Beruflich Dokumente
Kultur Dokumente
Sclera Structure
Hochgeladen von
ferdi151991Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Sclera Structure
Hochgeladen von
ferdi151991Copyright:
Verfügbare Formate
Experimental Eye Research 78 (2004) 609623 www.elsevier.
com/locate/yexer
Review
Scleral structure, organisation and disease. A review
Peter G. Watsona,*, Robert D. Youngb
b a 17 Adams Road, Cambridge CB3 9AD, UK Biophysics Group, Department of Optometry and Vision Sciences, Cardiff University, Redwood Building, King Edward VII Avenue, Cardiff, Wales CF10 3NB, UK
Received 25 June 2003; accepted 26 June 2003
Abstract Although disease of the sclera is unusual, when it occurs it can rapidly destroy both the eye and vision. However, normally the sclera provides an opaque protective coat for the intraocular tissues and a stable support during variations in internal pressure and eye movements, which would otherwise perturb the visual process through distortion of the retina and the lens/iris diaphragm. This stability, which is vital for clear vision is made possible by the organisation and viscoelastic properties of scleral connective tissue. Microscopically, the sclera displays distinct concentric layers including, from outside, Tenons capsule, episclera, the scleral stroma proper and lamina fusca, melding into underlying choroid. Two sites exhibit specialised structure and function: the perilimbal trabecular meshwork, through which aqueous lters into Schlemms canal, and the lamina cribrosa, which permits axons of the optic nerve to exit the posterior sclera. Throughout, sclera is densely collagenous, the stroma consisting of brils with various diameters combining into either interlacing bre bundles or dened lamellae in outer zones. Scleral brils are heterotypic structures made of collagen types I and III, with small amounts of types V and VI also present. Scleral elastic bres are especially abundant in lamina fusca and trabecular meshwork. The interbrillar matrix is occupied by small leucine-rich proteoglycans, decorin and biglycan, containing dermatan and dermatan/chondroitin sulphate glycosaminoglycans, together with the large proteoglycan, aggrecan, which also carries keratan sulphate sidechains. Decorin is closely associated with the collagen brils at specic binding sites situated close to the C-terminus of the collagen molecules. Proteoglycans inuence hydration, solute diffusion and uid movement through the sclera, both from the uvea and via the trabecular meshwork. As the sclera is avascular, nutrients come from the choroid and vascular plexi in Tenons capsule and episclera, where there is an artery to artery anastomosis in which blood oscillates, rather than ows rapidly. This predisposes to the development of vasculitis causing a spectrum of inammatory conditions of varying intensity which, in the most severe form, necrotising scleritis, may destroy all of the structural and cellular components of the sclera. Scleral cells become broblastic and the stroma is inltrated with inammatory cells dominated by macrophages and T-lymphocytes. This process resembles, and may be concurrent with, systemic disease affecting other connective tissues, particularly the synovial joints in rheumatoid arthritis. Current views support an autoimmune aetiology for scleritis. Whilst the role of immune complexes and the nature of initial proinammatory antigen(s) remain unknown, the latter may reside in scleral tissue components which are released or modied by viral infection, injury or surgical trauma. q 2003 Elsevier Ltd. All rights reserved.
Keywords: human sclera; review; structure; collagens; proteoglycans; vasculature; innervation; development; aging; inammatory diseases
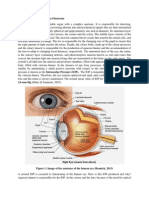
1. Introduction The human sclera, although relatively inert metabolically, is a remarkable structure which performs several important functions essential for the visual integrity of the eye. Primarily, the sclera provides a rm substrate for the delicate intraocular contents and protects them from injury.
