Beruflich Dokumente
Kultur Dokumente
Cryogenic Treatment PDF
Hochgeladen von
sivajirao70Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Cryogenic Treatment PDF
Hochgeladen von
sivajirao70Copyright:
Verfügbare Formate
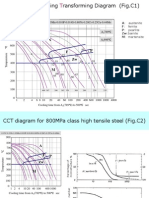
If for a round bar cross section of the chosen diameter the cooling curves are calculated at three or five
characteristic points (surface, (3/4)R, (1/2)R, (1/4)R, center), using the HEATTRANSF module, the CCT-DIAGR module enables the user to read off the hardness values after quenching at those points and to obtain the hardness distribution curve displayed graphically on the computer screen. In the case of delayed quenching with discontinuous change of cooling rate, the prediction of structural transformations and hardness values after quenching from an ordinary CCT diagram is not correct because the incubation time consumed (at any point of the cross section) before the cooling rate abruptly changes has not been taken into account. For a detailed explanation see Shimizu and Tamura [11].
6.3.3 RETAINED AUSTENITE
AND
CRYOGENIC TREATMENT
The martensite start (Ms) and martensite finish (Mf) temperatures for unalloyed steels depend on their carbon content, as shown in Figure 6.119. As can be seen from this diagram, when steels of more than 0.65% C are quenched the austenite-to-martensite transformation does not end at room temperature (208C (688F)) but at some lower temperature, even at temperatures much lower than 08C (328F). Consequently, after these steels are quenched to room temperature, a portion of austenite will remain untransformed; this is referred to as retained austenite. The greater the amount of carbon in the steel, the greater the amount of retained austenite after quenching, as shown in Figure 6.120c. Retained austenite, which is a softer constituent of the structure, decreases the steels hardness after quenching. If present in amounts of more than 10%, a substantial decrease in the hardness of the quenched steel may result (see curve a of Figure 6.120a). When quenching hypereutectoid steels from the usual hardening temperature (Figure 6.120b) i.e., from the g Fe3C region, the same hardness will result independently of carbon content (curve b in Figure 6.120a), because the hardness of martensite depends only on the carbon dissolved in austenite (g), which further depends (according to the solubility limit, line SE) on the hardening temperature. The structure of hardened hypereutectoid steels therefore consists of martensite Fe3C retained austenite. When quenching hypereutectoid steels from the region of pure austenite (g), i.e., from above the Acm temperature (which is not usual), the structure after hardening consists only of martensite and retained austenite, and the hardness decreases with carbon content as shown
600 500 Temperature, 8C 400 300 200 100 20 0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 C, wt% Mf Ms
FIGURE 6.119 Martensite start (Ms) and martensite finish (Mf) temperatures vs. carbon content in unalloyed steels.
2006 by Taylor & Francis Group, LLC.
c 1000 b Hardness, HV10 800 a 600 (a) Quenched from region to 0C (b) Quenched from + Fe3C region to 0C (c) After transformation to 100% martensite 0.2 0.4 0.6 0.8 1.0 1.2 1.4
400
200 0.0
(a)
Temperature, C
1000 900 800 700 600
Usual hardening temp.
+ S
A cm + Fe3C
(b)
Retained austenite, vol%
40 30 20 10 0 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
(c)
Carbon content, %
FIGURE 6.120 (a) Hardness of carbon (unalloyed) steels depending on carbon content and austenitizing temperature; (b) the range of usual hardening temperatures; (c) volume percent of retained austenite. rmebehandlung von Stahl, 2nd ed., VEB Deutscher Verlag (From H.J. Eckstein (Ed.), Technologie der Wa r Grundstoffindustrie, Leipzig, 1987.) fu
by curve a of Figure 6.120a. If the retained austenite is transformed (e.g., by subsequent cryogenic treatment) to 100% martensite, the hardness would follow curve c in Figure 6.120a. When after hardening the steel is kept at room temperature for some time or is heated to the temperature range corresponding to the first tempering stage, the retained austenite is stabilized, which implies that it has become more difficult to transform when subjected to cryogenic treatment. The stabilization of retained austenite is assumed to be due to the dissolution, at the arrest temperature, of the martensite nuclei formed during cooling from the austenitizing temperature. When martempering is performed, i.e., the quenching process is interrupted somewhere around the Ms temperature, a similar stabilization of retained austenite occurs. When the cooling to room temperature is then continued, the same effect, in principle, results as that obtained by the subzero cryogenic treatment with respect to the transformation of retained austenite to martensite. The initial amount of retained austenite (after quenching) is dependent to a very large extent on the austenitizing (hardening) temperature. The higher the hardening temperature, the greater the amount of retained austenite, but greater amounts of retained austenite may also be transformed to martensite by subzero cryogenic treatment for the same stabilizing temperature and same stabilizing time as shown in Figure 6.121. The stabilizing effect
2006 by Taylor & Francis Group, LLC.
