Beruflich Dokumente
Kultur Dokumente
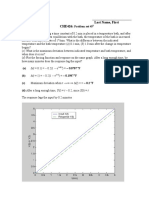
Chemical Engineering Design: Out Sat
Hochgeladen von
Mae BernadetteOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Chemical Engineering Design: Out Sat
Hochgeladen von
Mae BernadetteCopyright:
Verfügbare Formate
Chemical Engineering Design
Step 1:
Pout = 13.4 kPa, Tsat = 51.67C from steam tables.
Evaporator 3 with x = 0.7092
BPR3 = 1.78(0.7092) + 6.22(0.7092)2 = 4.39C
T3 = 51.67 + 4.39 = 56.06C
Step 2:
Overall and a solids balance to calculate total amount vaporized (V1 + V2 + V3) and L3,
F = 29,828.75 = L3 + (V1 + V2 + V3)
FxF = (29,828.75)(0.0332) = L3(0.7092) + (V1 + V2 + V3)(0)
L3 = 1,396.38 kg/h
Total vaporized = (V1 + V2 + V3) = 29,828.75 1,396.38 = 28,432.37 kg/h
Assuming equal amount vaporized in each effect, V1 = V2 = V3 = 9,477.46 kg/h
Making total material balance on effects 1, 2 and 3 and solving,
(1) F = 29, 828.75 = V1 + L1 = 9,477.46 + L1,
(2) L1 = 20,351.29 = V2 + L2 = 9,477.46 + L2,
(3) L2 = 10,873.83 = V3 + L3 = 9,477.46 + L3,
L1 = 20,351.29 kg/h
L2 = 10,873.83 kg/h
L3 = 1,396.37 kg/h
Making a solids balance on effects 1, 2 and 3 and solving,
(1) 29, 828.75(0.0332) = L1x1 = 20,351.29(x1),
(2) 20,351.29(0.0487) = L2x2 = 10,873.83(x2),
(3) 10,873.83(0.0911) = L3x3 = 1,396.37(x3),
Step 3: BPR in each effect
(1) 1.78(0.0487) + 6.22(0.0487)2 = 0.10 C
(2) 1.78(0.0911) + 6.22(0.0911)2 = 0.21 C
(3) 1.78(0.7092) + 6.22(0.7092)2 = 4.39 C
= 121.1 51.67 0.10 0.21 4.39 = 64.72 C
x1 = 0.0487
x2 = 0.0911
x3 = 0.7092
=12.16
2 = 19.12
3 = 33.44
Actual boiling point in each effect
(1) T1 = TS1 -
= 121.1 12.16 = 108.94
TS1 = 121.1 (condensing satd steam temperature to effect 1)
(2) T2 = T1 BPR1 -
2=
108.94 0.10 19.12 = 89.72
TS2 = T1 BPR1 = 108.94 0.10 = 108.84 (condensing satd steam temperature to effect 2)
(3) T3 = T2 BPR2 -
3=
89.72 0.21 33.44 = 56.07
TS3 = T2 BPR2 = 89.72 0.21 = 89.51 (condensing satd steam temperature to effect 3)
Step 4: Heat capacity, cp = 4.19 2.35x
F: cp = 4.19 2.35(0.0332) = 4.112
L1 : cp = 4.19 2.35(0.0487) = 4.076
L2 : cp = 4.19 2.35(0.0487) = 3.976
L3 : cp = 4.19 2.35(0.0487) = 2.523
Values of enthalpy H of various vapor streams (Datum temp: 0)
Effect 1:
T1 = 108.94, TS2 = 108.84, BPR1 = 0.10, TS1 = 121.1
H1 = HS2 (satn enthalpy at TS2) + 1.884(0.10) = 2689.7 + 0.1884 = 2689.9
S1
= HS1 (vapor satn enthalpy) hS1(liquid enthalpy at TS1) = 2707.8 508.39 = 2199.4
Effect 2:
T2 = 89.72, TS3 = 89.51, BPR2 = 0.21
H2 = HS3 + 1.884(0.21) = 2659.3 + 0.3956 = 2659.7
S2
= H1 hS2 = 2689.9 456.39 = 2233.51
Effect 3:
T3 = 56.07, TS4 =51.67, BPR3 = 4.39
H3 = HS4 + 1.884(4.39) = 2595.0 + 8.2708 = 2603.3
S3
= H2 hS3 = 2659.7 374.86 = 2284.8
Heat Balance on each effect:
(1) F cp (Tf 0) + S
S1 =
L1cp(T1 0) + V1H1
V1 = 29,828.75 L1
(29,828.75)(4.111)(28.0 0) + S
S1 =
L1(4.076)(108.94 0) + (29,828.75 L1)(H1)
3,433,527.76 + (2199.4)(S) = (444.04)(L1) + (80,236,354.63) (2689.9)(L1)
2199.4S + 2245.86L1 = 76,802,826.87