* Corresponding author. Dr Peter G. Watson, 17 Adams Road, Cambridge CB3 9AD, UK. E-mail address: peter.g.watson@btinternet.com (P.G. Watson). 0014-4835/$ - see front matter q 2003 Elsevier Ltd. All rights reserved. DOI:10.1016/S0014-4835(03)00212-4
Its opacity ensures that internal light scattering does not affect the retinal image. In addition, it facilitates rotation of the eyeball without signicant distortion through nearly 1808 by powerful muscles. The shape of the eye is, in part, maintained by the presence of the intraocular contents and the intraocular pressure. However, the sclera must be rigid enough to provide relatively constant conditions so that, when the eyeball is moved, the intraocular pressure does not uctuate and adversely affect vision. Scleral deformation would impair vision not only because of wrinkling of the retina itself, but also through irregular distortion of the lens
610
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
iris diaphragm. Theoretically the functional requirements of the sclera could be satised by a rigid globe, but they have been achieved in different ways throughout the animal kingdom. In most animals the globe is circular, the sclera thin and of even thickness throughout its circumference, but often supported with cartilage or even bone. It is possible that the less rigid brous structure of the sclera in mammals allows a more even distribution of the blood supply to the choroid, and thence the retina, during the large excursions of voluntary ocular movement. Optical stability is achieved through the balance of intraocular pressure and the curvatures of the sclera/corneal envelope. This relationship is so constant that it can be relied on when the power of a surgically implanted lens is calculated. Certainly, softening of the eye, for example through injury or inammation, can lead to a disproportionately greater visual loss than might be expected from the distortion of the intraocular contents alone. Conversely, when patients with dysthyroid ophthalmopathy look upwards, their intraocular pressure rises because of the pressure of the rigid rectus muscles on the globe and, in some cases, this is accompanied by a dramatic fall in vision. However, scleral folding on its own does not usually affect vision unless the macula is involved, as can be seen sometimes after retinal detachment surgery when the intraocular pressure is normal. The sclera is able to fulll these functions owing to the unique microscopical structure and arrangement of protein and carbohydrate molecules, which interact to form its connective tissue matrix. In common with other connective tissues, the sclera may also succumb to immuno-inammatory diseases which degrade its components. In these, and surgical procedures involving access to the intraocular contents via incision of scleral tissue, the potential of the sclera for regeneration and repair is crucial for the healthy eye. It is perhaps therefore surprising that the human sclera remains such an under-researched structure. Many of the new discoveries relating to connective tissue composition and organisation have come from studies on cornea, tendon and cartilage. In this article, we review current knowledge on the structure of the human sclera, make reference where applicable to new evidence from studies on other tissues and comment briey on contemporary aspects of scleral disease processes and repair.
with the scleral collagen. From the insertion of extraocular muscles towards the limbus, the sclera gradually increases in thickness up to 08 mm, where it blends with the cornea. Women have slightly thinner sclera than men. There is also an increase in scleral thickness, together with opacity, in relation to age. Opaque, yellowish-white sclera merges with transparent cornea across an intermediate zone extending about 2 mm, termed the limbus. Here, a sulcus is formed owing to the higher radius of curvature of cornea than the sclera. However, this is not readily visible as it is lled in by overlying episclera and conjunctiva. The sclera encroaches slightly more into the cornea in superior and inferior quadrants than it does laterally (corneal horizontal axis: 116 mm; vertical axis: 106 mm), but the internal diameter of the so-called scleral foramen is circular at 116 mm. Thus, the posterior edge of the scleral sulcus is almost parallel to the optic axis laterally, but lies obliquely elsewhere. Two vascularised fascial layers invest the outer surface of the sclera: Tenons capsule and the episclera. 2.1. Tenons capsule (Fascia bulbi) Tenons capsule is identied as a distinct hypocellular layer of radially-arranged, compact collagen bundles running parallel to the scleral surface. At its anterior origin in the limbus, the capsule is rmly attached to overlying conjunctival tissue and the episclera below. About 3 mm from the limbus, it thickens and becomes freely mobile over the underlying episclera to which it maintains attachment via ne interconnecting trabeculae. It extends from the limbus backwards to ensheath the rectus muscles and becomes continuous with their perimysium. The importance of Tenons capsule as a muscle pulley for the extraocular muscles, particularly in relation to strabismus, where collagen brils in the capsule show increased diameter and packing density (Shauly et al., 1992), was recently reemphasised (Roth et al., 2002). Continuing posteriorly as a simple condensation of collagenous bres, it probably merges with the dural sheath of the optic nerve and with brous bands connecting the eyeball to the orbit. Tenons capsule lies anteriorly between two vascular layers, the conjunctival plexus and the episcleral plexus, both of which nourish it. Ramications of the anterior ciliary vessels course throughout the matrix with the veins running supercially and the arteries coming close to the surface only near the limbal arcade. Towards the equator posteriorly, a ne tenuous network of vessels runs in this tissue from the posterior ciliary arteries. 