Beruflich Dokumente
Kultur Dokumente
SCAN
Hochgeladen von
Lucian TeodorCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
SCAN
Hochgeladen von
Lucian TeodorCopyright:
Verfügbare Formate
Immunological Complications
63
Table 11.2 Immune red cell destruction. Site of destruction Characteristics lytic of antibodies Red cell ruptures Predominantly intravascular Potent, Predominantly extravascular
Antibodies that do not activate or only partially activate complment (Anti-Rh, anti-K, anti-Jk, and others) Nausea, shivering
(Anti-A and -B) Substernal pain, lumbar pain, restlessness uncontrollable bleeding Haemoglobinuria
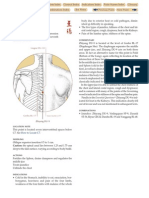
Haemoglobin liberated into plasma Figure 11.1 IgM anti-A binding to two adjacent A sites activtes th whole of complment pathway (C1 to C9); C8 and C9 make holes in th red cell membrane.
Symptoms
Signs: Immdiate
Hypotension, fever,
Fever
Jaundice
\ I g IgG anti-Rh
Later
. Rh sites Red cells lysed / Antibody coated red cells I \
antigen (anti-HLA) - do not activate
within macrophage; haemoglobin metabolised Bilirubin liberated
Red cells adhres to, and is lysed outside, macrophage
Red cells adhres to, and is lysed outside, killer lymphocyte Haemoglobin liberated
Haemoglobin liberated Figure 11.2 IgG anti-Rh does not activate complment. Red cells coated with antibody adhre to a macrophage or killer lymphocyte and are either ingested or lysed at th cell surface.
incompatible blood are unconscious at th time, so that there are no symptoms that might call attention to what is happening.
Mechanisms of red cell destruction
When ABO incompatible red cells are transfused - for example donor group A and rcipient group O - th incompatible IgM antibody (anti-A and anti-AB in this case) immediately binds to th donor's red cells (Figure 11.1). The bound antibody activtes complment leading to immdiate lysis of donor red cells in th plasma. When th antibody is potent, virtually ail th transfused red cells may be lysed within th bloodstream. Many other blood group allo-antibodies, leading to immdiate haemolytic reactions, are IgG and activate complment, but as a rule they do so only partially, up to C3b, and th coated cells, after binding to monocytes/macrophages, are either ingested or destroyed by cytotoxicity as they pass through th liver and spleen. Some antibodies - for example anti-Rh and a few anti-human leucocyte
complment (Figure 11.2), and thse cause macrophages to remove coated red cells or platelets in th spleen only.
Symptoms and signs The vast majority of patients experiencing an ABO incompatible transfusion reaction hve no symptoms. However, about 20-25% of patients, particularly if group O, will hve symptoms of vary-ing severity (Table 11.2). In th conscious patient, th transfusion of even a few millilitres of ABO incompatible blood may cause symptoms within 1 or 2 minutes. The patient becomes restless and complains of a feeling of oppression that is often accompanied by substernal pain, chills and fever. The patient may also complain of a burning face and may hve abdominal pain and may vomit. In th unconscious patient th most important signs are hypotension and uncontrollable bleeding. The main cause of th symptoms and of th hypotension is th release of th products of complment (C3a and C5a) into th plasma. Thse are polypeptides with a molecular weight of about 20000, which cause th contraction of smooth muscle and th production of nitric oxide, oxygen radicals and cytokines such as tumour necrosis factor (TNF) and interleukin-1 (IL-1); they also cause degranulation of mast cells leading to th release of vasoac-tive substances (bradykinin and serotonin). Bleeding is caused by disseminated intravascular coagulation, which is thought to be triggered by procoagulant substances released from red cell stroma after lysis and also, possibly, by th direct activation of th coagulation System by complment, by antigen-antibody complexes and by cytokines. Oliguria is common after th transfusion of ABO incompatible blood and is thought to be th resuit of changes in rnal blood fiow precipitated by hypotension, rather than haemoglobinuria (Box 11.5). As soon as it is suspected that incompatible blood has been transfused, th transfusion should be stopped, and an intra-venous infusion of crystalloid solution should be started to maintain urinary output. Frusemide 80-200 mg should be given
