Beruflich Dokumente
Kultur Dokumente
Iron-IronCarbide Phase Diagram
Hochgeladen von
umangmodi32Originalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Iron-IronCarbide Phase Diagram
Hochgeladen von
umangmodi32Copyright:
Verfügbare Formate
1496T_c09_252-310 11/29/05 11:33 Page 290
REVISED PAGES
290 Chapter 9 / Phase Diagrams
T h e I ro n C a r b o n S ys t e m
Of all binary alloy systems, the one that is possibly the most important is that for iron and carbon. Both steels and cast irons, primary structural materials in every technologically advanced culture, are essentially ironcarbon alloys. This section is devoted to a study of the phase diagram for this system and the development of several of the possible microstructures. The relationships among heat treatment, microstructure, and mechanical properties are explored in Chapters 10 and 11.
9.18 THE IRONIRON CARBIDE (FeFe3C) PHASE DIAGRAM
ferrite austenite
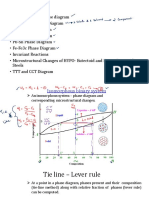
A portion of the ironcarbon phase diagram is presented in Figure 9.24. Pure iron, upon heating, experiences two changes in crystal structure before it melts. At room temperature the stable form, called ferrite, or a iron, has a BCC crystal structure. Ferrite experiences a polymorphic transformation to FCC austenite, or g iron, at 912C (1674F). This austenite persists to 1394C (2541F), at which temperature the FCC austenite reverts back to a BCC phase known as d ferrite, which finally
Composition (at% C) 1600 0 1538C 1493C L 2500 1394C 1200 Temperature (C) , Austenite 1000 912C + Fe3C 2.14 +L 5 10 15 20 25
1400
1147C 4.30 2000 Temperature (F)
800
+ 0.76 0.022
1500 727C
600
, Ferrite
+ Fe3C Cementite (Fe3C) 1000
400
0 (Fe)
3 4 Composition (wt% C)
6.70
Figure 9.24 The ironiron carbide phase diagram. [Adapted from Binary Alloy Phase Diagrams, 2nd edition, Vol. 1, T. B. Massalski (Editor-in-Chief), 1990. Reprinted by permission of ASM International, Materials Park, OH.]
1496T_c09_252-310 11/29/05 11:33 Page 291
REVISED PAGES
9.18 The IronIron Carbide (FeFe3C) Phase Diagram 291
Figure 9.25 Photomicrographs of (a) a ferrite (90) and (b) austenite (325). (Copyright 1971 by United States Steel Corporation.)
(a)
(b)
cementite
melts at 1538C (2800F). All these changes are apparent along the left vertical axis of the phase diagram.1 The composition axis in Figure 9.24 extends only to 6.70 wt% C; at this concentration the intermediate compound iron carbide, or cementite (Fe3C), is formed, which is represented by a vertical line on the phase diagram. Thus, the ironcarbon system may be divided into two parts: an iron-rich portion, as in Figure 9.24, and the other (not shown) for compositions between 6.70 and 100 wt% C (pure graphite). In practice, all steels and cast irons have carbon contents less than 6.70 wt% C; therefore, we consider only the ironiron carbide system. Figure 9.24 would be more appropriately labeled the FeFe3C phase diagram, since Fe3C is now considered to be a component. Convention and convenience dictate that composition still be expressed in wt% C rather than wt% Fe3C; 6.70 wt% C corresponds to 100 wt% Fe3C. Carbon is an interstitial impurity in iron and forms a solid solution with each of a and d ferrites, and also with austenite, as indicated by the a, d, and g singlephase fields in Figure 9.24. In the BCC a ferrite, only small concentrations of carbon are soluble; the maximum solubility is 0.022 wt% at 727C (1341F). The limited solubility is explained by the shape and size of the BCC interstitial positions, which make it difficult to accommodate the carbon atoms. Even though present in relatively low concentrations, carbon significantly influences the mechanical properties of ferrite. This particular ironcarbon phase is relatively soft, may be made magnetic at temperatures below 768C (1414F), and has a density of 7.88 g/cm3. Figure 9.25a is a photomicrograph of a ferrite.
