Beruflich Dokumente
Kultur Dokumente
0016 0018
Hochgeladen von
Anonymous FigYuONxuuOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
0016 0018
Hochgeladen von
Anonymous FigYuONxuuCopyright:
Verfügbare Formate
TetrahedronLetters, No. 7, pp* 16-18,1959,Pergamon Press Great Britain.
Ltd.
Printed in
THE CONFIGURATIONOF (-) TROPIC ACID A.NDOF ITS NA~LLY
G. Fodor
and
ADDING
ESTERS
Gy. Csepreghy
Stereochemical Laboratory,The Hungarian Academy of Scienoea, Budapest (Reaeived11 May 1959)
(-1 TROPIC acid is the saidic building stone of a number of important alkaloids, among others of hyoecyamineand hyoecine. In order to obtain a deeper inaight into the bioeyntheeieand mode of phyeiologicalaction of the latter compounde,eetabliehingof the absolute configurationof (-Itropicacid was required, bahlorohydratropic acid (II) was reeolved by MoKenzie and Strathern' more than three deoadee ago, and the levorotatoryacid wee aubaequently hydrolyeed t6 (-)tropioaoid (I) the reaction being accompaniedby alight racemiaation;hydrolyeiewith ammonium hydroxide gave (-)tromde still higher state of optioal purity. On the other hand, (+)a-methylphenyltetio acid (Iffb) wae treated
by the Curtiua reaction to give (-)phenylethylamine2 (IV), and the
in a
N-benzoyl derivativewas oxidized, in turn,3 to (+) alanine (V), 4
: A. McKenzie and R. C. Strathern, J. Chem. Sot, U1, 86 (1925). ii,I, Bernsteinand F. C. Whitmore, J. Amer. Chem. Soo, ii, 1324
(1939). 3 W, Leithe, Ber. Dteoh. Chem. Gee,, $4, 2827 16
(1931).
The configurationof (-)tropicacid
17
According to the unequivocal conventionof Cahn et a1,,4 (+)a-methylacid (IIIb), phenylaceticacid is designatedas 2 (+)a-methylphenylacetic where the g configurationcorrespondsto the levorotatoryform (IIIa), The single mieaing link between tropic acid and alanine wa8 now the eetabliehmentof the aorrelationof (-)&chlorohydratropicacid (II) with either 2 or a a-methylphenylacetic acid. This work has been done recently in the laboratoryof the authors. $-ohlorohydratropic acid obtained from atropic acid5 wae reeolved by 1 codeine instead of morphine used by McKenzie, The codeine salt of (-)&chlorohydratropicacid melted at 138' (decomp.),[a]: -95' (o = 0.4, in methanol), (Found: C, 67.9; H, 6.3; N, 2.7, C27H3005NC1requiree c, 67.0; H, Q.28 N, 2.98). The levorotatoryacid, m.p. 62', gave oorreat' rotationalvalues,
bl?
-115' (0 - 0.4; in 9& ethanol). Hydrogenolyeisof thie compound over a Pd-charcoaloatalyet in ethyl
aoetate in the presence of barium hydroxide resulted in the formation of g (-)u-methylphenylacetic acid, b.p.l_~k15, [a]? = -76' (o - 0.794 in 96 ethanol). (Found: C, 72.2; H, 6.7. C9Hlo02 requireer C, 72.0; H, 6.746.) TheCahn-Ingold - Prelog convention,4gives the unequivocal
configurationof 2 (-)tropicacid (I) for the levorotatoryform.
4 R.
S. Cahn, C. K. Ingold and V. Prelog, Rperientia U, 81 (1956). 5 A, McKenzie and J. K. Wood, J. Chem. Sot, Uj, 635 (1919).
18
The oonfiguratlon
of (-)tropioacid
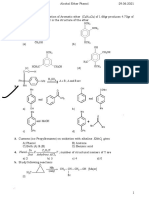
;6=5 PWH H-Cco2H a
76=5 ,-*
;6=5 H - c - C02H
k20E
1118 (-1
II (-1
I (-)-Troploaold
;6=5 Ifo2c - c - H __)
;6=5
BH2 -C-H "2-V"
co2H I
cH3
IIIb (+I Iv (-1 v (+)-AlanIne
All there formulae axe Firoher pPojeotion.