120
80
x
5% transt. 40
x x
40% 0
30
20
10
(a) Stablizing temperature, C
120
x
101
Stablizing time, min
x
102
103
104
80
x x
20%
40 70% transt. 0 101
x
60
50
40
30
(b)
Stablizing time, min
102
103
104
120
x x
30% 40
x
80
50 40
x x
70% transt. 0 101
60
(c)
Stablizing time, min
102
103
104
FIGURE 6.121 Influence of stabilizing temperature and time on the amount of retained austenite that transforms on being subzero treated at 1808C for the ball bearing steel AISI 52100. (a) Austenitizing temperature 7808C; 9.4% retained austenite after quenching; (b) austenitizing temperature 8408C; 18% retained austenite after quenching; (c) austenitizing temperature 9008C; 27% retained austenite after quenching. (From K.E. Thelning, Steel and Its Heat Treatment, 2nd ed., Butterworths, London, 1984.)
increases as the stabilizing temperature and time increase. After quenching from, say, 8408C (15448F) (Figure 6.121b), there is 18% retained austenite. If the subzero treatment is carried out within 5 min after the temperature of the steel has reached 208C (688F), about 70% of the retained austenite will be transformed. If 40 min is allowed to pass before the subzero treatment, 60% will be transformed, and after 50 h holding at 208C (688F) only 30% of the retained austenite will respond to the subzero cryogenic treatment. If the steel is held after quenching at a higher temperature, e.g., at 1208C (2488F), for only 10 min before subzero treatment, only 30% of the retained austenite will be transformed. In order to transform the greatest possible amount of retained austenite, the subzero cryogenic treatment should be performed immediately after quenching before tempering. The question of whether the retained austenite in the structure is always detrimental or whether in some cases it can be advantageous has still not been answered unambiguously. When dealing with carburized and case-hardened components, because of the high carbon content in the case, the problem of retained austenite is always a real one, especially with steels containing nickel. The higher alloy nickel steels, such as types AISI 4620, 4820, and
2006 by Taylor & Francis Group, LLC.
2400 2000 Load, kg 1600 1200 800
50% retained 0% retained austenite austenite 16MnCr5 (1.13Mn, 1.00Cr) 14NiCr14 (3.67Ni, 0.78Cr) 20MoCr4 (0.49Cr, 0.5Mo)
Ck15 (Carbon steel)
400 104
105 Cycles
106
107
FIGURE 6.122 Improvement of contact fatigue of carburized and case-hardened steels containing 50% retained austenite, according to C. Razim. (From J. Parrish, Adv. Mater. Process. 3:2528, 1994.)
