Beruflich Dokumente
Kultur Dokumente
Capsule Filling Guide Answers Common Questions
Hochgeladen von
luismanolo09Originalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Capsule Filling Guide Answers Common Questions
Hochgeladen von
luismanolo09Copyright:
Verfügbare Formate
C
o
p
y
r
i
g
h
t
,
C
S
C
P
u
b
l
i
s
h
i
n
g
.
T
a
b
l
e
t
s
&
C
a
p
s
u
l
e
s
1
capsule filling
Answers to 10 common questions
about capsule filling
Donald K. Lightfoot
Todays capsulefilling machines produceas many as 200,000
capsulesper hour thankstobetter equipment, better controls, and
a better understanding of theprocess. But if yourejust getting
started in capsulefilling, you probably havesomebasic ques-
tions about theoperation. This articleprovides answers to some
common questions about capsulefilling.
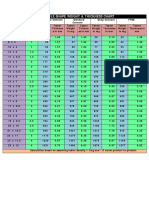
You need to determine the
density of the formulation to
answer this question. For pow-
der formulations, use the
tapped density value. For pel-
lets or granules, use the bulk
density. Once you have this
information and you know the
target fill weight, ask a capsule
supplier for a capacity chart,
such as the one shown in Table
1. Using this chart as an exam-
ple, encapsulating 500 mil-
ligrams of a powder with a
tapped density of 0.8 gram per
milliliter would require a size 0
capsule.
How do I determine the
appropriate capsule size for my
formulation?
Table 1
Capsule volumes and filling capacities
Size 000 00el 00 0el 0 1el 1 2el 2 3 4 5
Capsule 1.37 1.02 0.91 0.78 0.68 0.54 0.50 0.41 0.37 0.30 0.21 0.13
volume (ml)
Powder Capsule capacity (mg)
tapped
density
0.6 g/ml 822 612 546 468 408 324 300 246 222 180 126 78
0.8 g/ml 1096 816 728 624 544 432 400 308 296 240 168 104
1 g/ml 1370 1020 910 780 680 540 500 410 370 300 210 130
1.2 g/ml 1644 1224 1092 936 816 648 600 492 444 360 252 156
Figure 1
Example of a weight control chart
Product: Strength: mg Date:
Batch number: Operator/Checker / Page No. 1
Filling Limits(Avg.): UCL 260 mg LCL 248 mg Range: 20
Sample Time/Weights (mg)
11:17AM 11:18AM 11:30AM 11:45AM 12:00PM 12:15PM 12:30PM 12:45PM 1:00PM
1 254 262 259 251 254 257 252 255 258
2 261 263 257 246 258 255 252 255 252
3 254 257 252 260 256 256 250 249 250
4 258 259 248 253 251 255 251 254 251
5 256 254 255 251 246 255 260 257 255
6 256 261 258 255 254 254 253 259 251
7 257 254 253 250 250 253 254 252 240
8 256 259 249 251 248 251 248 250 261
9 258 258 256 258 255 253 255 248 259
10 253 259 258 252 248 252 253 252 260
Avg. 256 259 255 253 252 254 253 253 254
Range 8 9 11 14 12 6 12 11 21
C
o
p
y
r
i
g
h
t
,
C
S
C
P
u
b
l
i
s
h
i
n
g
.
T
a
b
l
e
t
s
&
C
a
p
s
u
l
e
s
weight variation has changed due to an assignable cause.
Such changes indicate that the capsule filling machine is
no longer in a state of statistical control.
2
You can accomplish this with statistical process con-
trol (SPC) techniques. SPC establishes the process capa-
bility for a specific formulation being encapsulated on a
designated filling machine. Most statistical textbooks and
publications on lean sigmaprovide the procedures and
formulas for establishing process control limits. Ive also
found a website that is particularly helpful [1], and a vari-
ety of SPC software is available.
Ive had excellent results monitoring capsule filling runs
by charting the average weights and range of weights of
the samples. Figure 1 provides an example of a control
chart (UCL stands for upper control limit. LCL stands for
lower control limit). Making a chart like this requires
using SPC procedures to determine the upper and lower
control weight limits and the upper and lower control lim-
its for the average weight and weight range. Then follow
these steps:
Take a sample of 10 capsules at regular intervals (every
15 to 30 minutes), calculate the average and range of
their weights, and plot the results on the control
chart. These plots provide a simple graphical tech-
nique for determining if the average or range val ues
are outside the control limits. See Figure 2.