Aooordingly,
(-hvo=ine
(-horopsmine ie
S (-)tropoyl-tropan-h-01, and
i@ a (-)tropo~l-3a-hHro~~6,7 B-epoxy-tropane.
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Erosion of Limestones Under Soil AND Vegetation Systems On LimestoneDokument11 SeitenThe Erosion of Limestones Under Soil AND Vegetation Systems On LimestoneAnonymous FigYuONxuuNoch keine Bewertungen
- Masthead (Earth Surface Processes, Vol. 1, Issue 1) (1976)Dokument1 SeiteMasthead (Earth Surface Processes, Vol. 1, Issue 1) (1976)Anonymous FigYuONxuuNoch keine Bewertungen
- Stability of Minels in Ring Reaneral Thchemical Approach (Earth Surface Processes, Vol. 1, Issue 1) (1976)Dokument8 SeitenStability of Minels in Ring Reaneral Thchemical Approach (Earth Surface Processes, Vol. 1, Issue 1) (1976)Anonymous FigYuONxuuNoch keine Bewertungen
- Of of of of 9% For: Short CommunicationsDokument1 SeiteOf of of of 9% For: Short CommunicationsAnonymous FigYuONxuuNoch keine Bewertungen
- Al Stream Relationships - A Case Study in The Westend Basin of The Southern Pennines, England (Earth Surface Processes, Vol. 1, Issue 1) (1976)Dokument7 SeitenAl Stream Relationships - A Case Study in The Westend Basin of The Southern Pennines, England (Earth Surface Processes, Vol. 1, Issue 1) (1976)Anonymous FigYuONxuuNoch keine Bewertungen
- A Scree Slope Rockfa (Esses, Vol. 1, Issue 1) (1976)Dokument20 SeitenA Scree Slope Rockfa (Esses, Vol. 1, Issue 1) (1976)Anonymous FigYuONxuuNoch keine Bewertungen
- Pedological Feeability of Hillydale, Yorire (, Vol. 1, Issue 1) (1976)Dokument14 SeitenPedological Feeability of Hillydale, Yorire (, Vol. 1, Issue 1) (1976)Anonymous FigYuONxuuNoch keine Bewertungen
- Earth Surface Processes, Vol. 39, Issue 5Dokument1 SeiteEarth Surface Processes, Vol. 39, Issue 5Anonymous FigYuONxuuNoch keine Bewertungen
- The Ground State Oi The Bose Gas By: AbstractDokument16 SeitenThe Ground State Oi The Bose Gas By: AbstractAnonymous FigYuONxuuNoch keine Bewertungen
- Hydrograph Peakedness and Basin Area (Es, Vol. 1, Issue 1) (1976)Dokument4 SeitenHydrograph Peakedness and Basin Area (Es, Vol. 1, Issue 1) (1976)Anonymous FigYuONxuuNoch keine Bewertungen
- Iterated Crossed Box Diagram in The Complex Angular Momentum Plane and Bethe-Salpeter EquationDokument15 SeitenIterated Crossed Box Diagram in The Complex Angular Momentum Plane and Bethe-Salpeter EquationAnonymous FigYuONxuuNoch keine Bewertungen
- Communications in Maths&Physics 6-13Dokument8 SeitenCommunications in Maths&Physics 6-13Anonymous FigYuONxuuNoch keine Bewertungen
- On The Connection Between The LSZ and Wightman Quantum Field TheoryDokument17 SeitenOn The Connection Between The LSZ and Wightman Quantum Field TheoryAnonymous FigYuONxuuNoch keine Bewertungen
- A Theorem Concerning The Positive Metric: Derek W. Robi NsonDokument6 SeitenA Theorem Concerning The Positive Metric: Derek W. Robi NsonAnonymous FigYuONxuuNoch keine Bewertungen