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5782)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (72)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Topic 8 Transport in Plants: 8.1 Vascular Tissue Functions of Xylem & PhloemDokument10 SeitenTopic 8 Transport in Plants: 8.1 Vascular Tissue Functions of Xylem & PhloemMuhammad Hassan TariqNoch keine Bewertungen
- Everything You Need to Know About the Muscular SystemDokument46 SeitenEverything You Need to Know About the Muscular SystemSide PoorNoch keine Bewertungen
- Immunity Worksheet PDFDokument3 SeitenImmunity Worksheet PDFCrayolaConsumer Sumer100% (1)
- ModuleDokument15 SeitenModuleKrisha Ann RosalesNoch keine Bewertungen
- Motor Proteins Power Cell TransportDokument6 SeitenMotor Proteins Power Cell TransportIndranil BanerjeeNoch keine Bewertungen
- ExamView - Chapter - 01Dokument2 SeitenExamView - Chapter - 01Devin MckayNoch keine Bewertungen
- OedemaDokument23 SeitenOedemaTavleen KaurNoch keine Bewertungen
- Immunedermatology MCQsDokument73 SeitenImmunedermatology MCQsDr.Tawheed90% (10)
- TestBank Norris Porths Essentials Pathophysiology 5e 2019Dokument505 SeitenTestBank Norris Porths Essentials Pathophysiology 5e 2019Cindy Perez0% (1)
- Thoa 1994Dokument24 SeitenThoa 199439 femina pcNoch keine Bewertungen
- 1 BAMS - MUHS Question PapersDokument45 Seiten1 BAMS - MUHS Question PapersShubham KaleNoch keine Bewertungen
- Response of The Oral Mucosa To Denture WearingDokument13 SeitenResponse of The Oral Mucosa To Denture WearingZachary DuongNoch keine Bewertungen
- C. J. Mieny, U. Mennen: Principles of Surgical Patient Care - Volume II Chapter 13: Organ Transplantation Principles of Organ Transplantation J. R. Botha and L. P. MargoliusDokument28 SeitenC. J. Mieny, U. Mennen: Principles of Surgical Patient Care - Volume II Chapter 13: Organ Transplantation Principles of Organ Transplantation J. R. Botha and L. P. MargoliusGordana UzelacNoch keine Bewertungen
- Physiology III - Exam 2 QuestionsDokument25 SeitenPhysiology III - Exam 2 QuestionsBrian Raymond0% (1)
- Previous Year Solved Question Papers of ICSE Biology Class X Board Paper 2008Dokument21 SeitenPrevious Year Solved Question Papers of ICSE Biology Class X Board Paper 2008Lokesh MalikNoch keine Bewertungen
- 11.1 Antibody Production and VaccinationDokument28 Seiten11.1 Antibody Production and VaccinationFRENCHONLYNoch keine Bewertungen
- Anatomy of ESRDDokument3 SeitenAnatomy of ESRDJheanAlphonsineT.MeansNoch keine Bewertungen
- Fish Anatomy and Physiology GuideDokument52 SeitenFish Anatomy and Physiology Guidelow luffecussNoch keine Bewertungen
- Du 9Dokument1 SeiteDu 9BDI92Noch keine Bewertungen
- Hormones Role in Male and Female Reproductive SystemsDokument15 SeitenHormones Role in Male and Female Reproductive SystemsJoeffrie G. CostalesNoch keine Bewertungen
- Science DLL Q2W1Dokument5 SeitenScience DLL Q2W1ANGELBERT PEDNGANoch keine Bewertungen
- Classification of PulpitisDokument18 SeitenClassification of PulpitisEkrar Okto ViarNoch keine Bewertungen
- Provide Nursing Care in First Line PDFDokument219 SeitenProvide Nursing Care in First Line PDFMisaw KasyeNoch keine Bewertungen
- Hemorrhagic Conditions of Late Pregnancy Other Related Problems 1Dokument5 SeitenHemorrhagic Conditions of Late Pregnancy Other Related Problems 1imnasNoch keine Bewertungen
- Plant Reproduction: Asexual vs Sexual (39Dokument28 SeitenPlant Reproduction: Asexual vs Sexual (39Lorine ChoiNoch keine Bewertungen
- Vanishing Twin SacDokument14 SeitenVanishing Twin SacAL MARIA MEDNoch keine Bewertungen
- St. Joseph's Co-Ed School Biology Lesson on Cell OrganellesDokument19 SeitenSt. Joseph's Co-Ed School Biology Lesson on Cell Organellesom gupta100% (1)
- Psychoneuroimmunology 2Dokument3 SeitenPsychoneuroimmunology 2bhasiNoch keine Bewertungen
- InteGRATED SCIENCE - ExcretionDokument2 SeitenInteGRATED SCIENCE - Excretionnehru09Noch keine Bewertungen
- The First Three Weeks of Human EmbryogenesisDokument46 SeitenThe First Three Weeks of Human EmbryogenesisJasmine KaurNoch keine Bewertungen