If the data points are within the acceptable limits,
dont adjust the weight settings unless there is a
trend where the previous six average weight checks
were consistently above or below the centerline.
If either the average or range of weights falls outside
the control limits, stop the filling run and investigate
to determine the cause(s).
Isolate all the production collected from the previous
satisfactory weight check and evaluate it to deter-
mine the disposition for
this segment of produc-
tion. In most cases, this
segment of production is
either discarded or weight-
sorted.
Resume processing only
when you have ascertained
the causes of the change
and taken the required
corrective action to bring
the average and range
back within control limits.
We would usually perform
two weight checks a
minute apart to verify this.
In summary, the upper and
lower control limits for both
the average weight and range
of weights are used to identify
conditions where the process
How do I establish proper filling limits to effectively
monitor and control a capsule filling run?
Figure 2
Weight control chart plot
Average capsule weights
Range
Time
A
v
g
.
(
m
g
)
R
a
n
g
e
(
m
g
)
Time
262
260
258
256
254
252
250
248
246
242
25
20
15
10
5
0
244
11:17 AM 11:18 AM 11:30 AM 12:00 PM 11:45 PM 12:15 PM 12:30 PM 12:45 PM 1:00 PM
11:17 AM 11:18 AM 11:30 AM 12:00 PM 11:45 PM 12:15 PM 12:30 PM 12:45 PM 1:00 PM
Upper control limit
Limit
Range
Target weight
Avg.
Lower control limit
3
How do I ensure capsule filling quality?
The traditional approach is to perform a quality check
on a small sample (usually 10 capsules) every 30 or 60
minutes when you take a weight-check sample. While
this is certainly a good procedure and one that my capsule
filling department followed for many years, we still visu-
ally inspected the batches to remove defects that were not
always found in the routine quality checks.
Every time we analyzed a quality problem, we discov-
ered a high correlation between the setup of the capsule
filling machine and the incidence rate of defects. Based on
this, we began to check a large sample for acceptable
quality level (AQL) after every machine setup and after
every major repair. After adjusting the machine to hit the
target fill weight, we would perform a sustained run for 5
to 10 minutes, stop, and then carefully inspect every cap-
sule for defects. Using such a large sample highlighted
specific defects that, in most cases, we could attribute to
machine setup.
For example, a high incidence rate of telescopedor
split capsules indicates either a misalignment of the upper
and lower capsule segments (or bushings) or an incorrect
setting of the cap hold-down pin (or plate) in the joining
station. Dents in the capsule body indicate either an
incorrect setting of the body joining pins or an incorrect
pin size or pin configuration. Most manufacturers of cap-
sule filling machines supply a troubleshooting guide to
assist with this kind of analysis.
Based on the information obtained from these proce-
dures, we developed a detailed checklist for machine
C
o
p
y
r
i
g
h
t
,
C
S
C
P
u
b
l
i
s
h
i
n
g
.
T
a
b
l
e
t
s
&
C
a
p
s
u
l
e
s
4
7
Where and how should I store my capsules?
Empty capsules. Empty two-piece gelatin capsules are
shipped with a moisture content between 13 to 16 per-
cent. It is important that this moisture content is main-
tained. Avoid exposing the capsules to high temperatures
or to cycles of high and low temperatures. There is also a
tremendous volume of air inside the capsule that can
extract or release moisture from the capsule. Maintain the
area where you store the capsules at 15 to 25C and 35
to 55 percent relative humidity (RH). Do not store empty
What are some of the key factors to make a formula-
tion run effectively on high-speed capsule filling
machines?
The majority of high-speed filling machines dose cap-
sules using either a dosator and piston system or a tamp-
ing and dosing disc method. Each has specific formula-
tion requirements.
Dosator and piston machines require a formulation
that is well lubricated to ensure clean ejection by the pis-
ton. It also must compact well so that the plug does not
break up when the dosator is withdrawn from the powder
bed.
Tamping and dosing disc machines need formulations
with adequate lubrication for efficient plug ejection to
prevent filming and to reduce friction of the sliding com-
ponents. While the formulation must have compactabil-
ity to make coherent plugs that eject cleanly, this is less
critical for tamping and dosing disc machines than it is
for dosator and piston systems. Tamping and dosing
machines also require formulations with good flow char-
acteristics to ensure a uniform powder bed level in the
large-diameter dosing bowl.
5
6
setup, which required operators to measure components
and settings precisely at each critical step. The operator
or mechanic then had to sign off that everything on the
checklist was indeed checked. These procedures allowed
us to build quality into the process and to reduce by 85
percent the time we spent visually inspecting for defects.
