Beruflich Dokumente
Kultur Dokumente
Chemical Engineering Laws
Hochgeladen von
Abhijit MoreOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Chemical Engineering Laws
Hochgeladen von
Abhijit MoreCopyright:
Verfügbare Formate
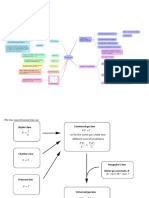
Raoult's law
the vapor pressure of an ideal solution is dependent on the vapor pressure of each chemical component and the mole fraction of the component present in the solution.[2] Once the components in the solution have reached equilibrium, the total vapor pressure p of the solution is:
and the individual vapor pressure for each component is
where pi is the partial pressure of the component i in the mixture (in the solution) p*i is the vapor pressure of the pure component i xi is the mole fraction of the component i in the mixture (in the solution)
Ideal Gas Law Calculation
The Ideal Gas Law(General Gas Equation) is the equation of state of a hypothetical ideal gas. Calculate the pressure, volume, temperature and moles of gas.
Ideal Gas Law: Gas Equation: PV = nRT where, P = pressure, V = volume, n = moles of gas, T = temperature, R = 8.314 J K-1 mol-1, ideal gas constant.
Boyle's/Mariotte's Law Calculation
Boyle's law states that the volume of a gas increases when the pressure decreases at a constant temperature.
Boyle's/Mariotte's Law: Gas Equation: PiVi = PfVf where, Pi = Initial Pressure, Vi = Initial Volume, Pf = Final Pressure, Vf = Final Volume.
Charles' Law Calculation
Charles' Law states that the volume of a given amount of gas is directly proportional to the temperature provided the amount of gas and the pressure remain fixed.
Charles' Law: Gas Equation: Vi/Ti = Vf/Tf or Vf/Vi = Tf/Ti or ViTf = VfTi where, Vi = Initial Volume, Ti = Initial Temperature, Vf = Final Volume. Tf = Final Temperature,
Dalton's Law Calculator
The Pressure of the mixture gas is equal to the sum of the pressure of the partial gases in a container'' is the statement of Dalton's partial pressures law. Calculate the pressure of combined gases with known values of temperature and mole of gas. Formula: Ptot=p1+p2+p3+...+pm (or) ptot={n1+n2+n3+...nm}RT/v
Where, p1,p2,p3, ...,pm= Partial pressures of the individual gases in the mixture. V = volume, T = temperature, n1,n2,n3, ...,nm=n is the total amount of gas of the m gases present in the mixture, R = 8.314 J K-1 mol-1, ideal gas constant. The Dalton's law calculator is derived from the law of partial pressures.
Gay-Lussac's Law Calculation
Gay-Lussac's Law states that the pressure of a fixed amount of gas at fixed volume is directly proportional to its temperature in kelvins. Gay-Lussac's Law: Gas Equation: Pi/Ti = Pf/Tf or PiTf = PfTi where, Pi = Initial Pressure, Ti = Initial Temperature, Pf = Final Pressure, Tf = Final Temperature.
Bernoulli equation
V= velocity P= pressure H= height g= gravity = density
Das könnte Ihnen auch gefallen
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsVon EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsBewertung: 5 von 5 Sternen5/5 (1)
- Week 3 PPT AD CHEMDokument8 SeitenWeek 3 PPT AD CHEMSophia Ysabelle EstradaNoch keine Bewertungen
- Thermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeVon EverandThermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeNoch keine Bewertungen
- Boyle's Law: Important: Charles's Law Only Works When The Pressure Is ConstantDokument3 SeitenBoyle's Law: Important: Charles's Law Only Works When The Pressure Is ConstantYlla GutierrezNoch keine Bewertungen
- Recommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsVon EverandRecommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsE. F. G. HeringtonNoch keine Bewertungen
- Chapter 5Dokument10 SeitenChapter 5Ayesha MohamudNoch keine Bewertungen
- Gas LawsDokument3 SeitenGas LawsSHALINI SINGHNoch keine Bewertungen
- (Lec5) Properties of GasesDokument52 Seiten(Lec5) Properties of GasesdinurjNoch keine Bewertungen