9310, are particularly likely to have retained austenite in their microstructures after heat treatment because nickel acts as an austenite stabilizer. Tests performed by M. Shea (cited in Ref. [24]), showed marked improvement in tensile bending strain values when retained austenite was present in the 2030% range for AISI 8620 and 4620 steels and up to 40% for AISI 3310 steel. This report indicated that the transformation of retained austenite in the range of more than 20% allows more plastic strain to be accommodated before crack initiation because the austenite deforms and subsequently transforms to martensite. The graph in Figure 6.122, taken from work done by C. Razim, shows where large quantities of retained austenite (in the range of 50%) improve contact fatigue of carburized and case-hardened steels. In another publication [24], several grades of carburized and casehardened steels were compared (both before and after subzero treatment), and a clear improvement in bend ductility is reported for those having retained austenite. As a result of the above-mentioned investigations, when dealing with carburized and casehardened gears, an amount of 1020%, and in some instances up to 25%, of retained austenite is not objectionable for most applications and may be beneficial. On the other hand, retained austenite can be detrimental, causing premature wear of sliding on the components surface or of sliding and rolling of gear teeth, because it is a softer constituent of the microstructure. The presence of retained austenite in cases of carburized and case-hardened components that are to be subsequently ground is definitely detrimental because under certain grinding conditions it causes severe grinding burns and cracking. The susceptibility of carburized and casehardened components to cracking during grinding becomes greater, the greater the amount of retained austenite, this amount further depending on the steel grade and carburizing temperature as shown in Figure 6.123. 6.3.3.1 Transforming the Retained Austenite When steels are tempered, retained austenite decomposes to bainite during the second tempering stage (2302808C (4465368F)). For high-alloy chromium steels, hot-work steels, and high-speed steels, the range of decomposition of retained austenite is shifted toward higher temperatures. The product of decomposition, i.e., whether it is bainite or martensite, depends on the tempering temperature and time. Bainite formation occurs isothermally, i.e., at
2006 by Taylor & Francis Group, LLC.
Das könnte Ihnen auch gefallen
- QuenchingDokument24 SeitenQuenchingHakan Yamanoğlu100% (4)
- Cold Rolled Steel StripDokument40 SeitenCold Rolled Steel StripshelarsanjayNoch keine Bewertungen
- Heat Treatment Definition and ObjectivesDokument56 SeitenHeat Treatment Definition and ObjectivesAakarsh RastogiNoch keine Bewertungen
- Heat-Treatment of High Carbon Steel Wire - PatentingDokument4 SeitenHeat-Treatment of High Carbon Steel Wire - Patentingعزت عبد المنعم100% (1)
- Gundappa Viswanath StatisticsDokument2 SeitenGundappa Viswanath Statisticssivajirao70100% (1)
- Mak214e hmw1Dokument3 SeitenMak214e hmw1çağla AydınNoch keine Bewertungen
- Annealing Heat Treatment PDFDokument5 SeitenAnnealing Heat Treatment PDFsmani170Noch keine Bewertungen
- SB Tooling Solution AHSSDokument21 SeitenSB Tooling Solution AHSSHugo RodriguezNoch keine Bewertungen
- Steel ImagesDokument49 SeitenSteel ImagesMustafa OğuzhanNoch keine Bewertungen
- Friction Stir Welding of High Strength 7XXX Aluminum AlloysVon EverandFriction Stir Welding of High Strength 7XXX Aluminum AlloysNoch keine Bewertungen
- Continuous Cooling Transforming DiagramDokument26 SeitenContinuous Cooling Transforming DiagramaunginternetNoch keine Bewertungen