That represented a significant labor savings.
What type of formulations may not be suitable for
two-piece gelatin capsules?
The best method for determining suitability is to con-
duct compatibility studies of the API and the excipients.
There are two major API characteristics that can be prob-
lematic: moisture sensitivity and hygroscopicity.
Moisture sensitivity. Two-piece gelatin capsule shells
have a moisture content between 13 and 16 percent, and
the moisture can interact with the encapsulated product
and cause stability problems.
Hygroscopicity. Once encapsulated, hygroscopic
products will remove moisture from the gelatin capsule
shell, which leads to brittleness once moisture content
drops below 10 percent.
There are two capsule alternatives to address the prob-
lems associated with these types of formulations:
Hydroxypropyl methylcellulose (HPMC) capsules and
gelatin-polyethylene glycol (PEG) capsules.
HPMC capsules have a low moisture content (4 to 6
percent), an attractive feature when dealing with mois-
ture-sensitive and hygroscopic formulations. HPMC cap-
sules have an excellent stability profile and resist physical
changes at low humidities.
There are also gelatin capsules that have been spe-
cially developed with PEG 400 to reduce brittleness
when exposed to low-moisture fills. These capsules are
more compatible with hygroscopic formulations or mois-
ture-sensitive ingredients than standard gelatin capsules.
What are some of the difficulties of filling capsules
with pellets?
The capsule filling and dosing mechanisms for pellets
and granules are based on volumetric filling, by using
either a dosing chamber or a vacuumdosator or by directly
filling into the capsule body. Inconsistency can result from
Large differences in the pellet/granule particle size
Agglomeration of the pellets/granules
Poor flow characteristics
Insufficient level of pellets in the supply hopper that
fills the dosing chamber
Electrostatic charge that retards the transfer from the
dosing chamber to the capsule body
In many cases, the pellets or granules have a functional
coating that controls release. Its important to verify that
the dosing system and the material handling system
(product feeder) are not abrading the coating, which
could affect the release profile.
Products that comprise a blend of different pellets
raise the issue of content uniformity, particularly when
the blend includes high- and low-dose pellet groups. The
pellets may segregate (de-blend) during feeding or encap-
sulation. Since many of the modern filling machines can
be equipped with more than one pellet feeding system,
you can resolve this problem by feeding the pellet prod-
ucts separately.
One challenging formulation from my experience
comprised four pellet groups: 1) Product A immediate
release, 75 milligrams; 2) Product A controlled release, 75
milligrams; 3) Product B immediate release, 12.5 mil-
ligrams; and 4) Product B controlled release, 12.5 mil-
ligrams. This formulation was filled using an MG Futura
machine equipped with two pellet dosing systems, each
of which had two dosing chambers. By dosing each pellet
group separately, we resolved the problems of blend uni-
formity and release profiles.
C
o
p
y
r
i
g
h
t
,
C
S
C
P
u
b
l
i
s
h
i
n
g
.
T
a
b
l
e
t
s
&
C
a
p
s
u
l
e
s
capsules in freezers. The empty capsules are also very sus-
ceptible to damage because the capsule walls are unsup-
ported.
Filled capsules. The storage requirements for filled
capsules are based on the product stability profile. The
warehouse storage areas should be temperature-mapped
and monitored to ensure the room temperature is under
control and that product-specific refrigeration/RH
requirements are maintained. Ideally, the storage area
would be equipped with alarms and there would be prod-
uct-specific product handling procedures that explain
how to deal with out-of-limit temperature/humidity inci-
dents. If the product is stored in bulk containers for a sig-
nificant period of time prior to packaging, you should
institute procedures to monitor bulk product stability.
General storage recommendations. Protect the cap-
sules from direct sunlight by storing them away from
windows and skylights. They should also be stored away
from radiators, heat registers, hot water pipes, and steam
pipes. Keep the capsules out from under potential sources
of water condensation, such as water pipes, and keep pal-
let loads off the floor.
8
9
10
What are the tamper-evident requirements for capsules?
The requirements for OTC capsule products are speci-
fied in 21 CFR Part 211. Section 211.132, paragraph (b)
(2) states that In addition to the tamper-evident packag-
ing feature described in paragraph (b) (1) of this section,
any two-piece, hard gelatin capsule covered by this sec-
tion must be sealed using an acceptable tamper-evident
technology.