- Basic Rules and Laws of Science For Food TechnologyDokument22 SeitenBasic Rules and Laws of Science For Food TechnologypokhralikanchhaNoch keine Bewertungen
- Lec 3Dokument14 SeitenLec 3Not EmeraruduNoch keine Bewertungen
- Chemistry Picture Vocabulary - Gas LawsDokument23 SeitenChemistry Picture Vocabulary - Gas Lawsapi-254514513Noch keine Bewertungen
- ME 161: Introduction To Mechanical Engineering: Asif KabirDokument21 SeitenME 161: Introduction To Mechanical Engineering: Asif KabirMohammad Asif KabirNoch keine Bewertungen
- NB: The 'Handful' Story Is My Own Simplified Way of Understanding This. It's Not Really A ThingDokument2 SeitenNB: The 'Handful' Story Is My Own Simplified Way of Understanding This. It's Not Really A ThingMohammed Zaakir AllyNoch keine Bewertungen
- Etd - Unit - VDokument60 SeitenEtd - Unit - VSenthilkumar SivasankaranNoch keine Bewertungen
- States of Matter 2. Gas Laws and Ideal Gas EquationDokument29 SeitenStates of Matter 2. Gas Laws and Ideal Gas EquationHani FawziNoch keine Bewertungen
- GasesDokument2 SeitenGaseschrisroldan012696Noch keine Bewertungen
- Chem 111-2Dokument10 SeitenChem 111-2lets.torque.laterNoch keine Bewertungen
- Gases and Other Properties: Lesson 5Dokument7 SeitenGases and Other Properties: Lesson 5lucifer angelNoch keine Bewertungen
- 4th QTR - Module - Week 3Dokument3 Seiten4th QTR - Module - Week 3Avirel Reynante PodadorNoch keine Bewertungen
- Chapter 5 GasesDokument3 SeitenChapter 5 GasesKevin HuangNoch keine Bewertungen
- 10 Gases 2b PDFDokument10 Seiten10 Gases 2b PDFchewazableNoch keine Bewertungen
- Class-11 Chemistry Chapter-5 States of Matter Part-IIDokument7 SeitenClass-11 Chemistry Chapter-5 States of Matter Part-IINevin ShajiNoch keine Bewertungen
- Chemistry QuizDokument3 SeitenChemistry QuizCielo PulmaNoch keine Bewertungen
- States of MatterDokument15 SeitenStates of MatterShaku JoshiNoch keine Bewertungen
- Victorio Oriel - Ideal Gas Law and Molar Mass EquationDokument5 SeitenVictorio Oriel - Ideal Gas Law and Molar Mass Equationapi-233267698Noch keine Bewertungen
- The Gas Laws: Equations of StateDokument32 SeitenThe Gas Laws: Equations of Stateyiye rubyNoch keine Bewertungen
- Lecture 4 Gas Laws and RelationsDokument28 SeitenLecture 4 Gas Laws and RelationsArsal SohrabNoch keine Bewertungen
- Applied Thermodynamics IIDokument40 SeitenApplied Thermodynamics IIMeka SaimaNoch keine Bewertungen
- Gas Behaviour EOSDokument59 SeitenGas Behaviour EOSMurugavel ChandranNoch keine Bewertungen
- Properties of GasesDokument11 SeitenProperties of GasessalasineNoch keine Bewertungen
- Non-Reacting Gas Mixtures: Reading ProblemsDokument10 SeitenNon-Reacting Gas Mixtures: Reading ProblemspavanraneNoch keine Bewertungen
- Study of Gas LawDokument15 SeitenStudy of Gas LawKushagra jaiswalNoch keine Bewertungen
- Partial Pressure ConceptDokument6 SeitenPartial Pressure Conceptburgesss87Noch keine Bewertungen
- 111 1st - 2 PDFDokument3 Seiten111 1st - 2 PDFPhilip Darwin ArcenalNoch keine Bewertungen
- The Ideal - Gas Equation of StateDokument13 SeitenThe Ideal - Gas Equation of StateAudu SanusiNoch keine Bewertungen
- Week 7: Dalton'S Law of Partial PressuresDokument16 SeitenWeek 7: Dalton'S Law of Partial PressuresLeonilo Olanda JrNoch keine Bewertungen
- Internal Energy of An Ideal GasDokument3 SeitenInternal Energy of An Ideal GasSelvakumar JanakiramanNoch keine Bewertungen
- Review of Phase Equilibria - NEWDokument19 SeitenReview of Phase Equilibria - NEWkarmawii taqatqaNoch keine Bewertungen
- Chemistry Notes Ideal Gas LawsDokument19 SeitenChemistry Notes Ideal Gas LawsAbhishek MasneNoch keine Bewertungen
- Lec 21 22 - CH 13Dokument21 SeitenLec 21 22 - CH 13samhameed2Noch keine Bewertungen
- Properties of Mixtures Mixture of Perfect GasesDokument7 SeitenProperties of Mixtures Mixture of Perfect GasesAudu SanusiNoch keine Bewertungen
- Gas Laws: M. L. WatsonDokument25 SeitenGas Laws: M. L. WatsonAbhishek ChakrabartiNoch keine Bewertungen
- CH 10 Gases StudentDokument48 SeitenCH 10 Gases StudentTrọng NguyễnNoch keine Bewertungen