- Dimensional Changes After Heat TreatmentDokument8 SeitenDimensional Changes After Heat TreatmentRahul garjeNoch keine Bewertungen
- Hardness PreheatDokument16 SeitenHardness PreheatLaurentiu ZgripceaNoch keine Bewertungen
- En 353Dokument93 SeitenEn 353kumarsathish2009Noch keine Bewertungen
- Normalizing Heat Treatment PDFDokument6 SeitenNormalizing Heat Treatment PDFsmani170100% (1)
- Review On Thermomechanical Processing of Advanced High Strength SteelsDokument130 SeitenReview On Thermomechanical Processing of Advanced High Strength SteelsharishNoch keine Bewertungen
- Microstructure and Mechanical Properties of ASTM A743 CA6NM Steel Welded by FCAW ProcessDokument8 SeitenMicrostructure and Mechanical Properties of ASTM A743 CA6NM Steel Welded by FCAW ProcessretrogradesNoch keine Bewertungen
- Welding TMCP SteelsDokument7 SeitenWelding TMCP SteelsElias Kapa100% (1)
- f6nm PDFDokument5 Seitenf6nm PDFasprclms durgapur100% (1)
- Grong - Metallurgical Modelling of WeldingDokument620 SeitenGrong - Metallurgical Modelling of Weldinggeerhardusvos100% (1)
- Hardenability of SteelDokument59 SeitenHardenability of SteelKhaula M RausyanNoch keine Bewertungen
- Bisalloy Technical Manual SectionsDokument65 SeitenBisalloy Technical Manual Sectionsramaus100% (1)
- Development of Coarse-Grained Structure During RecrystallizationDokument20 SeitenDevelopment of Coarse-Grained Structure During Recrystallizationsivajirao70100% (1)
- Soft Annealing Heat Treatment PDFDokument6 SeitenSoft Annealing Heat Treatment PDFsivajirao70Noch keine Bewertungen
- Introduction To Ductile IronDokument8 SeitenIntroduction To Ductile IronNatalino FonsecaNoch keine Bewertungen
- Isothermal Heat Treatment PDFDokument6 SeitenIsothermal Heat Treatment PDFsmani170Noch keine Bewertungen
- Cmi215.2017 - Guia TTDokument9 SeitenCmi215.2017 - Guia TTalex123456789009850% (2)
- Quiz 1 G1 + ANSWERDokument3 SeitenQuiz 1 G1 + ANSWERTayaChandranNoch keine Bewertungen
- TEST 1marking Guide: Mass Effect of Heat-Treatment. This Eliminates The More Costly Need To Quench-Harden With ADokument5 SeitenTEST 1marking Guide: Mass Effect of Heat-Treatment. This Eliminates The More Costly Need To Quench-Harden With ARhea GaiaNoch keine Bewertungen
- Aws Preheat Calculations From HardnessDokument6 SeitenAws Preheat Calculations From HardnessElias KapaNoch keine Bewertungen
- O HC HCDokument101 SeitenO HC HCIndustrial Infra Jobs100% (1)
- CA 6NM CastingsDokument11 SeitenCA 6NM CastingsvasanthiNoch keine Bewertungen
- TA9 Heat Treatment 2-Rev31Dokument5 SeitenTA9 Heat Treatment 2-Rev31Carlene ToaNoch keine Bewertungen
- Mechanical Properties Improvement of Low Carbon Steel by Combined Heat TreatmentsDokument15 SeitenMechanical Properties Improvement of Low Carbon Steel by Combined Heat TreatmentsReyza PrasetyoNoch keine Bewertungen
- Continuous Cooling Transformations in Nuclear Pressure Vessel SteelsDokument10 SeitenContinuous Cooling Transformations in Nuclear Pressure Vessel SteelsVanina GiselaNoch keine Bewertungen
- Effect of Copper on Hot Ductility Loss of Low Carbon SteelsDokument5 SeitenEffect of Copper on Hot Ductility Loss of Low Carbon SteelsBalakrishna G SettyNoch keine Bewertungen
- MSE3094 HW#8 Solutions April 1, 2011 1) : A) The Data IsDokument5 SeitenMSE3094 HW#8 Solutions April 1, 2011 1) : A) The Data IsradarskiNoch keine Bewertungen
- Materials and Cold Work of Forming: 2.1 Steel StandardsDokument23 SeitenMaterials and Cold Work of Forming: 2.1 Steel Standardshunter8080Noch keine Bewertungen
- Structural Steels and Their PropertiesDokument27 SeitenStructural Steels and Their Propertiesellesor macalamNoch keine Bewertungen