Paragraph (c) (i) states that container labels must
include a statement that identifies all tamper-evident fea-
Table 2
Capsule banders
Supplier Model Capsules/hour Other features
IMA North America Hermetica 50,000 Automatic single-
Bristol, PA (two models) 100,000 band sealer
Built-in viscometer
Sealing and drying
plates have double row
of capsules to
www.ima.it reduce change parts
Qualicaps Lab scale 3,500 Automatic double-
Whitsett, NC band sealer
S-40 40,000 Automatic double-
band sealer
S-100 100,000 Automatic double-
www.qualicaps.com band sealer
Schaefer STI Lab Depends on Semi-automatic
Technologies top bander number of double-band sealer
Indianapolis, IN banding slats
STI CB-15 15,000 Automatic double-
band sealer
Bonapace 3,000 Automatic single-
BD-3000 band sealer
www.schaefer-technologies.com
Table 3
Excipients and packaging materials containing low levels
of aldehydes
Excipient Formaldehyde Packaging Formaldehyde
content (ppm) material content (ppm)
Starch 2.4 Rayon 2.0
Lactose 0.1 Plastic caps 2.4
Croscarmellose 0.3 Inserts 0.8
HPMC 1.1
Tween 0.3
Sodium 0.3
lauryl sulfate
ture(s) and any capsule sealing technologies used to com-
ply with paragraph (b) of this section.
Capsule banders or sealers that apply a gelatin band
around the seam area of the capsule (cap-body interface)
are considered an acceptable tamper-evident technology.
Table 2 lists the available capsule banders.
One company offers a capsule sealing system alterna-
tive to banding [2]. The FDA is currently evaluating
whether this system provides acceptable tamper-evi-
dence. See the article on page 12 for more information.
What are some things that can go wrong that people
dont anticipate?
I spent more than 30 years of my career involved with
capsule filling, so I could probably fill a novel with my
response to this question. Instead, Ill describe just two
challenging issues I encountered.
The first involved pinholes and cracks that resulted in
gradual leakage of the product from the capsule.
Recovering the batch by weight-sorting was not totally
effective, because some of the acceptable capsules would
continue to leak and would eventually become low-fills in
the package, which could trigger a product recall. Instead,
we first subjected the capsules to excessive vibration in a
vibratory sieve. That accelerated the leakage, creating low-
fills, and then we could successfully weight-sort the batch.
In most cases, pinholes and cracks occur when the
dome of the capsule body fractures due to excessive vac-
uum during separation. The problem can also stem from
incorrect setup of the joining pin. Preventing these situa-
tions is easy: Follow the setup procedures described in the
response to question 3. Installing a check valve on the vac-
uum separator line to limit the maximum suction is also
helpful.
The second incident involved the cross-linking of filled
capsules. The problemcame to our attention during a site
transfer of some products, when an accelerated stability
evaluation indicated a significant decrease in dissolution
profiles. An investigation revealed that this was the result of
the gelatin cross-linking under stress conditions. In addi-
tion, it was discovered that some of the formulations excipi-
C
o
p
y
r
i
g
h
t
,
C
S
C
P
u
b
l
i
s
h
i
n
g
.
T
a
b
l
e
t
s
&
C
a
p
s
u
l
e
s
ents contained trace amounts of aldehyde, which caused
chemical interactions with the gelatin.
Fromsearching the literature we learned that low levels
of aldehydes had been detected both in excipients and in
packaging materials and that they could cause gelatin cross-
linking. See Table 3. Consequently, we added aldehyde
testing to our acceptance specifications for all excipent and
packaging materials used with capsule products.
It should be noted that recent studies of the cross-link-
ing phenomena indicate that the bio-availability of the
drug is not significantly altered [3, 4]. The first of these
referenced studies suggests conducting two-stage in vitro
dissolution tests on dosage forms that contain hard
gelatin. In the first test, use a dissolution medium without
enzymes and in the second, use a dissolution media that
contains enzymes (gastric and intestinal media).
What is the best way to recover product fromcapsules?
Any method of recovering fill materials from capsules
must
Minimize gelatin fines;
Avoid grinding or particle size reduction of the fill
material;
Minimize attrition of the pellet/granule coating system;
Allow for validation and stability evaluation of the
recovered product; and
Be included in the filing or update if the product is
an NDA.
The traditional methods of milling and sieving do not
adequately address the above issues. You may want to
evaluate the recently introduced capsule separator/recov-
ery units listed in Table 4. These machines pull the cap-
sules apart mechanically without damaging the contents.