- CHM131 - Chapter 6 - The Gaseous StateDokument37 SeitenCHM131 - Chapter 6 - The Gaseous StateNotes NotesNoch keine Bewertungen
- Chapter3 IdealgaslawDokument45 SeitenChapter3 Idealgaslaw翁绍棠Noch keine Bewertungen
- 5.1 Pressure: Chapter 5: GasesDokument4 Seiten5.1 Pressure: Chapter 5: GasesSam ChungNoch keine Bewertungen
- Topic 1 - Gas Laws (Part 2)Dokument45 SeitenTopic 1 - Gas Laws (Part 2)Joshua LaBordeNoch keine Bewertungen
- Ideal Gas: General Chemistry 1Dokument9 SeitenIdeal Gas: General Chemistry 1Daniel Corcino100% (1)
- States of Matter Notes PDFDokument14 SeitenStates of Matter Notes PDFalien xNoch keine Bewertungen
- IPUE 208 Introduction To Process and Utilities Engineering: Gmol CM VDokument8 SeitenIPUE 208 Introduction To Process and Utilities Engineering: Gmol CM VRandy SooknananNoch keine Bewertungen
- Chemistry Gas Laws ExercisesDokument6 SeitenChemistry Gas Laws Exercisesjag1231Noch keine Bewertungen
- Mechanical Engineering ThermofluidsDokument175 SeitenMechanical Engineering Thermofluidsemad11518100% (1)
- Gas Laws: Temperature: Co-Ordinates: Boyle's LawDokument4 SeitenGas Laws: Temperature: Co-Ordinates: Boyle's LawVenu GopalNoch keine Bewertungen
- Ideal Gas Law With ExamplesDokument10 SeitenIdeal Gas Law With Examplesayan hazarikaNoch keine Bewertungen
- Amagat's Law: Ideal Gas MixtureDokument2 SeitenAmagat's Law: Ideal Gas MixtureAar AeyNoch keine Bewertungen
- Chapter 14Dokument42 SeitenChapter 14Aubrey LanotNoch keine Bewertungen
- Gaseous States of Matter (HINTS) 2Dokument2 SeitenGaseous States of Matter (HINTS) 2hchawla421Noch keine Bewertungen
- Reactors Dimensions - 1Dokument10 SeitenReactors Dimensions - 1Abhijit MoreNoch keine Bewertungen
- Design of Experiments: A 360 Development ApproachDokument24 SeitenDesign of Experiments: A 360 Development ApproachAbhijit More100% (1)
- Advance ReactionDokument6 SeitenAdvance ReactionAbhijit MoreNoch keine Bewertungen
- Encon in BoilerDokument51 SeitenEncon in BoilerAbhijit MoreNoch keine Bewertungen
- Edit Commands: Autocad 2D TutorialDokument15 SeitenEdit Commands: Autocad 2D Tutorialamini307Noch keine Bewertungen
- Natural Gas Combustion Reaction: CH + 2 O - CO + 2 H O + EnergyDokument3 SeitenNatural Gas Combustion Reaction: CH + 2 O - CO + 2 H O + EnergyAbhijit MoreNoch keine Bewertungen
- Organic ChemistryDokument76 SeitenOrganic Chemistryapi-3738901100% (2)
- Performance Evaluation of An Oil Fired Boiler A Case Study in Dairy Industry.Dokument8 SeitenPerformance Evaluation of An Oil Fired Boiler A Case Study in Dairy Industry.atul100% (8)
- Performance Evaluation of An Oil Fired Boiler A Case Study in Dairy Industry.Dokument8 SeitenPerformance Evaluation of An Oil Fired Boiler A Case Study in Dairy Industry.atul100% (8)
- Design of AgitatorDokument3 SeitenDesign of AgitatorManish PatelNoch keine Bewertungen
- PRV Sizing For Gas & VapourDokument22 SeitenPRV Sizing For Gas & VapourAbhijit MoreNoch keine Bewertungen
- Natural Gas Combustion Reaction: CH + 2 O - CO + 2 H O + EnergyDokument3 SeitenNatural Gas Combustion Reaction: CH + 2 O - CO + 2 H O + EnergyAbhijit MoreNoch keine Bewertungen
- Adsorption: P M. A NjitDokument69 SeitenAdsorption: P M. A NjitRevathy KanasinNoch keine Bewertungen
- 11 Glove SelecDokument19 Seiten11 Glove SelecAbhijit MoreNoch keine Bewertungen
- PumpsDokument105 SeitenPumpsAbhijit More100% (1)
- Evaporation Rate EADokument2 SeitenEvaporation Rate EAAbhijit MoreNoch keine Bewertungen
- Heat ExchangersDokument35 SeitenHeat ExchangersBaher Elsheikh90% (20)
- AutoCAD 2013 2D Tutorials by Kristen S. KurlandDokument255 SeitenAutoCAD 2013 2D Tutorials by Kristen S. KurlandnotevaleNoch keine Bewertungen
- FluidDokument3 SeitenFluidarieldelvecchioNoch keine Bewertungen
- Cooling Tower CalculationsDokument2 SeitenCooling Tower CalculationsAbhijit MoreNoch keine Bewertungen
- Revised Brochure BStat (2016)Dokument36 SeitenRevised Brochure BStat (2016)ManishKumarNoch keine Bewertungen