- Applying Computer Simulation in Improving Heat Treating Condition of Thin High-Carbon Steel PartsDokument5 SeitenApplying Computer Simulation in Improving Heat Treating Condition of Thin High-Carbon Steel PartssathishelakkiyaNoch keine Bewertungen
- 4 Harden AbilityDokument12 Seiten4 Harden AbilityFadlin QisthiNoch keine Bewertungen
- Heat Treatment Grossmann Hardenability PDFDokument11 SeitenHeat Treatment Grossmann Hardenability PDFsivajirao70100% (2)
- Hardenability concepts and testing methodsDokument64 SeitenHardenability concepts and testing methodssujit_sekharNoch keine Bewertungen
- Dual High StrengthDokument8 SeitenDual High StrengthAhmed El-SaiedNoch keine Bewertungen
- Stretch - Angeability of A High-Strength TRIP Type Bainitic SheetDokument7 SeitenStretch - Angeability of A High-Strength TRIP Type Bainitic SheetKhomasan JumpasriNoch keine Bewertungen
- Wear Behavior of 100cr6-PolatDokument6 SeitenWear Behavior of 100cr6-PolatAntonioNoch keine Bewertungen
- Martensitic Stainless SteelsDokument8 SeitenMartensitic Stainless SteelsAdilmar E. NatãnyNoch keine Bewertungen
- IntroDokument9 SeitenIntroKeyshia KoishiNoch keine Bewertungen
- 105994998770347981Dokument11 Seiten105994998770347981Edgar HornusNoch keine Bewertungen
- Materials: 6.1 Hull SteelDokument13 SeitenMaterials: 6.1 Hull SteelJuan SilvaNoch keine Bewertungen
- Journal of Manufacturing Processes: Hadi Ghasemi Nanesa, Julien Boulgakoff, Mohammad JahaziDokument5 SeitenJournal of Manufacturing Processes: Hadi Ghasemi Nanesa, Julien Boulgakoff, Mohammad JahazisamNoch keine Bewertungen
- Wear Resistance of High C High Si Steel With Low RDokument11 SeitenWear Resistance of High C High Si Steel With Low RChristina Christina ChristinaNoch keine Bewertungen
- 1931 6834 1 SMDokument12 Seiten1931 6834 1 SMchpinto10% (1)
- Development of Bake-Hardenable Al-Killed Steel Sheet by Box Annealing ProcessDokument10 SeitenDevelopment of Bake-Hardenable Al-Killed Steel Sheet by Box Annealing ProcessRamírez WillebaldoNoch keine Bewertungen
- SD Article 46Dokument8 SeitenSD Article 46Mustafa MoussaouiNoch keine Bewertungen
- Laser Beam Welding of Quenched and Tempered Astm A 517 GR.B SteelDokument7 SeitenLaser Beam Welding of Quenched and Tempered Astm A 517 GR.B SteelbiancogallazziNoch keine Bewertungen
- Weldab 13%CRDokument7 SeitenWeldab 13%CRcawid100% (1)
- Chapter 9, Problem 1Dokument7 SeitenChapter 9, Problem 1Cam MillerNoch keine Bewertungen
- On Welding Gray Cast Iron Using SMAW and GTAW Process: Articles You May Be Interested inDokument11 SeitenOn Welding Gray Cast Iron Using SMAW and GTAW Process: Articles You May Be Interested inDoty RisantiNoch keine Bewertungen
- Introduction of Heat and Surface Treatment PDFDokument45 SeitenIntroduction of Heat and Surface Treatment PDFScott BakerNoch keine Bewertungen
- Memorandum Assignment 3Dokument2 SeitenMemorandum Assignment 3Michael G ErasmusNoch keine Bewertungen
- Modern PowerplantsDokument12 SeitenModern PowerplantsTibor KeményNoch keine Bewertungen
- Welding PreheatingDokument13 SeitenWelding PreheatingkenzsugiyantoNoch keine Bewertungen
- Mechanical Properties and Retained Austenite in Intercritically Heat-Treated Bainite-Transformed SteelDokument10 SeitenMechanical Properties and Retained Austenite in Intercritically Heat-Treated Bainite-Transformed SteelJhohan JimenezNoch keine Bewertungen
- Engineering Metallurgy Chapter-8 Ref: Introduction To Physical MetallurgyDokument34 SeitenEngineering Metallurgy Chapter-8 Ref: Introduction To Physical MetallurgyMD Al-AminNoch keine Bewertungen
- India Vs New Zealand 2020 ODI WhitewashDokument2 SeitenIndia Vs New Zealand 2020 ODI Whitewashsivajirao70Noch keine Bewertungen