T&C
References
1. See www.murtongroup.com. At the bottom of the
page, click on Free-SPC Companion to download a
helpful 10-page document.
2. Liquid encapsulation microspray sealing (LEMS),
Capsugel, Greenwood, SC.
3. Digenis, G.A., Gold, T.B., and Shah, V.P. Cross-
linking of gelatin capsules and its relevance to their in
vitro-in vivo performance. J Pharm Sci 83 (7) 915-921
(1994).
4. Meyer, M.C., Straughn, A.B., Mhatre, R.M.,
Hussain, A., Shah, V.P., Bottom, C.C., Cole, E.T., Lesko,
L.L., Mallinowski, H., and Williams, R.L. The effect of
gelatin cross-linking on the bioequivalence of hard and
soft gelatin acetaminophen capsules. Pharm Res 17 (8)
962-966 (2000).
Donald K. Lightfoot is an independent consultant, 2826 W.
Placita Paciente, Tucson, AZ 85742. Tel. 520 229 3506. E-
mail: donald.lightfoot@comcast.net. Herecently retired from
GlaxoSmithKlineafter 46 years of service. As a director of
manufacturingandR&D, hegainedextensiveproduction expe-
riencein capsulefilling and other processes, including granula-
tion, tablet compression, tablet coating, pellet coating, andsup-
pository manufacturing. His expertisespans pharmaceutical
manufacturing of both clinical and commercial supplies.
Table 4
Capsule separators/recovery machinery
Manufacturer/US supplier Maximum output
Sejong Pharmatech 40,000 capsules/hr
(CMS Technologies)
Cranbury, NJ
E-mail: chiminsunwoo@hotmail.com
Vanguard Pharmaceutical 36,000 capsules/hr
Machinery
Spring, TX
www.pharmaceutical-equipment.com
Das könnte Ihnen auch gefallen
- Capsule & Tablet Size ChartDokument1 SeiteCapsule & Tablet Size ChartMahesh Patil KahatulNoch keine Bewertungen
- Corn Starch and Pregelatinized StarchDokument8 SeitenCorn Starch and Pregelatinized StarchSagita WidiyastutiNoch keine Bewertungen
- Tablet Technology EditedDokument42 SeitenTablet Technology EditedPramod Kc100% (1)
- Bruno Hancock Arden House Final HandoutsDokument18 SeitenBruno Hancock Arden House Final Handoutsdepardieu1973Noch keine Bewertungen
- 0.2011 IMA Kilian R+D Concept PDFDokument37 Seiten0.2011 IMA Kilian R+D Concept PDFPaqui Miranda Gualda100% (1)
- GS Coating Equipment enDokument8 SeitenGS Coating Equipment enAkber LakhaniNoch keine Bewertungen
- Capsugel DBcaps Sizing Information 1Dokument1 SeiteCapsugel DBcaps Sizing Information 1luismanolo09Noch keine Bewertungen
- Optimum Tablet Press Optimization - Machine Versus GranulationDokument10 SeitenOptimum Tablet Press Optimization - Machine Versus GranulationbookulNoch keine Bewertungen
- Dissolution - DisintegrationDokument10 SeitenDissolution - DisintegrationKathryn Faith Malabag100% (1)
- CapsulesDokument12 SeitenCapsulesDr-Jagadeesh MangamooriNoch keine Bewertungen
- Flow Through Cell Dissolution ApparatusDokument22 SeitenFlow Through Cell Dissolution ApparatusKimberly MccoyNoch keine Bewertungen
- Capsugel ConiSnap Sizing Information 2Dokument2 SeitenCapsugel ConiSnap Sizing Information 2CRD NEOMEDICNoch keine Bewertungen
- 01 Disintegration Test For Tablets and CapsulesDokument30 Seiten01 Disintegration Test For Tablets and CapsulesmefroNoch keine Bewertungen
- 3 Performa P Basic Machines: 4.1.2 Optional Features and Equipment (See Section 5.2)Dokument23 Seiten3 Performa P Basic Machines: 4.1.2 Optional Features and Equipment (See Section 5.2)Sangram KendreNoch keine Bewertungen
- Techniques of Tablet Coating Concepts and Advancements A Comprehensive Review 1 6Dokument6 SeitenTechniques of Tablet Coating Concepts and Advancements A Comprehensive Review 1 6Stefany Luke0% (1)