- On The Vacuum State in Quantum Field Theory. II: H. J. BorchersDokument23 SeitenOn The Vacuum State in Quantum Field Theory. II: H. J. BorchersAnonymous FigYuONxuuNoch keine Bewertungen
- Divergence of Perturbation Theory For Bosons: ArthurDokument23 SeitenDivergence of Perturbation Theory For Bosons: ArthurAnonymous FigYuONxuuNoch keine Bewertungen
- Inhomogeneous: SL (N, C)Dokument9 SeitenInhomogeneous: SL (N, C)Anonymous FigYuONxuuNoch keine Bewertungen
- Communications in Math&Physics 49-56Dokument8 SeitenCommunications in Math&Physics 49-56Anonymous FigYuONxuuNoch keine Bewertungen
- Upper Andlower Limitsforthe Number Ofbound States in A Given Central PotentialDokument9 SeitenUpper Andlower Limitsforthe Number Ofbound States in A Given Central PotentialAnonymous FigYuONxuuNoch keine Bewertungen
- Evaluating Go Game Records For Prediction of Player AttributesDokument7 SeitenEvaluating Go Game Records For Prediction of Player AttributesAnonymous FigYuONxuuNoch keine Bewertungen
- Communications in Math&Physics 1-5Dokument5 SeitenCommunications in Math&Physics 1-5Anonymous FigYuONxuuNoch keine Bewertungen
- Communications in Math&Physics14-48Dokument35 SeitenCommunications in Math&Physics14-48Anonymous FigYuONxuuNoch keine Bewertungen
- A Photoelectric Method The Phosphorus': For DeterminationDokument3 SeitenA Photoelectric Method The Phosphorus': For DeterminationAnonymous FigYuONxuuNoch keine Bewertungen
- Jou Chem of SaltsDokument1 SeiteJou Chem of SaltsAnonymous FigYuONxuuNoch keine Bewertungen
- Simple Ozonizer: LaboratoryDokument1 SeiteSimple Ozonizer: LaboratoryAnonymous FigYuONxuuNoch keine Bewertungen
- Measurement Distensibilitv Organic Finishes: NE Is A of A TDokument4 SeitenMeasurement Distensibilitv Organic Finishes: NE Is A of A TAnonymous FigYuONxuuNoch keine Bewertungen
- 44 GÇô45Dokument2 Seiten44 GÇô45Anonymous FigYuONxuuNoch keine Bewertungen
- An Improved Semi-Micro and Micro-Carius Determination: HE L. and of A and ToDokument2 SeitenAn Improved Semi-Micro and Micro-Carius Determination: HE L. and of A and ToAnonymous FigYuONxuuNoch keine Bewertungen
- 46 GÇô47Dokument2 Seiten46 GÇô47Anonymous FigYuONxuuNoch keine Bewertungen
- Preparation of Vehicle Films Free of Supporting Foundation: Semi-MicromethodDokument1 SeitePreparation of Vehicle Films Free of Supporting Foundation: Semi-MicromethodAnonymous FigYuONxuuNoch keine Bewertungen
- Important 90 Questions of Garments Washing & Dyeing.Dokument4 SeitenImportant 90 Questions of Garments Washing & Dyeing.Shamim HossainNoch keine Bewertungen
- 3.3 - Lockhart & Crescenzi - Sour Oil and Gas Management PDFDokument34 Seiten3.3 - Lockhart & Crescenzi - Sour Oil and Gas Management PDFsantiagoNoch keine Bewertungen
- Demo Flinn Co2 SolubilitydemoDokument3 SeitenDemo Flinn Co2 Solubilitydemoapi-321107093Noch keine Bewertungen
- Vol - 8 - 1 - 044-056 - FAREED AHMED MEMON PDFDokument13 SeitenVol - 8 - 1 - 044-056 - FAREED AHMED MEMON PDFAnonymous e2wolbeFsNoch keine Bewertungen
- Sample Question 3 With AnswerDokument18 SeitenSample Question 3 With AnswerPyae Sone Kyaw100% (1)