The reader may wonder why no b phase is found on the FeFe3C phase diagram, Figure 9.24 (consistent with the a, b, g, etc. labeling scheme described previously). Early investigators observed that the ferromagnetic behavior of iron disappears at 768C and attributed this phenomenon to a phase transformation; the b label was assigned to the high-temperature phase. Later it was discovered that this loss of magnetism did not result from a phase transformation (see Section 20.6) and, therefore, the presumed b phase did not exist.
1496T_c09_252-310 11/29/05 11:33 Page 292
REVISED PAGES
292 Chapter 9 / Phase Diagrams The austenite, or g phase of iron, when alloyed with carbon alone, is not stable below 727C (1341F), as indicated in Figure 9.24. The maximum solubility of carbon in austenite, 2.14 wt%, occurs at 1147C (2097F). This solubility is approximately 100 times greater than the maximum for BCC ferrite, since the FCC interstitial positions are larger (see the results of Problem 4.5), and, therefore, the strains imposed on the surrounding iron atoms are much lower. As the discussions that follow demonstrate, phase transformations involving austenite are very important in the heat treating of steels. In passing, it should be mentioned that austenite is nonmagnetic. Figure 9.25b shows a photomicrograph of this austenite phase. The d ferrite is virtually the same as a ferrite, except for the range of temperatures over which each exists. Since the d ferrite is stable only at relatively high temperatures, it is of no technological importance and is not discussed further. Cementite (Fe3C) forms when the solubility limit of carbon in a ferrite is exceeded below 727C (1341F) (for compositions within the a Fe3C phase region). As indicated in Figure 9.24, Fe3C will also coexist with the g phase between 727 and 1147C (1341 and 2097F). Mechanically, cementite is very hard and brittle; the strength of some steels is greatly enhanced by its presence. Strictly speaking, cementite is only metastable; that is, it will remain as a compound indefinitely at room temperature. However, if heated to between 650 and 700C (1200 and 1300F) for several years, it will gradually change or transform into a iron and carbon, in the form of graphite, which will remain upon subsequent cooling to room temperature. Thus, the phase diagram in Figure 9.24 is not a true equilibrium one because cementite is not an equilibrium compound. However, inasmuch as the decomposition rate of cementite is extremely sluggish, virtually all the carbon in steel will be as Fe3C instead of graphite, and the ironiron carbide phase diagram is, for all practical purposes, valid.As will be seen in Section 11.2, addition of silicon to cast irons greatly accelerates this cementite decomposition reaction to form graphite. The two-phase regions are labeled in Figure 9.24. It may be noted that one eutectic exists for the ironiron carbide system, at 4.30 wt% C and 1147C (2097F); for this eutectic reaction,
Eutectic reaction for the iron-iron carbide system
L g Fe3C
heating
cooling
(9.18)
the liquid solidifies to form austenite and cementite phases. Of course, subsequent cooling to room temperature will promote additional phase changes. It may be noted that a eutectoid invariant point exists at a composition of 0.76 wt% C and a temperature of 727C (1341F).This eutectoid reaction may be represented by