- Catalogue of Leak Test ApparatusDokument4 SeitenCatalogue of Leak Test ApparatustixocNoch keine Bewertungen
- CPR-18 - Ed 2010 IIDokument4 SeitenCPR-18 - Ed 2010 IIMin Min ZawNoch keine Bewertungen
- Tablet: Types of TabletsDokument82 SeitenTablet: Types of TabletsKema Akma100% (1)
- Formulation of Topical Products With Antiviral and AntibacterialDokument114 SeitenFormulation of Topical Products With Antiviral and AntibacterialMuhammad Masoom AkhtarNoch keine Bewertungen
- Prospective Validation of Paracetamol Tablet Dosage FormDokument10 SeitenProspective Validation of Paracetamol Tablet Dosage Formedgar palominoNoch keine Bewertungen
- Process Validation: Solid Dosage Forms Part IIDokument84 SeitenProcess Validation: Solid Dosage Forms Part IILien Hong0% (1)
- CTD Checklist DummyDokument1 SeiteCTD Checklist Dummydaizhussain004Noch keine Bewertungen
- Patented Technology in Soft Gelatin Capsule A ReviewDokument16 SeitenPatented Technology in Soft Gelatin Capsule A ReviewFatimahAhmat100% (1)
- Kasus-Film Coated TabletsDokument25 SeitenKasus-Film Coated TabletsCitra Ariani EdityaningrumNoch keine Bewertungen
- Tablet Compression Machine: Prepared By: - Akhilesh Rai (Engineering Officer) Department of Engineering Medreich LimitedDokument36 SeitenTablet Compression Machine: Prepared By: - Akhilesh Rai (Engineering Officer) Department of Engineering Medreich LimitedAkhilesh Rai100% (2)
- Standard Operating Procedure Department: ProductionDokument2 SeitenStandard Operating Procedure Department: Productionasit_mNoch keine Bewertungen
- Uncoated TabletsDokument1 SeiteUncoated Tabletsfopcu91Noch keine Bewertungen
- Pilot Scaleup Techniques For Solid Dosage Form - An Overview For TabletsDokument7 SeitenPilot Scaleup Techniques For Solid Dosage Form - An Overview For TabletsAsifNoch keine Bewertungen
- Process Validation For Atorvastatin Tablet - ArticleDokument13 SeitenProcess Validation For Atorvastatin Tablet - ArticleAnalyst NerdNoch keine Bewertungen
- Guide to Hard Gelatin Capsule ProductionDokument33 SeitenGuide to Hard Gelatin Capsule Productionalay_brahmbhattNoch keine Bewertungen
- Question and Answer For In-Process Parameters For Tablets and CapsulesDokument7 SeitenQuestion and Answer For In-Process Parameters For Tablets and CapsulesMubarak PatelNoch keine Bewertungen
- 2.PV Semisolid Fda PDFDokument37 Seiten2.PV Semisolid Fda PDFYuli SukmawatiNoch keine Bewertungen
- Brexpiprazole - Chem RevDokument112 SeitenBrexpiprazole - Chem RevSam SonNoch keine Bewertungen
- Cleaning Validation ProcessDokument11 SeitenCleaning Validation Processsamia khanNoch keine Bewertungen
- Dissolution MethodsDokument59 SeitenDissolution MethodsShiraz KhanNoch keine Bewertungen
- SOP For HPLC Shimadzu LabSolutionsDokument6 SeitenSOP For HPLC Shimadzu LabSolutionsfawaz khalilNoch keine Bewertungen
- Abstract Book JCDMCOP FinalDokument173 SeitenAbstract Book JCDMCOP FinalKapil SoniNoch keine Bewertungen
- STP Montelukast Sa.Dokument6 SeitenSTP Montelukast Sa.ShagorShagor100% (1)
- Vostem REPORT RegistDokument22 SeitenVostem REPORT RegistFajarRachmadiNoch keine Bewertungen
- Posterior Probability of Passing Compendial TestDokument47 SeitenPosterior Probability of Passing Compendial Testvg_vvgNoch keine Bewertungen
- WHO Certificate E120 2019Dokument4 SeitenWHO Certificate E120 2019Risen ChemicalsNoch keine Bewertungen
- Gkf3000s enDokument6 SeitenGkf3000s enPiotrSikoraNoch keine Bewertungen
- Carrs Index Hausner RatioDokument1 SeiteCarrs Index Hausner Ratiopharmashri5399Noch keine Bewertungen