- India Australia 2017 First ODIDokument4 SeitenIndia Australia 2017 First ODIsivajirao70Noch keine Bewertungen
- IPL 2021 RCB Vs CSKDokument1 SeiteIPL 2021 RCB Vs CSKsivajirao70Noch keine Bewertungen
- India Women Cricket Beats Australia Women Feb2020Dokument7 SeitenIndia Women Cricket Beats Australia Women Feb2020sivajirao70Noch keine Bewertungen
- England Australia 2018 Fourth One Day MatchDokument2 SeitenEngland Australia 2018 Fourth One Day Matchsivajirao70Noch keine Bewertungen
- India surrender at Edgbaston - 5 past Test heartbreaks that add to India's lossDokument4 SeitenIndia surrender at Edgbaston - 5 past Test heartbreaks that add to India's losssivajirao70Noch keine Bewertungen
- India Vs Australia: Prolific Cheteshwar Pujara Slams 18th Test Century, Scripts New RecordDokument2 SeitenIndia Vs Australia: Prolific Cheteshwar Pujara Slams 18th Test Century, Scripts New Recordsivajirao70Noch keine Bewertungen
- Shikar Dhawan ICC 4th CenturyDokument2 SeitenShikar Dhawan ICC 4th Centurysivajirao70Noch keine Bewertungen
- Rohit Sharma 200 ODIDokument3 SeitenRohit Sharma 200 ODIsivajirao70Noch keine Bewertungen
- England win ODI series against India behind Root, Morgan centuriesDokument3 SeitenEngland win ODI series against India behind Root, Morgan centuriessivajirao70Noch keine Bewertungen
- Virat Kohli Fastest To Reach 10000 RunsDokument2 SeitenVirat Kohli Fastest To Reach 10000 Runssivajirao70Noch keine Bewertungen
- England Australia 2018 Third One Day MatchDokument2 SeitenEngland Australia 2018 Third One Day Matchsivajirao70Noch keine Bewertungen
- India Vs West Indies Virat Splendid PerformanceDokument3 SeitenIndia Vs West Indies Virat Splendid Performancesivajirao70Noch keine Bewertungen
- India Vs England 2018 Virat The Top ScorerDokument2 SeitenIndia Vs England 2018 Virat The Top Scorersivajirao70Noch keine Bewertungen
- India Vs England, 1st ODI Nottingham, Full Cricket Score: Rohit, Kuldeep Guide IND To VictoryDokument2 SeitenIndia Vs England, 1st ODI Nottingham, Full Cricket Score: Rohit, Kuldeep Guide IND To Victorysivajirao70Noch keine Bewertungen
- India Vs England - England WinsDokument3 SeitenIndia Vs England - England Winssivajirao70Noch keine Bewertungen
- IPL 2018 Orange Cap: Kane Williamson ends season as top run scorerDokument2 SeitenIPL 2018 Orange Cap: Kane Williamson ends season as top run scorersivajirao70Noch keine Bewertungen
- Virat Kohli hits first Test century in EnglandDokument2 SeitenVirat Kohli hits first Test century in Englandsivajirao70Noch keine Bewertungen
- IPL 2018 Rishabh Pant FiftiesDokument2 SeitenIPL 2018 Rishabh Pant Fiftiessivajirao70Noch keine Bewertungen
- David Warner scores 600+ runs in IPL 2017Dokument1 SeiteDavid Warner scores 600+ runs in IPL 2017sivajirao70Noch keine Bewertungen
- England Australia 2018 First One Day MatchDokument4 SeitenEngland Australia 2018 First One Day Matchsivajirao70Noch keine Bewertungen
- England Australia 2018 Second One Day MatchDokument2 SeitenEngland Australia 2018 Second One Day Matchsivajirao70Noch keine Bewertungen
- India Vs Afghanistan Historic Cricket TestDokument3 SeitenIndia Vs Afghanistan Historic Cricket Testsivajirao70Noch keine Bewertungen
- IPL 2018 Kane Williamson Scores 500 RunsDokument2 SeitenIPL 2018 Kane Williamson Scores 500 Runssivajirao70Noch keine Bewertungen
- IPL 2018 Shane Watson CenturyDokument2 SeitenIPL 2018 Shane Watson Centurysivajirao70Noch keine Bewertungen
- MS Dhoni Reaches Another Milestone in IPLDokument2 SeitenMS Dhoni Reaches Another Milestone in IPLsivajirao70Noch keine Bewertungen
- Erapalli Prasanna StatisticsDokument1 SeiteErapalli Prasanna Statisticssivajirao70Noch keine Bewertungen