Eutectoid reaction for the iron-iron carbide system
g 1 0.76 wt% C 2 a 1 0.022 wt% C 2 Fe3C 1 6.7 wt%C 2
heating
cooling
(9.19)
or, upon cooling, the solid g phase is transformed into a iron and cementite. (Eutectoid phase transformations were addressed in Section 9.14.) The eutectoid phase changes described by Equation 9.19 are very important, being fundamental to the heat treatment of steels, as explained in subsequent discussions. Ferrous alloys are those in which iron is the prime component, but carbon as well as other alloying elements may be present. In the classification scheme of ferrous alloys based on carbon content, there are three types: iron, steel, and cast iron. Commercially pure iron contains less than 0.008 wt% C and, from the phase diagram, is composed almost exclusively of the ferrite phase at room temperature. The
Das könnte Ihnen auch gefallen
- Callister7E - pp290 301 (The Iron Carbon System)Dokument12 SeitenCallister7E - pp290 301 (The Iron Carbon System)iglumacNoch keine Bewertungen
- Fundamentals of Electrocatalyst Materials and Interfacial Characterization: Energy Producing Devices and Environmental ProtectionVon EverandFundamentals of Electrocatalyst Materials and Interfacial Characterization: Energy Producing Devices and Environmental ProtectionNoch keine Bewertungen
- Handout Chapter 5 Iron Carbon SystemDokument7 SeitenHandout Chapter 5 Iron Carbon SystemBikram MuduliNoch keine Bewertungen
- Iron Carbon Equilibrium DiagramDokument11 SeitenIron Carbon Equilibrium Diagramganesh82Noch keine Bewertungen
- Dokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dDokument16 SeitenDokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dAfrizal Adithya PNoch keine Bewertungen
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDokument39 SeitenFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualcacoonnymphaea6wgyct100% (15)
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDokument79 SeitenFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualdextrermachete4amgqgNoch keine Bewertungen
- Chapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMDokument13 SeitenChapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMPAUL NDIRITUNoch keine Bewertungen
- Phase Diagram - : Dr. Aneela Wakeel 02-01-2018Dokument42 SeitenPhase Diagram - : Dr. Aneela Wakeel 02-01-2018Hassan KhanNoch keine Bewertungen
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDokument13 SeitenUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongNoch keine Bewertungen
- Introduction To Fe-C Equilibrium Phase Diagram: Chia-Chang ShihDokument42 SeitenIntroduction To Fe-C Equilibrium Phase Diagram: Chia-Chang ShihTuấnPhạmNoch keine Bewertungen
- Welding Technology English (Cont.)Dokument143 SeitenWelding Technology English (Cont.)Long Beautéophile100% (1)
- Capili Jefferson 10Dokument5 SeitenCapili Jefferson 10Christian Al EncarnacionNoch keine Bewertungen
- MSM GTU Study Material E-Notes Unit-5 23112020052908AMDokument14 SeitenMSM GTU Study Material E-Notes Unit-5 23112020052908AMVijayNoch keine Bewertungen
- Chapter 2 - TTA - TTT - DiagramsDokument13 SeitenChapter 2 - TTA - TTT - DiagramsPrasad Mhatre100% (1)
- Lec 7 Fe C DiagramDokument45 SeitenLec 7 Fe C DiagramAdnan MehmoodNoch keine Bewertungen