- Development and Validation of RP-HPLC Method For Simultaneous Determination of Guaifenesin Impurities in Multi Drug CombinationsDokument9 SeitenDevelopment and Validation of RP-HPLC Method For Simultaneous Determination of Guaifenesin Impurities in Multi Drug CombinationsRouag AbdelkarimNoch keine Bewertungen
- URS Deviation ListDokument4 SeitenURS Deviation ListjaiminNoch keine Bewertungen
- VG Glatt PDFDokument14 SeitenVG Glatt PDFPankesin WonkaNoch keine Bewertungen
- LEON PHARMACEUTICALS PRODUCT DATA SHEETSDokument2 SeitenLEON PHARMACEUTICALS PRODUCT DATA SHEETSShagorShagorNoch keine Bewertungen
- Tablets: Popular Dosage FormsDokument7 SeitenTablets: Popular Dosage FormsYuppie Raj100% (1)
- Validation Protocol for Duke Process FRM3Dokument10 SeitenValidation Protocol for Duke Process FRM3Prashansa ShresthaNoch keine Bewertungen
- Art. Enteric CoatingDokument13 SeitenArt. Enteric CoatingDiana Marcela Parra BaronaNoch keine Bewertungen
- PV 01Dokument20 SeitenPV 01Logan KandanNoch keine Bewertungen
- CapsulesDokument35 SeitenCapsulesRochelle AntigNoch keine Bewertungen
- Solid Dosage Forms - CapsulesDokument14 SeitenSolid Dosage Forms - CapsulesHellcroZNoch keine Bewertungen
- Effect of Force Feeder On Tablet Strength During CompressionDokument9 SeitenEffect of Force Feeder On Tablet Strength During CompressionJosé RojasNoch keine Bewertungen
- Addendum 2019 to IP 2018: 66 New MonographsDokument2 SeitenAddendum 2019 to IP 2018: 66 New MonographspawnammalNoch keine Bewertungen
- Tamsulosina + DutasterideDokument13 SeitenTamsulosina + DutasterideJavier Hernandez100% (1)
- Asiklovir tablet product profileDokument16 SeitenAsiklovir tablet product profileAnita RuliyaniNoch keine Bewertungen
- Cleaning and disinfection of food factories: a practical guideVon EverandCleaning and disinfection of food factories: a practical guideNoch keine Bewertungen
- GCP Sect8 Packaging PerformanceDokument10 SeitenGCP Sect8 Packaging Performancelevanvui161Noch keine Bewertungen
- Performa II 07022590005Dokument180 SeitenPerforma II 07022590005Rodney Vitorino DiasNoch keine Bewertungen
- 50 Communication Activities Icebreakers and ExercisesDokument248 Seiten50 Communication Activities Icebreakers and ExercisesVaithiyanathan HaranNoch keine Bewertungen
- RM-Background-Eng Registro Medicamentos BackgroundDokument5 SeitenRM-Background-Eng Registro Medicamentos Backgroundluismanolo09Noch keine Bewertungen
- Final Report IcrelDokument207 SeitenFinal Report Icrelluismanolo09Noch keine Bewertungen
- D Cade BAS 2010Dokument4 SeitenD Cade BAS 2010luismanolo09Noch keine Bewertungen
- MRCT Proyect ReportDokument92 SeitenMRCT Proyect Reportluismanolo09Noch keine Bewertungen
- QA-QC viewpoint favors capsules over tablets for pharmaceutical preparationsDokument4 SeitenQA-QC viewpoint favors capsules over tablets for pharmaceutical preparationsluismanolo09Noch keine Bewertungen
- Capsugel DBcaps Sizing Information 1Dokument1 SeiteCapsugel DBcaps Sizing Information 1luismanolo09Noch keine Bewertungen
- QA-QC viewpoint favors capsules over tablets for pharmaceutical preparationsDokument4 SeitenQA-QC viewpoint favors capsules over tablets for pharmaceutical preparationsluismanolo09Noch keine Bewertungen
- Laboratory Techniques Option One Report SolvedDokument5 SeitenLaboratory Techniques Option One Report SolvedYasmeen AlnajjarNoch keine Bewertungen
- Calculation of Surface Area and Volume of Ponds and AssociatDokument16 SeitenCalculation of Surface Area and Volume of Ponds and AssociatLai SerranoNoch keine Bewertungen
- Department of Mechanical EngineeringDokument7 SeitenDepartment of Mechanical EngineeringMd TouhidNoch keine Bewertungen