(2) L1cp(T1 0) + V1
S2 =
L2cp(T2 0) + V2H2
V2 = L1 L2
L1 (4.076)(108.94 0) + (29,828.75 L1)
S2 =
L2(3.976)(89.72 0) + (L1 L2)(H2)
(444.04)(L1) + 66,622,811.41 (2233.51)(L1) = (356.73)(L2) + (2659.7)(L1) (2659.7)(L2)
-4449.17L1 + 2302.97L2 = -66,622,811.41
(3) L2cp(T2 0) + V2
S3 =
L3cp(T3 0) + V3H3
V3 = L2 - 1,396.37
L3 = 1,396.37
L2 (3.976)(89.72 0) + ( L1 L2)
S3 =
L3(2.523)(56.07 0) + (L2 - 1,396.37)(H3)
(356.73)(L2) + (2284.8)(L1) (2284.8)(L2) = 197,536.94 + (2603.3)(L2) (3,635,170.02)
2284.8L1 4631.37L2 = -3,437,633.08
Solving last two equations simultaneously for L1 and L2 and substituting into the first equation,
L1 = 20,625.21
S = 13,859.00
L2 = 10,917.31
V1 = 9,203.54
L3= 1,396.37
V2 = 9,707.9
Step 5:
S1
= 2199.4
S2
= 2233.51
S3
= 2284.8
Solving for the values of area and q in each effect,
q1 = S
S1 =
8.467 x106 W
V3 = 9,520.94
q2 = V1
S2 =
5.71 x106 W
q3 = V2
S3 =
6.161x106 W
= 222.96 m2
= 150.30 m2
= 162.18 m2
(4) F cp (Tf 0) + S
S1 =
L1cp(T1 0) + V1H1
V1 = 29,828.75 L1
(29,828.75)(4.112)(28.0 0) + S
S1 =
L1(4.077)(105.91 0) + (29,828.75 L1)(H1)
3,433,362.96 + (2199.4)(S) = (431.80)(L1) + (80,096,189.33) (2685.20)(L1)
2199.4S + 2253.4L1 = 76,662,826.37
(5) L1cp(T1 0) + V1
S2 =
L2cp(T2 0) + V2H2
V2 = L1 L2
L1 (4.077)(105.91 0) + (29,828.75 L1)
S2 =
L2(3.977)(89.71 0) + (L1 L2)(H2)
(431.80)(L1) + 66,860,248.26 (2241.47)(L1) = (356.78)(L2) + (2659.6)(L1) (2659.6)(L2)
-4469.27L1 + 2302.82L2 = -66,860,248.26
(6) L2cp(T2 0) + V2
S3 =
L3cp(T3 0) + V3H3
V3 = L2 - 1,396.37
L3 = 1,396.37
L2 (3.977)(89.71 0) + ( L1 L2)
S3 =
L3(2.523)(51.11 0) + (L2 - 1,396.37)(H3)
(356.78)(L2) + (2284.5)(L1) (2284.5)(L2) = 180,062.65 + (2608.1)(L2) (3,641,872.60)
2284.5L1 4535.82L2 = -3,461,809.95
Solving last two equations simultaneously for L1 and L2 and substituting into the first equation,
L1 = 20,733.98
S = 13,613.20
L2 = 11,206.04
V1 = 9,094.77
L3= 1,396.37
V2 = 9,527.94
V3 = 9,809.67
S1
= 2199.4
S2
= 2241.47
S3
= 2284.5
Solving for the values of area and q in each effect,
q1 = S
S1 =
8.317 x106 W
q2 = V1
S2 =
5.663 x106 W
q3 = V2
S3 =
6.046x106 W
= 175.32 m2
= 177.01 m2
= 175.15 m2
175.83 m2
Steam economy =
Average ratio of heat transfer area and volume capacity 4.9 5.9
Choosing 5.9 arbitrarily:
29.8 m3
Volume capacity with 30% safety factor:
(
)(
Density = 1003.14 kg/m3
Volumetric flow rate
1st effect:
2nd effect:
m3
3rd
effect:
Outlet:
Residence time
1st effect:
2nd effect:
3rd effect:
Total residence time in evaporator = 1.30 + 1.87 + 3.47 = 6.65h
Mechanical Engineering Design
Tubular designs: 2.in (51mm) OD tube, 30ft (9m) long
Material: Stainless Steel 316
Das könnte Ihnen auch gefallen
- Sbi Afi 2012Dokument48 SeitenSbi Afi 2012Moneylife FoundationNoch keine Bewertungen
- Chapter 4 SolutionsDokument34 SeitenChapter 4 Solutionskdd1218w100% (1)
- Marketing Measurement Done RightDokument16 SeitenMarketing Measurement Done RightWasim Ullah0% (1)
- GENUS Clock Gating Timing CheckDokument17 SeitenGENUS Clock Gating Timing Checkwasimhassan100% (1)