- AFS Thermal Analysis of CupsDokument12 SeitenAFS Thermal Analysis of Cupsyash_ganatraNoch keine Bewertungen
- MEC 414 - Iron Phase Diagram Experiment 2Dokument7 SeitenMEC 414 - Iron Phase Diagram Experiment 2boatcomNoch keine Bewertungen
- Lron-Carbon: Iron - RonDokument1 SeiteLron-Carbon: Iron - RonSreejith NairNoch keine Bewertungen
- Phase Transformation in Metals: Dr. Aneela WakeelDokument29 SeitenPhase Transformation in Metals: Dr. Aneela WakeelmazharNoch keine Bewertungen
- Iron Carbon Phase DiagramDokument4 SeitenIron Carbon Phase DiagramMizanur RahmanNoch keine Bewertungen
- TTT Curves 1Dokument101 SeitenTTT Curves 1ibrahimNoch keine Bewertungen
- Constr Materials B PDFDokument72 SeitenConstr Materials B PDFAgniva DuttaNoch keine Bewertungen
- 4Dokument63 Seiten4ARJUN PESARU 19BME0161Noch keine Bewertungen
- Phase Diagrams:: The Iron-Iron Carbide (Fe-Fe3C) Diagram or Iron-Carbon (Fe-C) Equilibrium DiagramDokument46 SeitenPhase Diagrams:: The Iron-Iron Carbide (Fe-Fe3C) Diagram or Iron-Carbon (Fe-C) Equilibrium DiagramUsman FarooqNoch keine Bewertungen
- Weldability of Metals - NPTELDokument18 SeitenWeldability of Metals - NPTELKaushal Gandhi0% (1)
- The IronCarbide DiagramDokument11 SeitenThe IronCarbide DiagramshajjikhalidNoch keine Bewertungen
- Characterization of The Weld Structure in A Duplex Stainless Steel Using Color MetallographyDokument11 SeitenCharacterization of The Weld Structure in A Duplex Stainless Steel Using Color MetallographyAndrea CalderaNoch keine Bewertungen
- EMAT 10 (2k21)Dokument41 SeitenEMAT 10 (2k21)Kumail AbbasNoch keine Bewertungen
- 05 - MetE 414-Phase Transformations-Microstructures of Steels-Fall 2023Dokument70 Seiten05 - MetE 414-Phase Transformations-Microstructures of Steels-Fall 2023egesenturk2000Noch keine Bewertungen
- 06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramDokument42 Seiten06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramTalaat Ahmed Mohamed El-Benawy100% (2)
- Engineering Material-II: Iron Carbide Phase DiagramDokument16 SeitenEngineering Material-II: Iron Carbide Phase DiagramAla ZiNoch keine Bewertungen
- Iron Carbon DiagramDokument10 SeitenIron Carbon DiagramsivakumarNoch keine Bewertungen
- Iron Carbon Part1 PDFDokument33 SeitenIron Carbon Part1 PDFErick HoganNoch keine Bewertungen
- PP Set 1 & 2Dokument3 SeitenPP Set 1 & 2Raveendra kumar KosireddiNoch keine Bewertungen
- The Effects of Alloying Elements On SteelsDokument36 SeitenThe Effects of Alloying Elements On SteelsRahul PandeyNoch keine Bewertungen
- Iron-Carbon Phase Diagram Explained BrieflyDokument4 SeitenIron-Carbon Phase Diagram Explained BrieflyZicoNoch keine Bewertungen
- Iron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingDokument7 SeitenIron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingRajulapati Sunil KumarNoch keine Bewertungen
- Review MetalDokument38 SeitenReview MetalnisannisaNoch keine Bewertungen
- Iron-Carbon Phase Diagram: 1-Peritectic Reaction: Occur at Temperature 1493Dokument5 SeitenIron-Carbon Phase Diagram: 1-Peritectic Reaction: Occur at Temperature 1493حسين كاظم ياسينNoch keine Bewertungen
- Ch-27.3 Iron Carbon Equilibrium DiagramDokument58 SeitenCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfNoch keine Bewertungen
- Iron Carbon Phase DiagramDokument4 SeitenIron Carbon Phase DiagramFuhad HasanNoch keine Bewertungen