2.2. Episclera The episclera is a thin and dense, but well-vascularised layer of connective tissue, with bres blending imperceptibly with the underlying stroma of the sclera itself. In contrast with Tenons capsule, the bundles of collagen are
2. Anatomy of human sclera The sclera comprises ve-sixths of the outer tunic of the eye extending posteriorly from the corneal perimeter to the optic foramen, perforated by the optic nerve. It is approximately spherical with an average vertical diameter of 24 mm. The thickness of the adult human sclera is not uniform. It is thickest at the posterior pole (1 135 mm), decreasing gradually to 04 06 mm at the equator and thinnest under the recti muscles (03 mm), increasing again to 06 mm, where the parallel shiny tendon bres merge
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
611
circumferentially arranged with tight attachments to the walls of the blood vessels, preventing its independent movement over the sclera. The attachments to Tenons capsule are dense near the limbus and weaken progressively towards the equator, where the episclera is bound to the capsule only by very thin bands of collagen. A small amount of elastic tissue can be found in the episclera together with melanocytes and a few macrophages. A few myelinated and unmyelinated nerve bres also ramify within the episclera terminating mostly around the vessels. 2.3. Scleral stroma The strength and resilience of the scleral stroma is achieved by bundles of parallel-aligned collagen brils, in supercial sites grouped into dense superimposed lamellae, which run mostly parallel to the surface of the eyeball (Fig. 1). The majority of bundles exhibit a circular orientation, but lie meridionally at the limbus (Hogan et al., 1971). In contrast to cornea, scleral lamellae branch and interlace extensively and exhibit wide-ranging dimensions, up to 50 mm wide and 6 mm thick (Komai and Ushiki, 1991). Increased interweaving and density of bres replaces the lamellar arrangement in the deep sclera, while scattered elastic bres are present between and within the collagen bundles throughout the stroma. Tendon bres of the extraocular muscles intermingle with the scleral bres, extending anteriorly as far as the limbus. The innermost layer of the sclera adjacent to the uvea is known as the lamina fusca. In this region the collagen bundles are again smaller and branch extensively to blend into the underlying choroidal stroma. The sclera is traversed by blood vessels and nerves. Anterior ciliary vessels penetrate anterior to the rectus muscles while long and short posterior ciliary
vessels, vortex veins and nerves enter posterior to the muscles. This tissue organisation provides the sclera with considerable visco-elastic properties. Indentation of the tissue initially causes a rapid lengthening of bres and a rebound, followed by a slow stretching on prolonged pressure. It also confers strong tensional properties consistent with the requirement to resist the stresses and strains imposed on it by the extraocular muscles. Scleral visco-elasticity protects the eye from injury during transient elevations of intraocular pressure. This is evident when pressure is raised articially by injecting uid into the eye, the pressure will rise rapidly and then gradually fall to its original level without signicant distortion of the eye. An initial lengthening of the bres is followed by slow sliding of the bres one upon another (Friberg and Lace, 1988); the amount of stretch is not directly proportional to the change in pressure, rigidity increasing as the bres are stretched. 2.4. Scleral spur Supercial bres of the sclera blend with the episcleral bres at the limbus. The deep bres condense in a ring to form the scleral spur, which is an important anatomic landmark, recognised by all ophthalmic surgeons in relation to post cataract astigmatism. This rigid ring structure, together with the corneal annulus, which is formed by a circumferential swathe of limbal brils originating in the cornea (Newton and Meek, 1998), probably accounts for the stability of the corneal contour. The trabecular tissue is inserted into the scleral spur anteriorly and it receives the longitudinal part of the ciliary muscle posteriorly. The collagen bres of the scleral spur, which are continuous with the bres of the corneoscleral trabecular meshwork,
Fig. 1. Outer layers of normal, supero-temporal sclera from 40-year-old man showing lamellar structure. Collagen brils are present in longitudinal (Lc), transverse (Tc) and oblique section (Oc) and exhibit wide variation in diameter. A brocyte (F) and elastin bre (E) are also visible. Bar represents , 15 mm. From Wolffs Anatomy of the Eyes and Orbit, 8th Ed., A.C. Bron, R.C. Tripathi, B.J. Tripathi, 1997. Reproduced by permission of Hodder Arnold.