- Heat Rate Calc in ExcelDokument13 SeitenHeat Rate Calc in ExcelMukesh VadaliaNoch keine Bewertungen
- Revised Nata de CocoDokument7 SeitenRevised Nata de CocoMae BernadetteNoch keine Bewertungen
- Caterpillar Sis (01.2014) MultilanguageDokument10 SeitenCaterpillar Sis (01.2014) MultilanguageTemmy Candra Wijaya100% (1)
- 2 Otto Diesel & Dual CyclesDokument39 Seiten2 Otto Diesel & Dual CyclesFausto ArambuloNoch keine Bewertungen
- The Impact of Social Networking Sites To The Academic Performance of The College Students of Lyceum of The Philippines - LagunaDokument15 SeitenThe Impact of Social Networking Sites To The Academic Performance of The College Students of Lyceum of The Philippines - LagunaAasvogel Felodese Carnivora64% (14)
- Cooling TowerDokument6 SeitenCooling Towerʞǝǝs Uǝ Ǝpıɥ100% (4)
- Rotary DryersDokument5 SeitenRotary DryersAgung Siswahyu0% (1)
- CS1 Entity Level Controls SolutionsDokument16 SeitenCS1 Entity Level Controls SolutionsPakistan Breaking News100% (6)
- Tutorium Refrigeration SolutionDokument20 SeitenTutorium Refrigeration SolutionwanpudinNoch keine Bewertungen
- Tugas PPM Deny Saputro Arifin 113170039Dokument9 SeitenTugas PPM Deny Saputro Arifin 113170039Vira IrnandaNoch keine Bewertungen
- Problem Solving (Adrian Recamadas) : FindDokument9 SeitenProblem Solving (Adrian Recamadas) : FindPJ PerezNoch keine Bewertungen
- Practice Quiz Reflection Project Initiation and Key ComponentsDokument3 SeitenPractice Quiz Reflection Project Initiation and Key ComponentsFalastin Tanani67% (3)
- Cooling TowerDokument6 SeitenCooling TowerLucas FurlanNoch keine Bewertungen
- Rotary Dryer CalculationDokument5 SeitenRotary Dryer Calculationsushant_jhawer100% (2)
- Evaporators 8.5-4 SolnDokument12 SeitenEvaporators 8.5-4 SolnLissa HannahNoch keine Bewertungen
- Exer 1 FinalDokument15 SeitenExer 1 FinalChelsea Martinez100% (1)
- Thermodinamika Dan Mesin KalorDokument12 SeitenThermodinamika Dan Mesin KalorMade AgusNoch keine Bewertungen
- (Using Tributary Areas) : Dead Load Computations Transverse Direction (W) (W)Dokument67 Seiten(Using Tributary Areas) : Dead Load Computations Transverse Direction (W) (W)Randy ViolaNoch keine Bewertungen
- Tube Side: G Jam LB Jam 1 2Dokument5 SeitenTube Side: G Jam LB Jam 1 2Nanda Fahrur RoziNoch keine Bewertungen
- Energy Balance FINAL SUBMITDokument34 SeitenEnergy Balance FINAL SUBMITRelaxing MusicNoch keine Bewertungen
- Lab 04Dokument8 SeitenLab 04Gautham SezhianNoch keine Bewertungen
- Tugas Pp2 Reny OktaviantiDokument9 SeitenTugas Pp2 Reny Oktaviantibimo_alkautsarNoch keine Bewertungen
- The Heat Engine CyclesDokument8 SeitenThe Heat Engine CyclesPaseka GodlyNoch keine Bewertungen
- Coulson Solution ManualDokument13 SeitenCoulson Solution Manualmachine20Noch keine Bewertungen
- AnswersDokument8 SeitenAnswersMika Pelagio100% (1)
- Homework 3Dokument12 SeitenHomework 3Yıldırım KURTULUŞNoch keine Bewertungen
