mokide100% (1)
- Determine TOC Water SamplesDokument5 SeitenDetermine TOC Water Samplesanilkumar995472Noch keine Bewertungen
- Gas AnalyzersDokument41 SeitenGas Analyzersdark knight50% (2)
- Research On On-Line DGA Using FTIRDokument6 SeitenResearch On On-Line DGA Using FTIRJames McVeighNoch keine Bewertungen
- AmmoniaDokument24 SeitenAmmoniaCamilo GarzonNoch keine Bewertungen
- Combustion of Pulverised Coal in a Mixture of Oxygen and Recycled Flue GasVon EverandCombustion of Pulverised Coal in a Mixture of Oxygen and Recycled Flue GasNoch keine Bewertungen
- Method 19 - Determination of Sulfur Dioxide Removal Efficiency and Particulate Matter, Sulfur Dioxide, and Nitrogen Oxide Emission RatesDokument15 SeitenMethod 19 - Determination of Sulfur Dioxide Removal Efficiency and Particulate Matter, Sulfur Dioxide, and Nitrogen Oxide Emission RatesSarfaraz KhanNoch keine Bewertungen
- Measurement of ExhaustDokument25 SeitenMeasurement of ExhaustSreeram NandakumarNoch keine Bewertungen
- Method 19Dokument15 SeitenMethod 19JeeEianYannNoch keine Bewertungen
- Unit III Engine Exhaust Emission ControlDokument49 SeitenUnit III Engine Exhaust Emission ControlMani Karthi100% (1)
- Emissions MeasurementDokument24 SeitenEmissions Measurementprinceshital100% (3)
- FT-IR Sampling Parameters For Exhaust Gas Measurements: TechnicalDokument3 SeitenFT-IR Sampling Parameters For Exhaust Gas Measurements: TechnicalAdriano BludegardNoch keine Bewertungen
- Stack Emission and Automobile Emission.Dokument29 SeitenStack Emission and Automobile Emission.Kumar GauravNoch keine Bewertungen
- Study of Exhaust Gas AnalyserDokument7 SeitenStudy of Exhaust Gas Analyser01parth100% (1)
- The Analysis of CarbonDioxide in Natural GasDokument4 SeitenThe Analysis of CarbonDioxide in Natural GasBimo Gunners RespatiNoch keine Bewertungen
- Comparison of CyclesDokument32 SeitenComparison of CyclesS.Ashwin DanielNoch keine Bewertungen
- Sensors 19 04260 v2Dokument14 SeitenSensors 19 04260 v2johnny chuNoch keine Bewertungen
- Presented By:: Satabdy Jena Mtech (Power and Energy Systems) ROLL NO.: T14EE003 Nit MeghalayaDokument23 SeitenPresented By:: Satabdy Jena Mtech (Power and Energy Systems) ROLL NO.: T14EE003 Nit MeghalayaHamza Ali MinhasNoch keine Bewertungen
- Submitted To: Submitted By: Name: Topic: Class: Reg. No: DateDokument11 SeitenSubmitted To: Submitted By: Name: Topic: Class: Reg. No: DateIshfaq AhmadNoch keine Bewertungen
- Analysis and Control Methods of CO, CO & OzoneDokument11 SeitenAnalysis and Control Methods of CO, CO & OzoneKanchanNoch keine Bewertungen
- REDUCING CO2 IN OFFICE BUILDINGSDokument18 SeitenREDUCING CO2 IN OFFICE BUILDINGSZahid EmuNoch keine Bewertungen
- Automotive Lab 3Dokument6 SeitenAutomotive Lab 3Muhammad J SherwaniNoch keine Bewertungen
- Species Emission and Its Corrected Value Emission Control Methods SO Emission and Its ControlDokument7 SeitenSpecies Emission and Its Corrected Value Emission Control Methods SO Emission and Its ControlKrozeNoch keine Bewertungen
- R9907 Algorithm MethaneDokument29 SeitenR9907 Algorithm MethanemxnoxnNoch keine Bewertungen
- CARBON DIOXIDE MONITORING IN OFFICE BUILDINGSDokument17 SeitenCARBON DIOXIDE MONITORING IN OFFICE BUILDINGSNaheedNoch keine Bewertungen
- Analysis of Motor Vehicle Exhaust Emissions: Hpr-20 Application NoteDokument2 SeitenAnalysis of Motor Vehicle Exhaust Emissions: Hpr-20 Application Notevzimak2355Noch keine Bewertungen
- Toc PDFDokument20 SeitenToc PDFarun aryaNoch keine Bewertungen
- Am Tool 06 v1Dokument14 SeitenAm Tool 06 v1Suchart TarasapNoch keine Bewertungen