- Iron Carbon Phase Diagram TEDDokument6 SeitenIron Carbon Phase Diagram TEDAnonymous dh6DITNoch keine Bewertungen
- Iron Carbon Equillibrium Diagram GandhidhamDokument22 SeitenIron Carbon Equillibrium Diagram Gandhidhamcal2_uniNoch keine Bewertungen
- Engineering Materials 27-29Dokument40 SeitenEngineering Materials 27-29Sanu SouravNoch keine Bewertungen
- Future of Advanced High Strength SteelDokument7 SeitenFuture of Advanced High Strength Steeldzb2022Noch keine Bewertungen
- 3 Iron Carbon DiaDokument21 Seiten3 Iron Carbon DiaChhavi SharmaNoch keine Bewertungen
- Principles of Heat Treating of SteelsDokument30 SeitenPrinciples of Heat Treating of Steelssatish_trivediNoch keine Bewertungen
- Ch-27.5 Iron Carbon Equilibrium DiagramDokument52 SeitenCh-27.5 Iron Carbon Equilibrium DiagramManojNoch keine Bewertungen
- Iron - Iron Carbide Iron Iron Carbide Phase Diagram: Today's TopicsDokument11 SeitenIron - Iron Carbide Iron Iron Carbide Phase Diagram: Today's TopicsembargioNoch keine Bewertungen
- Lesson 5 - Fe-C Diagram - Rev. 0Dokument11 SeitenLesson 5 - Fe-C Diagram - Rev. 0Arga SetyaNoch keine Bewertungen
- Fulltext 9Dokument12 SeitenFulltext 9Hasan TaslimiNoch keine Bewertungen
- Heat Treatment of 1045 Steel PDFDokument17 SeitenHeat Treatment of 1045 Steel PDFH_DEBIANENoch keine Bewertungen
- Ferrous AlloysDokument7 SeitenFerrous AlloysKeshav DesaiNoch keine Bewertungen
- 05 Heat Treatments To Produce Ferrite and PerliteDokument23 Seiten05 Heat Treatments To Produce Ferrite and PerliteRicardo Fidel Duarte SánchezNoch keine Bewertungen
- Hardening From The Liquid StateDokument5 SeitenHardening From The Liquid StateSinhrooNoch keine Bewertungen
- Medium MN SteelDokument8 SeitenMedium MN Steeldzb2022Noch keine Bewertungen
- Iron-Carbon DiagramDokument3 SeitenIron-Carbon DiagramnaniNoch keine Bewertungen
- The Bay Phenomenon in Steels With Reasonably Strong Carbide FormersDokument12 SeitenThe Bay Phenomenon in Steels With Reasonably Strong Carbide FormersbluecreteNoch keine Bewertungen
- Engineering Unit Conversion PDFDokument5 SeitenEngineering Unit Conversion PDFBarcepandoNoch keine Bewertungen
- Lect 3 4Dokument28 SeitenLect 3 4umangmodi32100% (1)
- Lect 1 2Dokument35 SeitenLect 1 2umangmodi32Noch keine Bewertungen
- ch-6 (Lect.-21)Dokument38 Seitench-6 (Lect.-21)rushangdaveNoch keine Bewertungen
- Lect 20 PolymerDokument42 SeitenLect 20 Polymerumangmodi32Noch keine Bewertungen
- Lect 8 9 10Dokument61 SeitenLect 8 9 10umangmodi32Noch keine Bewertungen
- CAREDokument11 SeitenCARELuis SementeNoch keine Bewertungen
- Multimedia System DesignDokument95 SeitenMultimedia System DesignRishi Aeri100% (1)
- Gastroesophagea L of Reflux Disease (GERD)Dokument34 SeitenGastroesophagea L of Reflux Disease (GERD)Alyda Choirunnissa SudiratnaNoch keine Bewertungen
- Newsletter 1-2021 Nordic-Baltic RegionDokument30 SeitenNewsletter 1-2021 Nordic-Baltic Regionapi-206643591100% (1)
- Omnitron CatalogDokument180 SeitenOmnitron Catalogjamal AlawsuNoch keine Bewertungen
- FINAL BÁO-CÁO-THỰC-TẬP.editedDokument38 SeitenFINAL BÁO-CÁO-THỰC-TẬP.editedngocthaongothi4Noch keine Bewertungen
- KMKT Pra PSPM ANS SCHEMEDokument16 SeitenKMKT Pra PSPM ANS SCHEMEElda AldaNoch keine Bewertungen
- Marion Nicoll: Life & Work by Catharine MastinDokument147 SeitenMarion Nicoll: Life & Work by Catharine MastinArt Canada InstituteNoch keine Bewertungen