- Free Body DiagramDokument10 SeitenFree Body Diagramjames arajaNoch keine Bewertungen
- Mark Scheme: Q Scheme Marks Aos Pearson Progression Step and Progress Descriptor 1 B1Dokument10 SeitenMark Scheme: Q Scheme Marks Aos Pearson Progression Step and Progress Descriptor 1 B1Nasser ElmanzalawyNoch keine Bewertungen
- Tutorial Exercises: Central ForcesDokument7 SeitenTutorial Exercises: Central ForcesShweta SridharNoch keine Bewertungen
- MA1002Dokument1 SeiteMA1002Cherry CharanNoch keine Bewertungen
- Solution Manual For Applied Partial Differential Equations With Fourier Series and Boundary Value Problems, 5/E Richard HabermanDokument36 SeitenSolution Manual For Applied Partial Differential Equations With Fourier Series and Boundary Value Problems, 5/E Richard Habermanpoupetonlerneanoiv0ob100% (18)
- Separation of Variables PowerPointDokument50 SeitenSeparation of Variables PowerPointhazimraad50% (2)
- Steady Supersonic Flow Over Right Circular ConeDokument9 SeitenSteady Supersonic Flow Over Right Circular Conecal linNoch keine Bewertungen
- Separable Differential EquationsDokument10 SeitenSeparable Differential EquationsAthar NawazNoch keine Bewertungen
- Lesson 11 Algebraic SubstitutionDokument3 SeitenLesson 11 Algebraic SubstitutionJose Barrera GaleraNoch keine Bewertungen
- M2 Unit 1Dokument56 SeitenM2 Unit 1Hitwardhan DhadwalNoch keine Bewertungen
- Decline Curve Analysis John LeeDokument33 SeitenDecline Curve Analysis John LeeEngr Sadiq WazirNoch keine Bewertungen
- Morris Method With Improved Sampling Strategy and Sobol' Variance-Based Method, As Validation Tool On Numerical Model of Richard's EquationDokument18 SeitenMorris Method With Improved Sampling Strategy and Sobol' Variance-Based Method, As Validation Tool On Numerical Model of Richard's EquationjordorNoch keine Bewertungen
- Solving QE (Extracting The Square Roots)Dokument2 SeitenSolving QE (Extracting The Square Roots)TheKnow04Noch keine Bewertungen
- Linear Equation in One VariableDokument22 SeitenLinear Equation in One VariablethinkiitNoch keine Bewertungen
- Calculator Techniques Seminar: BY: Engr. Jose Lorenzo D. BuctonDokument55 SeitenCalculator Techniques Seminar: BY: Engr. Jose Lorenzo D. BuctonIra Cervo100% (1)
- Free Body Diagram and Newton's LawDokument4 SeitenFree Body Diagram and Newton's Lawseeknana2833Noch keine Bewertungen
- Equation of Circle in General Form: Prepared By: Edmar A. Teel Teacher IIIDokument21 SeitenEquation of Circle in General Form: Prepared By: Edmar A. Teel Teacher IIIRomeo DiestaNoch keine Bewertungen
- The Essentials of Finite Element Modeling and Adaptive RefinementDokument29 SeitenThe Essentials of Finite Element Modeling and Adaptive RefinementMomentum PressNoch keine Bewertungen
- Analysis of SDOF Systems: Numerical MethodsDokument22 SeitenAnalysis of SDOF Systems: Numerical MethodsFernanda LagoNoch keine Bewertungen
- A Complete Generalized Solution To The Inverse Kinematics of Robots-UgADokument7 SeitenA Complete Generalized Solution To The Inverse Kinematics of Robots-UgAArijit RoyNoch keine Bewertungen
- Aditya 1201423653Dokument5 SeitenAditya 1201423653Lakshay SharmaNoch keine Bewertungen
- How-Do-I fxCG50AUDokument116 SeitenHow-Do-I fxCG50AUIGCSE NotesNoch keine Bewertungen
- A Study of Heat and Mass Transfer On Magnetohydrodamic MHD Flow of NanoparticlesDokument6 SeitenA Study of Heat and Mass Transfer On Magnetohydrodamic MHD Flow of Nanoparticlesdewi widyastutiNoch keine Bewertungen
- 6.1 Chapter 2 Galaxy LP Problem UpdatedDokument76 Seiten6.1 Chapter 2 Galaxy LP Problem UpdatedDharmendra PrajapatiNoch keine Bewertungen
- GM Week2 Day3Dokument4 SeitenGM Week2 Day3Good vouNoch keine Bewertungen
- Csec Additional Mathematics May 2021 PDFDokument132 SeitenCsec Additional Mathematics May 2021 PDFJoshua SaundersNoch keine Bewertungen
- Creeping Flow in Two-Dimensional Network - 1982Dokument29 SeitenCreeping Flow in Two-Dimensional Network - 1982Alireza Attari MNoch keine Bewertungen