- C + O CO: Enthalpy of FormationDokument29 SeitenC + O CO: Enthalpy of Formationvidya chakitwarNoch keine Bewertungen
- © Ncert Not To Be Republished: A I E, A N M MDokument17 Seiten© Ncert Not To Be Republished: A I E, A N M MrajatguptNoch keine Bewertungen
- Fig. 3.1-Different Furnace W/ Fuel Usage and Suggested Dimensions (Isometrics, Plan and Elevation)Dokument8 SeitenFig. 3.1-Different Furnace W/ Fuel Usage and Suggested Dimensions (Isometrics, Plan and Elevation)Che AguilarNoch keine Bewertungen
- Sugarcane Design 2520of 2520equipmentsDokument35 SeitenSugarcane Design 2520of 2520equipmentsSudiksha aroraNoch keine Bewertungen
- Perhitungan Rotary DryersDokument5 SeitenPerhitungan Rotary DryersAgung SiswahyuNoch keine Bewertungen
- علي قاسم صاحب علي Dokument5 Seitenعلي قاسم صاحب علي حمزة رعد حسنNoch keine Bewertungen
- Runge-Kutta 4 Order Method: Example: K DT DDokument10 SeitenRunge-Kutta 4 Order Method: Example: K DT DJhun Dhon Mari Abihay100% (1)
- MTL Gen Ode PPT Runge4thDokument14 SeitenMTL Gen Ode PPT Runge4thAfiq ImranNoch keine Bewertungen
- Chapter - 08 (RAC by Hipolito)Dokument6 SeitenChapter - 08 (RAC by Hipolito)John Jonel CasupananNoch keine Bewertungen
- Tugas ArisDokument8 SeitenTugas ArisAris SusantoNoch keine Bewertungen
- Cebu Institute of Technology: UniversityDokument10 SeitenCebu Institute of Technology: UniversityArchie HisolerNoch keine Bewertungen
- CHE654 2012 Homework5 SolutionsDokument37 SeitenCHE654 2012 Homework5 Solutionsmadithak100% (1)
- Answers: TEST - 3 (Paper-I)Dokument13 SeitenAnswers: TEST - 3 (Paper-I)pachuNoch keine Bewertungen
- Termo 3Dokument12 SeitenTermo 3Leti HanajNoch keine Bewertungen
- Técnicas de Mezclado Resistencia A La Tensión (Lb/Pulg)Dokument38 SeitenTécnicas de Mezclado Resistencia A La Tensión (Lb/Pulg)GARCÍA VERGARA KEYKO ELIANENoch keine Bewertungen
- Lab 1 ThermofluidDokument11 SeitenLab 1 Thermofluidizham hakimiNoch keine Bewertungen
- Head Loss of SyphonDokument6 SeitenHead Loss of SyphonAnkit SinglaNoch keine Bewertungen
- Chemistry Class Xi Exe. ProblemsDokument227 SeitenChemistry Class Xi Exe. ProblemsramchanderNoch keine Bewertungen
- Workbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsDokument14 SeitenWorkbook Workbook Workbook Workbook Workbook: Try Yourself QuestionsShubham mishraNoch keine Bewertungen
- Run 55 Celcius HE 1-1Dokument22 SeitenRun 55 Celcius HE 1-1Arini Dwi Astuti NainggolanNoch keine Bewertungen
- Modelling of CombustionDokument7 SeitenModelling of CombustionDynamix SolverNoch keine Bewertungen
- Example: Lead Compensator Design: SolutionDokument5 SeitenExample: Lead Compensator Design: SolutionJBBARNoch keine Bewertungen
- H-01 Design: Propylene-Propane Heat ExchangerDokument11 SeitenH-01 Design: Propylene-Propane Heat ExchangerappleabrahamNoch keine Bewertungen
- OriginalDokument6 SeitenOriginalyigaf49105Noch keine Bewertungen
- Chemical Engineering: SO 2+ O2 SO 3Dokument6 SeitenChemical Engineering: SO 2+ O2 SO 3أحمد العالمNoch keine Bewertungen