- Near Real Time Monitoring of Diesel Particulate Matter in Underground MinesDokument6 SeitenNear Real Time Monitoring of Diesel Particulate Matter in Underground MinesChandra RiadyNoch keine Bewertungen
- 1.0 Scope and ApplicationDokument28 Seiten1.0 Scope and Applicationlalonded1372Noch keine Bewertungen
- Auto Exhaust AnalyzerDokument20 SeitenAuto Exhaust AnalyzerAnugya RampuriaNoch keine Bewertungen
- Session 25 Emission Measuring Equipment - HC, Co and No Oxides of NitrogenDokument4 SeitenSession 25 Emission Measuring Equipment - HC, Co and No Oxides of NitrogenvijayakumarNoch keine Bewertungen
- Non-Dispersive Infra-Red Absorption (Ndir)Dokument9 SeitenNon-Dispersive Infra-Red Absorption (Ndir)thilip-kumar-6622Noch keine Bewertungen
- Practical handbook for heating measurement technologyDokument64 SeitenPractical handbook for heating measurement technologySara ZaedNoch keine Bewertungen
- Technical Code On Control of Emission of Nitrogen Oxides From Marine Diesel EnginesDokument97 SeitenTechnical Code On Control of Emission of Nitrogen Oxides From Marine Diesel EnginesPradeepPrincerajNoch keine Bewertungen
- Sibench NotesDokument3 SeitenSibench NotesAdil MajidNoch keine Bewertungen
- Lab Report 04Dokument7 SeitenLab Report 04Nouma SalehaNoch keine Bewertungen
- Pulmonary Gas AnalyzersDokument24 SeitenPulmonary Gas AnalyzersJohn Carlo ConsultaNoch keine Bewertungen
- Methane-Specific Gas Detectors: The Effect of Natural Gas CompositionDokument22 SeitenMethane-Specific Gas Detectors: The Effect of Natural Gas CompositionAhmed AwwadNoch keine Bewertungen
- Recent Development in MarineDokument44 SeitenRecent Development in MarinerajishrrrNoch keine Bewertungen
- Mine Ventilation AssignmentDokument6 SeitenMine Ventilation AssignmentNeelabh AbhishekNoch keine Bewertungen
- CFD Simulation of Pollutant Emission in Power Plant BoilersDokument8 SeitenCFD Simulation of Pollutant Emission in Power Plant BoilersRajneesh VachaspatiNoch keine Bewertungen
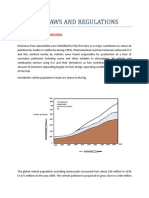
- Unit: 1 ..Laws and Regulations: Historical BackgroundsDokument9 SeitenUnit: 1 ..Laws and Regulations: Historical BackgroundsmanojNoch keine Bewertungen
- Application of Infrared Spectroscopy To Exhaust Gas AnalysisDokument10 SeitenApplication of Infrared Spectroscopy To Exhaust Gas AnalysisDerek BoegnerNoch keine Bewertungen
- Recent Development in Marine EnginesDokument81 SeitenRecent Development in Marine EnginesShashidhar ChandraiahNoch keine Bewertungen
- SSRN Id4284219Dokument8 SeitenSSRN Id4284219engr.okaforaugustineNoch keine Bewertungen
- Ambient Air Analysis and Measurement TechniquesDokument28 SeitenAmbient Air Analysis and Measurement TechniquesqncargbNoch keine Bewertungen
- PDGA BrochureDokument3 SeitenPDGA Brochureultramassive300Noch keine Bewertungen
- Fire SafetyDokument5 SeitenFire SafetyMaheshNoch keine Bewertungen
- Role of CFD in SCR NOX Reduction PerformanceDokument8 SeitenRole of CFD in SCR NOX Reduction PerformanceMadusuthanan SababathyNoch keine Bewertungen
- Numerical Analysis of Nox Reduction Using Ammonia Injection and Comparison With Water InjectionDokument14 SeitenNumerical Analysis of Nox Reduction Using Ammonia Injection and Comparison With Water InjectionSteve WanNoch keine Bewertungen
- Infrared Sensors Explained for Measuring CO2, CH4, and SO2Dokument3 SeitenInfrared Sensors Explained for Measuring CO2, CH4, and SO2sarvjeetmorNoch keine Bewertungen
- File 33272Dokument44 SeitenFile 33272EmOosh MohamedNoch keine Bewertungen