- Bishan Bedi Bowling StatisticsDokument1 SeiteBishan Bedi Bowling Statisticssivajirao70Noch keine Bewertungen
- Sunil Gavaskar StatisticsDokument2 SeitenSunil Gavaskar Statisticssivajirao70Noch keine Bewertungen
- Steel Making Prof. Deepak Mazumdar Prof. S. C. Koria Department of Materials Science and Engineering Indian Institute of Technology, KanpurDokument16 SeitenSteel Making Prof. Deepak Mazumdar Prof. S. C. Koria Department of Materials Science and Engineering Indian Institute of Technology, KanpurSyed HasniNoch keine Bewertungen
- Standard Terminology Relating To MetallographyDokument35 SeitenStandard Terminology Relating To MetallographyviverefeliceNoch keine Bewertungen
- GB 2585-2007 PDFDokument58 SeitenGB 2585-2007 PDFJames FaizNoch keine Bewertungen
- Welding Training Intro 20151010 PDFDokument74 SeitenWelding Training Intro 20151010 PDFBita MohajerniaNoch keine Bewertungen
- ME 210 Metallurgy and Materials EngineeringDokument5 SeitenME 210 Metallurgy and Materials Engineeringnandan144Noch keine Bewertungen
- Jominy TestDokument13 SeitenJominy Testihulme_2006139Noch keine Bewertungen
- Time-Temperature-Transformation Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalDokument82 SeitenTime-Temperature-Transformation Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalEdo DestradaNoch keine Bewertungen
- 5 Iron-Cementite Phase DiagramDokument46 Seiten5 Iron-Cementite Phase DiagramsmrutiNoch keine Bewertungen
- Heat TreatmentDokument38 SeitenHeat TreatmentTushar RoyNoch keine Bewertungen
- (Doi 10.1016 - B978-0!08!096532-1.01209-7) Ericsson, T. - Comprehensive Materials Processing - Residual Stresses Produced by Quenching of Martensitic SteelsDokument28 Seiten(Doi 10.1016 - B978-0!08!096532-1.01209-7) Ericsson, T. - Comprehensive Materials Processing - Residual Stresses Produced by Quenching of Martensitic SteelsmohamadNoch keine Bewertungen
- FeC and TTT DiagramsDokument12 SeitenFeC and TTT DiagramsMohamed El-WakilNoch keine Bewertungen
- The Iron-Carbon Phase DiagramDokument16 SeitenThe Iron-Carbon Phase DiagramMeena SivasubramanianNoch keine Bewertungen
- MBH Metallurgical Consultancy BrochureDokument2 SeitenMBH Metallurgical Consultancy BrochureZulfadhli bin MohdNoch keine Bewertungen
- 86 - The Formation of Panel Cracks in Steel Ingots - A State-Of-The-Art Review - II. Mid-Face and Off-Corner CracksDokument9 Seiten86 - The Formation of Panel Cracks in Steel Ingots - A State-Of-The-Art Review - II. Mid-Face and Off-Corner CracksdzizicNoch keine Bewertungen
- Is 1865Dokument13 SeitenIs 1865RAKESH SRIVASTAVANoch keine Bewertungen
- Effect of Alloying Elements on Corrosion Resistance of Reinforcing Bar SteelDokument7 SeitenEffect of Alloying Elements on Corrosion Resistance of Reinforcing Bar SteelJosé AntonioNoch keine Bewertungen
- Engineering MaterialsDokument39 SeitenEngineering MaterialsDivyaNoch keine Bewertungen
- NBS18 Heat TreatmentDokument46 SeitenNBS18 Heat Treatmentshailesh_tiwari_mechNoch keine Bewertungen
- Comp Mode of Jominy Test Paper ID1396Dokument5 SeitenComp Mode of Jominy Test Paper ID1396fdcarazoNoch keine Bewertungen
- Effect of Cooling Medium and Tempering On Microstructures and Hardness of SK3 High Carbon SteelDokument12 SeitenEffect of Cooling Medium and Tempering On Microstructures and Hardness of SK3 High Carbon Steelabdul azhimNoch keine Bewertungen
- Strengthening Effects and Processing Optimization of Niobium On High-Strength RebarsDokument12 SeitenStrengthening Effects and Processing Optimization of Niobium On High-Strength RebarsJJNoch keine Bewertungen
- Improved Etching Technique For The Determination of Percent MarDokument6 SeitenImproved Etching Technique For The Determination of Percent Marpinheiro_pinheiro6780Noch keine Bewertungen