- Soil Mechanic - Ch2Dokument31 SeitenSoil Mechanic - Ch2Yassine Kandoussi100% (1)
- Aspects of Lead Acid Battery Technology 6 Designing For Capacity PDFDokument43 SeitenAspects of Lead Acid Battery Technology 6 Designing For Capacity PDFtjkiddNoch keine Bewertungen
- ASTM D4253-16 Maximum Index Density and Unit Weight of Soil Using A Vibratory TableDokument19 SeitenASTM D4253-16 Maximum Index Density and Unit Weight of Soil Using A Vibratory TableAbeiku Naanye Bondziedu ImpraimNoch keine Bewertungen
- Lab Manual Fluid MechanicsDokument26 SeitenLab Manual Fluid MechanicsFarahSyazwaniNoch keine Bewertungen
- Extraction of Ethanol From Mixtures With N-Hexane by DesDokument8 SeitenExtraction of Ethanol From Mixtures With N-Hexane by Dessweety vermaNoch keine Bewertungen
- Introduction and Basic Concepts: Thermodynamics: An Engineering ApproachDokument49 SeitenIntroduction and Basic Concepts: Thermodynamics: An Engineering Approachtevin sessa100% (1)
- 1083ch6 1Dokument9 Seiten1083ch6 1Emad EmadNoch keine Bewertungen
- Physico-Mechanical Properties of Sapota (Achras Sapota L.)Dokument5 SeitenPhysico-Mechanical Properties of Sapota (Achras Sapota L.)Shailendra RajanNoch keine Bewertungen
- Geotechnical Engineering: Lab ManualDokument78 SeitenGeotechnical Engineering: Lab ManualajaykumarNoch keine Bewertungen
- Is 2386 2 1963 PDFDokument21 SeitenIs 2386 2 1963 PDFsherlin100% (1)
- A Different Approach To Estimate Air MoistureDokument7 SeitenA Different Approach To Estimate Air MoistureVal MosNoch keine Bewertungen
- Lab Manual For Soil TestingDokument58 SeitenLab Manual For Soil TestingSanjay MuthekarNoch keine Bewertungen
- Chapters 1, 2, & 3 - MUODokument99 SeitenChapters 1, 2, & 3 - MUOamanuelNoch keine Bewertungen
- Understanding Pressure: Units, Measurements, and CalculationsDokument2 SeitenUnderstanding Pressure: Units, Measurements, and Calculationsirma elNoch keine Bewertungen
- Measuring Real Yarn Diameter and Its Effect on Fabric QualityDokument6 SeitenMeasuring Real Yarn Diameter and Its Effect on Fabric QualityBoubker Kharchafi100% (1)
- Saso Din 51562 3 2016 eDokument13 SeitenSaso Din 51562 3 2016 eMaria AspriNoch keine Bewertungen
- Effect of Wetting Conditions On The in Situ DensitDokument10 SeitenEffect of Wetting Conditions On The in Situ DensitAlexander Angel Valda MelgarNoch keine Bewertungen
- Fluid Mechanics FundamentalsDokument15 SeitenFluid Mechanics FundamentalsJoseph ChanNoch keine Bewertungen
- Density of Soil and Rock In-Place at Depths Below Surface by Nuclear MethodsDokument7 SeitenDensity of Soil and Rock In-Place at Depths Below Surface by Nuclear MethodsIonut VasilescuNoch keine Bewertungen
- Astm D 2726 - 2000Dokument4 SeitenAstm D 2726 - 2000NazarethNoch keine Bewertungen
- Past Exam Soil MechDokument19 SeitenPast Exam Soil MechRyne TatendaNoch keine Bewertungen
- Formation of Integral Skin Polyurethane FoamsDokument11 SeitenFormation of Integral Skin Polyurethane FoamsHariHaran RajendranNoch keine Bewertungen
- Physics Daily Practice ProblemsDokument6 SeitenPhysics Daily Practice ProblemsRahulNoch keine Bewertungen
- Two Phase Flow RegimeDokument4 SeitenTwo Phase Flow RegimeRafael ReyesNoch keine Bewertungen
- Physics Grade 8Dokument5 SeitenPhysics Grade 8AshaNoch keine Bewertungen
- Mix Design Report For M-30 Grade of Concrete: M/s Aegis Logistics LTDDokument5 SeitenMix Design Report For M-30 Grade of Concrete: M/s Aegis Logistics LTD123shripadNoch keine Bewertungen
- Drying Lab ReportDokument26 SeitenDrying Lab ReportAriel Joseph Stern100% (2)