- Instructions: Industrial EcologyDokument5 SeitenInstructions: Industrial EcologyAdamNoch keine Bewertungen
- aAPC 2marks With AnswerDokument17 SeitenaAPC 2marks With AnswerERKATHIR0% (1)
- CHAPTER 3 CombustionDokument23 SeitenCHAPTER 3 CombustionChris ZiyuenNoch keine Bewertungen
- Iridium(III) in Optoelectronic and Photonics ApplicationsVon EverandIridium(III) in Optoelectronic and Photonics ApplicationsEli Zysman-ColmanNoch keine Bewertungen
- ATEX StandartiDokument12 SeitenATEX StandartiRadoslav RashevNoch keine Bewertungen
- Fig. 9.1: Control Volume of The Gas Flow in A Con-: Stant Cross SectionDokument34 SeitenFig. 9.1: Control Volume of The Gas Flow in A Con-: Stant Cross SectionEihabARaoufMustafaNoch keine Bewertungen
- Dear Pump Guy' : Feature Pumping Withlow NPSHDokument4 SeitenDear Pump Guy' : Feature Pumping Withlow NPSHReginaldo AmaroNoch keine Bewertungen
- Boiling and Condensation ProblemsDokument24 SeitenBoiling and Condensation Problemskeerthi srijithNoch keine Bewertungen
- Engine Exhaust Valves DamagedDokument3 SeitenEngine Exhaust Valves DamagedAmit Kumar GuptaNoch keine Bewertungen
- Selecting the Right Screw CompressorDokument3 SeitenSelecting the Right Screw Compressorli xianNoch keine Bewertungen
- APPENDIX B: Short Cut Key FunctionsDokument2 SeitenAPPENDIX B: Short Cut Key Functionsak_thimiriNoch keine Bewertungen
- SCBA protects users from gases and vaporsDokument1 SeiteSCBA protects users from gases and vaporsdulichsinhthaiNoch keine Bewertungen
- Industrial or Medical Oxygen GasDokument10 SeitenIndustrial or Medical Oxygen GasDelta DigitronicsNoch keine Bewertungen
- Marlotherm BrochureDokument24 SeitenMarlotherm Brochuresaka dewaNoch keine Bewertungen
- Vapor Pressure of WaterDokument3 SeitenVapor Pressure of WaterDương Hoàng100% (1)
- Refining Process: Hasan Djadid Assegaff (1431010056)Dokument2 SeitenRefining Process: Hasan Djadid Assegaff (1431010056)Hassan AssaqafNoch keine Bewertungen
- Gentle Delivery and Metering: Peristaltic Pumps DULCO Flex For Laboratory and Industry ApplicationsDokument8 SeitenGentle Delivery and Metering: Peristaltic Pumps DULCO Flex For Laboratory and Industry ApplicationsMizziNoch keine Bewertungen
- MansouriaDokument221 SeitenMansourianashwannoori100% (1)
- Pptonpumpspresentedbytamanash 131003045133 Phpapp01Dokument26 SeitenPptonpumpspresentedbytamanash 131003045133 Phpapp01Buddy EkoNoch keine Bewertungen
- Fluid Mechanics (BTME-301-18)Dokument13 SeitenFluid Mechanics (BTME-301-18)Surjit Kumar GandhiNoch keine Bewertungen
- EME1026 Assignment 1Dokument6 SeitenEME1026 Assignment 1Thaya IdhyomaticNoch keine Bewertungen
- EOS01204Dokument1 SeiteEOS01204Lesley-Anne RowandNoch keine Bewertungen
- Thesis 2016 GongDokument168 SeitenThesis 2016 GongBrahimABDNoch keine Bewertungen
- Techniques To Measure Dynamic Surface Tension PDFDokument8 SeitenTechniques To Measure Dynamic Surface Tension PDFjvchiqueNoch keine Bewertungen
- Trabajo de ReadingDokument3 SeitenTrabajo de ReadingPerez DavidNoch keine Bewertungen
- Twin Vs Single Screw ComparisonDokument2 SeitenTwin Vs Single Screw ComparisonRajeshNoch keine Bewertungen
- (SCAC Tropical) Cycle Logic (Eng) - 180914Dokument33 Seiten(SCAC Tropical) Cycle Logic (Eng) - 180914Tehnika SiingNoch keine Bewertungen
- Lecture 9: Steam Turbines - Impulse TurbineDokument8 SeitenLecture 9: Steam Turbines - Impulse TurbineIjazzzAliNoch keine Bewertungen
- Bouygues Construction Nigeria Limited: Weld History SheetDokument4 SeitenBouygues Construction Nigeria Limited: Weld History SheetnwohaNoch keine Bewertungen