- Transparency and Digitalization in The Public Administration of RomaniaDokument8 SeitenTransparency and Digitalization in The Public Administration of RomaniaMădălina MarincaşNoch keine Bewertungen
- Republic of The Philippines Division of Bohol Department of Education Region VII, Central VisayasDokument6 SeitenRepublic of The Philippines Division of Bohol Department of Education Region VII, Central VisayasJOHN MC RAE RACINESNoch keine Bewertungen
- Oceanarium: Welcome To The Museum Press ReleaseDokument2 SeitenOceanarium: Welcome To The Museum Press ReleaseCandlewick PressNoch keine Bewertungen
- Quanta To QuarksDokument32 SeitenQuanta To QuarksDaniel Bu100% (5)
- Brahms Symphony No 4Dokument2 SeitenBrahms Symphony No 4KlausNoch keine Bewertungen
- Ethical Conflicts in Psychology PDF DownloadDokument2 SeitenEthical Conflicts in Psychology PDF DownloadAvory0% (2)
- Duo Interpretation Class PresentationDokument31 SeitenDuo Interpretation Class PresentationPlanetSparkNoch keine Bewertungen
- LFF MGDokument260 SeitenLFF MGRivo RoberalimananaNoch keine Bewertungen
- WBCS 2023 Preli - Booklet CDokument8 SeitenWBCS 2023 Preli - Booklet CSurajit DasNoch keine Bewertungen
- DAA UNIT 1 - FinalDokument38 SeitenDAA UNIT 1 - FinalkarthickamsecNoch keine Bewertungen
- All India Civil Services Coaching Centre, Chennai - 28Dokument4 SeitenAll India Civil Services Coaching Centre, Chennai - 28prakashNoch keine Bewertungen
- MDI - Good Fellas - ScriptDokument20 SeitenMDI - Good Fellas - ScriptRahulSamaddarNoch keine Bewertungen
- June 2017 (IAL) MS - Unit 1 Edexcel Physics A-LevelDokument16 SeitenJune 2017 (IAL) MS - Unit 1 Edexcel Physics A-LevelNyraStardollNoch keine Bewertungen
- X - WORMWOOD EVENT IMMEDIATE - Paranormal - 4chanDokument7 SeitenX - WORMWOOD EVENT IMMEDIATE - Paranormal - 4chanAnonymous dIjB7XD8ZNoch keine Bewertungen
- BSH 7005-15Dokument129 SeitenBSH 7005-15Mark InnesNoch keine Bewertungen
- RPH Week 31Dokument8 SeitenRPH Week 31bbwowoNoch keine Bewertungen
- Latest ResumeDokument2 SeitenLatest Resumesamy1234567Noch keine Bewertungen
- Generalized Class of Sakaguchi Functions in Conic Region: Saritha. G. P, Fuad. S. Al Sarari, S. LathaDokument5 SeitenGeneralized Class of Sakaguchi Functions in Conic Region: Saritha. G. P, Fuad. S. Al Sarari, S. LathaerpublicationNoch keine Bewertungen
- GCP Vol 2 PDF (2022 Edition)Dokument548 SeitenGCP Vol 2 PDF (2022 Edition)Sergio AlvaradoNoch keine Bewertungen
- Positive Psychology in The WorkplaceDokument12 SeitenPositive Psychology in The Workplacemlenita264Noch keine Bewertungen
- Table of Specification 1st QDokument5 SeitenTable of Specification 1st QVIRGILIO JR FABINoch keine Bewertungen
- Directorate of Technical Education, Admission Committee For Professional Courses (ACPC), GujaratDokument2 SeitenDirectorate of Technical Education, Admission Committee For Professional Courses (ACPC), GujaratgamailkabaaaapNoch keine Bewertungen
- Transformed: Moving to the Product Operating ModelVon EverandTransformed: Moving to the Product Operating ModelBewertung: 4 von 5 Sternen4/5 (1)
- The Design Thinking Playbook: Mindful Digital Transformation of Teams, Products, Services, Businesses and EcosystemsVon EverandThe Design Thinking Playbook: Mindful Digital Transformation of Teams, Products, Services, Businesses and EcosystemsNoch keine Bewertungen