- Automata - Term - Paper - Arden's TheoremDokument11 SeitenAutomata - Term - Paper - Arden's TheoremArvjeet100% (1)
- Power System Generation, Transmission and Distribution Prof. D. P. Kothari Centre For Energy Studies Indian Institute of Technology, DelhiDokument19 SeitenPower System Generation, Transmission and Distribution Prof. D. P. Kothari Centre For Energy Studies Indian Institute of Technology, DelhiVISHAL TELANGNoch keine Bewertungen
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincVon EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincBewertung: 3.5 von 5 Sternen3.5/5 (137)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolVon EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNoch keine Bewertungen
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeVon EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeBewertung: 5 von 5 Sternen5/5 (4)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeVon EverandChemistry for Breakfast: The Amazing Science of Everyday LifeBewertung: 4.5 von 5 Sternen4.5/5 (90)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsVon EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNoch keine Bewertungen
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideVon EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNoch keine Bewertungen
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactVon EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactBewertung: 5 von 5 Sternen5/5 (5)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeVon EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeBewertung: 4 von 5 Sternen4/5 (1)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsVon EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsBewertung: 4 von 5 Sternen4/5 (146)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeVon EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeBewertung: 5 von 5 Sternen5/5 (1)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeVon EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNoch keine Bewertungen
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsVon EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsBewertung: 5 von 5 Sternen5/5 (3)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableVon EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableBewertung: 3.5 von 5 Sternen3.5/5 (22)
- Taste: Surprising Stories and Science About Why Food Tastes GoodVon EverandTaste: Surprising Stories and Science About Why Food Tastes GoodBewertung: 3 von 5 Sternen3/5 (20)
- The Periodic Table: A Very Short IntroductionVon EverandThe Periodic Table: A Very Short IntroductionBewertung: 4.5 von 5 Sternen4.5/5 (3)
- Tribology: Friction and Wear of Engineering MaterialsVon EverandTribology: Friction and Wear of Engineering MaterialsBewertung: 5 von 5 Sternen5/5 (1)
- Water-Based Paint Formulations, Vol. 3Von EverandWater-Based Paint Formulations, Vol. 3Bewertung: 4.5 von 5 Sternen4.5/5 (6)
- Ingredients: A Visual Exploration of 75 Additives & 25 Food ProductsVon EverandIngredients: A Visual Exploration of 75 Additives & 25 Food ProductsBewertung: 4 von 5 Sternen4/5 (1)
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookVon EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNoch keine Bewertungen
- Chemistry for Breakfast: The Amazing Science of Everyday LifeVon EverandChemistry for Breakfast: The Amazing Science of Everyday LifeBewertung: 4.5 von 5 Sternen4.5/5 (14)
- Bioplastics: A Home Inventors HandbookVon EverandBioplastics: A Home Inventors HandbookBewertung: 4 von 5 Sternen4/5 (2)
- High School Chemistry: Comprehensive Content for High School ChemistryVon EverandHigh School Chemistry: Comprehensive Content for High School ChemistryNoch keine Bewertungen
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactVon EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactBewertung: 5 von 5 Sternen5/5 (1)
- Chemistry: a QuickStudy Laminated Reference GuideVon EverandChemistry: a QuickStudy Laminated Reference GuideBewertung: 5 von 5 Sternen5/5 (1)