- Chem 315 - Lab 10 - Qualitative Organic AnalysisDokument20 SeitenChem 315 - Lab 10 - Qualitative Organic Analysisk50% (2)
- Lab Report 2 - Corrosion of IronDokument5 SeitenLab Report 2 - Corrosion of IronATHALIAH JENINE TABUCLIN BANTUGNoch keine Bewertungen
- MINERAL SerpentineDokument3 SeitenMINERAL SerpentineMichi Michi FitriyanaNoch keine Bewertungen
- Water and Acid-Base System: By: Dr. Mohd Fakharul ZamanDokument35 SeitenWater and Acid-Base System: By: Dr. Mohd Fakharul ZamanAbdul Ashraf RasidNoch keine Bewertungen
- Environmental Lab ManualDokument45 SeitenEnvironmental Lab Manualkeshav198083% (6)
- Chemistry SPM Trial Paper 3Dokument7 SeitenChemistry SPM Trial Paper 3Kaneson IyarooNoch keine Bewertungen
- Oxidation Reactions of AlcoholsDokument1 SeiteOxidation Reactions of AlcoholsxantogenatNoch keine Bewertungen
- CAPE Chemistry 2015 U2 P2Dokument21 SeitenCAPE Chemistry 2015 U2 P2Kyle YearwoodNoch keine Bewertungen
- Dyes and Chemicals As On 28-05-2020Dokument8 SeitenDyes and Chemicals As On 28-05-2020mohammedNoch keine Bewertungen
- IsoxazolesDokument13 SeitenIsoxazolesAntônio Neto MachadoNoch keine Bewertungen
- Conceptual Solution of Flexible Plant For Copper (Ii) Chloride ProductionDokument4 SeitenConceptual Solution of Flexible Plant For Copper (Ii) Chloride ProductionTuấnNguyễnNoch keine Bewertungen
- MARK SCHEME For The November 2004 Question PaperDokument8 SeitenMARK SCHEME For The November 2004 Question PaperVarun PanickerNoch keine Bewertungen
- Total Acid Number/ Total Base Number Standards & ReagentsDokument3 SeitenTotal Acid Number/ Total Base Number Standards & ReagentsMajed DawaNoch keine Bewertungen
- Jabir Ibn Hayyan ContributionsDokument4 SeitenJabir Ibn Hayyan ContributionshusnaNoch keine Bewertungen
- IVA Group Elements PDFDokument3 SeitenIVA Group Elements PDFGhulam RabbaniNoch keine Bewertungen
- Chemistry and Enzymology of Vitamin B12Dokument76 SeitenChemistry and Enzymology of Vitamin B12prakas.rao39695Noch keine Bewertungen
- Inventory of Kerala - IndustriesDokument348 SeitenInventory of Kerala - IndustriesMuthu Praveen SarwanNoch keine Bewertungen
- Capt. Vadakyil Wall Wash MethodDokument3 SeitenCapt. Vadakyil Wall Wash Methodpushkarsidhaye100% (2)
- Wk4-Biodegradation of Azo DyesDokument49 SeitenWk4-Biodegradation of Azo DyesGaurav DhawanNoch keine Bewertungen
- Tutorial 6 AlcoholDokument5 SeitenTutorial 6 Alcoholwan arifahNoch keine Bewertungen
- 9701 w04 QP 2Dokument12 Seiten9701 w04 QP 2Hubbak KhanNoch keine Bewertungen
- Acids and Alkalis Exam Style Questions 1Dokument2 SeitenAcids and Alkalis Exam Style Questions 1elezabethNoch keine Bewertungen
- Organic Compounds ActivitiesDokument11 SeitenOrganic Compounds Activitiesjoan marie PeliasNoch keine Bewertungen
- Incom. Sr. 29.06.2021 AssignmentDokument5 SeitenIncom. Sr. 29.06.2021 AssignmentSrikar SatyaNoch keine Bewertungen
- Calcium Chloride: Commercial ChemicalDokument3 SeitenCalcium Chloride: Commercial ChemicalIqbal batchaNoch keine Bewertungen