- CATIA V5-6R2015 Basics - Part I : Getting Started and Sketcher WorkbenchVon EverandCATIA V5-6R2015 Basics - Part I : Getting Started and Sketcher WorkbenchBewertung: 4 von 5 Sternen4/5 (10)
- Lean vs Agile vs Design Thinking: What You Really Need to Know to Build High-Performing Digital Product TeamsVon EverandLean vs Agile vs Design Thinking: What You Really Need to Know to Build High-Performing Digital Product TeamsBewertung: 4 von 5 Sternen4/5 (2)
- Articulating Design Decisions: Communicate with Stakeholders, Keep Your Sanity, and Deliver the Best User ExperienceVon EverandArticulating Design Decisions: Communicate with Stakeholders, Keep Your Sanity, and Deliver the Best User ExperienceBewertung: 4 von 5 Sternen4/5 (19)
- Laws of UX: Using Psychology to Design Better Products & ServicesVon EverandLaws of UX: Using Psychology to Design Better Products & ServicesBewertung: 5 von 5 Sternen5/5 (9)
- Analog Design and Simulation Using OrCAD Capture and PSpiceVon EverandAnalog Design and Simulation Using OrCAD Capture and PSpiceNoch keine Bewertungen
- Understanding Automotive Electronics: An Engineering PerspectiveVon EverandUnderstanding Automotive Electronics: An Engineering PerspectiveBewertung: 3.5 von 5 Sternen3.5/5 (16)
- The Jobs To Be Done Playbook: Align Your Markets, Organization, and Strategy Around Customer NeedsVon EverandThe Jobs To Be Done Playbook: Align Your Markets, Organization, and Strategy Around Customer NeedsBewertung: 5 von 5 Sternen5/5 (1)
- The Art of Welding: Featuring Ryan Friedlinghaus of West Coast CustomsVon EverandThe Art of Welding: Featuring Ryan Friedlinghaus of West Coast CustomsNoch keine Bewertungen
- Electrical Engineering 101: Everything You Should Have Learned in School...but Probably Didn'tVon EverandElectrical Engineering 101: Everything You Should Have Learned in School...but Probably Didn'tBewertung: 4.5 von 5 Sternen4.5/5 (27)
- Heat Exchanger Design Guide: A Practical Guide for Planning, Selecting and Designing of Shell and Tube ExchangersVon EverandHeat Exchanger Design Guide: A Practical Guide for Planning, Selecting and Designing of Shell and Tube ExchangersBewertung: 4 von 5 Sternen4/5 (13)
- Design for How People Think: Using Brain Science to Build Better ProductsVon EverandDesign for How People Think: Using Brain Science to Build Better ProductsBewertung: 4 von 5 Sternen4/5 (8)
- Artificial Intelligence Revolution: How AI Will Change our Society, Economy, and CultureVon EverandArtificial Intelligence Revolution: How AI Will Change our Society, Economy, and CultureBewertung: 4.5 von 5 Sternen4.5/5 (2)
- The Maker's Field Guide: The Art & Science of Making Anything ImaginableVon EverandThe Maker's Field Guide: The Art & Science of Making Anything ImaginableNoch keine Bewertungen
- Interfacing PIC Microcontrollers: Embedded Design by Interactive SimulationVon EverandInterfacing PIC Microcontrollers: Embedded Design by Interactive SimulationNoch keine Bewertungen
- Basic Electric Circuits: Pergamon International Library of Science, Technology, Engineering and Social StudiesVon EverandBasic Electric Circuits: Pergamon International Library of Science, Technology, Engineering and Social StudiesBewertung: 3 von 5 Sternen3/5 (1)
- Dynamic Aquaria: Building Living EcosystemsVon EverandDynamic Aquaria: Building Living EcosystemsBewertung: 4 von 5 Sternen4/5 (4)