- Report HóaDokument14 SeitenReport Hóatranbaohan0412Noch keine Bewertungen
- Gate Previous Year QuestionsDokument53 SeitenGate Previous Year QuestionsPOOJA VERMANoch keine Bewertungen
- Perancangan Turap (Sheet Pile Wall) Dan Tiang Pancang (Pile Foundation)Dokument5 SeitenPerancangan Turap (Sheet Pile Wall) Dan Tiang Pancang (Pile Foundation)Fadel Weah MuhammadNoch keine Bewertungen
- Técnicas de Mezclado Resistencia A La Tensión (Lb/Pulg)Dokument51 SeitenTécnicas de Mezclado Resistencia A La Tensión (Lb/Pulg)GARCÍA VERGARA KEYKO ELIANENoch keine Bewertungen
- He SelectionDokument7 SeitenHe SelectionKaizerNoch keine Bewertungen
- Hematra SolutionDokument7 SeitenHematra SolutionDarlene FranciaNoch keine Bewertungen
- Assignment 1 - Suggested Answers 1.0 Numerical AnalysisDokument13 SeitenAssignment 1 - Suggested Answers 1.0 Numerical AnalysisPrince Takyi ArthurNoch keine Bewertungen
- Cebu Institute of Technology: UniversityDokument10 SeitenCebu Institute of Technology: UniversityArchie HisolerNoch keine Bewertungen
- Técnicas de Mezclado Resistencia A La Tensión (Lb/Pulg)Dokument33 SeitenTécnicas de Mezclado Resistencia A La Tensión (Lb/Pulg)GARCÍA VERGARA KEYKO ELIANENoch keine Bewertungen
- Appendix Exp 3Dokument14 SeitenAppendix Exp 3markNoch keine Bewertungen
- Bab Iv Hasil Percobaan Dan PembahasanDokument4 SeitenBab Iv Hasil Percobaan Dan PembahasanTriNoch keine Bewertungen
- Ten-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesVon EverandTen-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesNoch keine Bewertungen
- Vessel Assembly DrawingDokument1 SeiteVessel Assembly DrawingMae BernadetteNoch keine Bewertungen
- Evap NotesDokument1 SeiteEvap NotesMae BernadetteNoch keine Bewertungen
- Vessel Assembly DrawingDokument1 SeiteVessel Assembly DrawingMae BernadetteNoch keine Bewertungen
- Texas City DisasterDokument6 SeitenTexas City DisasterMae BernadetteNoch keine Bewertungen
- Exp7 PDFDokument16 SeitenExp7 PDFAnurag BajpaiNoch keine Bewertungen
- Obligations: General Provisions, Nature and EffectDokument2 SeitenObligations: General Provisions, Nature and EffectMae BernadetteNoch keine Bewertungen
- Triz Workshop: Productivity vs. Waste EnergyDokument5 SeitenTriz Workshop: Productivity vs. Waste EnergyMae BernadetteNoch keine Bewertungen
- CA Recovery and PurificationDokument2 SeitenCA Recovery and PurificationMae BernadetteNoch keine Bewertungen
- CA ConcentrationDokument2 SeitenCA ConcentrationMae BernadetteNoch keine Bewertungen
- Table 1. Mass of Caco Caco Concentration Trial # Mass of CacoDokument26 SeitenTable 1. Mass of Caco Caco Concentration Trial # Mass of CacoMae BernadetteNoch keine Bewertungen
- Melting Point of SubstancesDokument2 SeitenMelting Point of SubstancesMae BernadetteNoch keine Bewertungen
- Chem1lab ChromiumDokument5 SeitenChem1lab ChromiumMae BernadetteNoch keine Bewertungen
- 313L Coconut Oil ExtractionDokument5 Seiten313L Coconut Oil ExtractionMae BernadetteNoch keine Bewertungen
- Proposed 4way D54 Proposed 2way D56: Issue Date DescriptionDokument3 SeitenProposed 4way D54 Proposed 2way D56: Issue Date DescriptionADIL BASHIRNoch keine Bewertungen
- Mech VibrationDokument14 SeitenMech VibrationSquakx BescilNoch keine Bewertungen
- RCD ManagementDokument6 SeitenRCD ManagementPindoterONoch keine Bewertungen
- Norma Geral Astm Bronze Alumínio - b150b150m.19198Dokument7 SeitenNorma Geral Astm Bronze Alumínio - b150b150m.19198EduardoNoch keine Bewertungen
- 234567890Dokument37 Seiten234567890erppibuNoch keine Bewertungen
- Module 1Dokument64 SeitenModule 1Jackyson RajkumarNoch keine Bewertungen
- Spike Magazine Cup PackDokument5 SeitenSpike Magazine Cup PackBungle MarleyNoch keine Bewertungen
- JAMB Syllabus For BiologyDokument27 SeitenJAMB Syllabus For BiologyOluebube UchennaNoch keine Bewertungen
- Paper Format IJRDTDokument3 SeitenPaper Format IJRDTrock starNoch keine Bewertungen
- Unseen Passage 2Dokument6 SeitenUnseen Passage 2Vinay OjhaNoch keine Bewertungen
- SmoothWall Express 2.0 Quick-Start GuideDokument6 SeitenSmoothWall Express 2.0 Quick-Start Guideinfobits100% (1)
- Rich Gas and Lean GasDokument7 SeitenRich Gas and Lean GasManish GautamNoch keine Bewertungen
- PROP CASES OUTLINE 7 - Right of Way - Light & ViewDokument108 SeitenPROP CASES OUTLINE 7 - Right of Way - Light & ViewKringle Lim - DansalNoch keine Bewertungen
- Game On Series BibleDokument28 SeitenGame On Series Bibleapi-513832615Noch keine Bewertungen
- Classical Encryption TechniqueDokument18 SeitenClassical Encryption TechniquetalebmuhsinNoch keine Bewertungen
- Buy Wholesale China Popular Outdoor Football Boot For Teenagers Casual High Quality Soccer Shoes FG Ag Graffiti Style & FootballDokument1 SeiteBuy Wholesale China Popular Outdoor Football Boot For Teenagers Casual High Quality Soccer Shoes FG Ag Graffiti Style & Footballjcdc9chh8dNoch keine Bewertungen
- Expansions Meet Health Care Needs: Economists Question Trump Plan FiguresDokument10 SeitenExpansions Meet Health Care Needs: Economists Question Trump Plan FiguresThe Daily Tar HeelNoch keine Bewertungen
- APS PresentationDokument32 SeitenAPS PresentationRozack Ya ZhackNoch keine Bewertungen
- Test Cases: Project Name: Virtual ClassroomDokument5 SeitenTest Cases: Project Name: Virtual ClassroomTina HernandezNoch keine Bewertungen
- Modal Verbs of Ability and PossibilityDokument12 SeitenModal Verbs of Ability and PossibilitymslolinrNoch keine Bewertungen
- Nokia 3g Full Ip CommissioningDokument30 SeitenNokia 3g Full Ip CommissioningMehul JoshiNoch keine Bewertungen
- 064 DIR - Launching Whipping Creme & Skimmed Milk Di Channel Horeka (Subdist Masuya)Dokument3 Seiten064 DIR - Launching Whipping Creme & Skimmed Milk Di Channel Horeka (Subdist Masuya)indra sapta PrahardikaNoch keine Bewertungen
- Grade 8 Mathematics Checkpoint Booklet AY 23-24Dokument270 SeitenGrade 8 Mathematics Checkpoint Booklet AY 23-24Arta riaNoch keine Bewertungen