612
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
increase in size from 40 nm in the trabecular sheets to 80 nm close to the sclera, so that the scleral spur feels rm under surgery. The inner layers, the so-called scleral roll, surround Schlemms canal in its whole circumference. From the posterior end of Schlemms canal a small circumferential band of scleral bres projects towards the anterior chamber. This is the part of the scleral spur to which the meridional bres of the ciliary muscle are attached. The ne anterior tips of the ciliary muscle form a tendinous structure inserting into the posterior part of the scleral spur and hence into the trabecular meshwork. This tendon has the same composition as the trabecular beams and consists of collagen and elastin. It is through this connection that contraction of the ciliary muscle can pull on the scleral spur opening the trabecular meshwork. The rigidity of the scleral spur may also help prevent closure of the trabecular meshwork when the ciliary muscle relaxes (Hamanaka, 1989). 2.5. The limbus Overlying the trabecular zone, across the limbus, a gradation of changes in the matrix reects the transition from sclera to cornea in an ill-dened region, 1 2 mm in width. The changes in tissue structure, composition and biomechanical properties at this site also incur increased susceptibility to injury and disease. Fibrils of the deep sclera extend beyond the trabecular bands running across the limbus to the region of Descemets membrane traversing, and some interacting with, the circumcorneal annulus of brils (Newton and Meek, 1998). The limbus is of importance not only as the translucent surgical landmark, but also because of the unusual cellular composition and the presence of stem cells within the tightly adherent conjunctival and episcleral tissues which overly it. It is from this region that new corneal epithelium is derived and because of the high content of antigen presenting cells in this tissue, it is of major importance in the immunological changes which occur in both sclera and cornea during inammation. 2.6. Posterior sclera The posterior sclera is perforated 3 mm medial to the midline and 1 mm below the horizontal by the optic nerve. The aperture is cone-shaped, being 2 mm wide on the internal surface and 35 mm externally. Posteriorly the outer two thirds of the scleral bres are continuous with the dural sheath of the optic nerve and the rest form the lamina cribrosa, a collagenous scaffold supporting the optic nerve. Multiple openings, lined by bundles of scleral bres covered by glial tissue, form short canals that provide a passage for the axons of the optic nerve. One of the openings in the lamina is larger than the rest and contains the central retinal artery and vein. The collagen bres are vertically arranged and condensed as septa, already present in the 160 mm
embryo, where the nerve bre bundles pass through them (Anderson, 1969). There is no doubt that structural abnormalities in the lamina cribrosa contribute to the collapse of the collagenous framework of the optic disc, associated with the cupping which occurs in glaucoma. However, considerable debate continues on the respective importance of collagen remodelling, raised intraocular pressure, ischaemia and other factors in the aetiology of this disease. A better understanding of matrix turnover in the posterior sclera could also help explain the changes found in progressive myopia and in some cases of low tension glaucoma in which the disc head collapses. Inammatory oedema of the sclera in the region of the optic disc leads to strangulation of the nerve bres and blood vessels, as they run in brous channels within the scleral tissue. These brous channels penetrate the sclera at three main sites: around the optic nerve, for the passage of the long and short posterior ciliary vessels and nerves; 4 mm behind the equator for the venae vorticosae; and between the limbus and the muscle insertions, for the transmission of the anterior ciliary vessels, nerves and perivascular lymphatics.
3. Blood supply and lymphatics of the sclera and episclera The sclera has a low metabolic requirement because of the slow turnover of the collagen of which it is composed. The scleral stroma receives no blood capillaries in the normal healthy state, although the long posterior ciliary arteries and nerves and the vortex veins pass through it in brous canals. The stroma derives its nutrition from the episcleral and choroidal vascular networks. Similarly, inammatory cells inltrating the sclera come from both of these sources. The reason for this total absence of direct blood supply and the reluctance of new blood vessels to enter the sclera even after injury is obscure and unresearched. The episclera and Tenons capsule derive their blood supply from the anterior ciliary arteries and the long posterior ciliary arteries posteriorly, with some contribution from the conjunctival arteries at the limbus. These major vessels contribute to the episcleral arterial circle, an often incomplete arterial network situated about 4 mm from the limbus (Morrison and Van Birskirk, 1983). The episcleral arterial circle in turn contributes to the limbal arcade of vessels. This unusual artery to artery anastomosis ensures that the anterior segment of the eye is always supplied with blood whatever the pressures on the globe may be. It does, however, have the disadvantage that, in the regions between the rectus muscles, arterial blood may not ow through the vessel, but rather oscillate within it (Meyer, 1988). As a consequence, extravasation of uid or cells in the region of these vessels stagnates and creates conditions in which immune reactions can readily occur. In the posterior
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
613
segment, both the episcleral tissue and the overlying Tenons capsule are thin, which results in relative avascularity of the supercial layers of the posterior sclera. There are well-formed lymphatic channels in conjunctiva, but they are absent in the episclera and sclera, although it has been suggested that spaces between bre bundles might enable the sclera itself to act as a lymphatic medium. The conjunctival lymphatic channels are in two layers, a well formed supercial network and a network of channels adjacent to Tenons capsule (Bussacca, 1947). Lymph from the supercial episcleral tissue drains into the subconjunctival space and thence to the parotid node nasally and to the submandibular nodes temporally. Lymph from elsewhere in the sclera and episclera passes into the orbit via the perivascular space around the veins to empty into the jugular lymph trunks and the deep cervical nodes.
the episcleral arteries and to a lesser extent on the veins (Stone et al., 1987). Nerve bres staining for neuroactive peptide Y (NPY), vasoactive intestinal peptide (VIP), vesicular acetyl choline transporter (VACHT), calcitonin gene-related peptide (CGRP) and substance P (SP) are also found largely on the arteries and at arterio-venous anastomoses In the episclera, anterior to the vascular circle, numerous free nerve endings staining for SP and CGRP are also found (Selbach et al., 1998). The purpose of these endings adjacent to the vessels and aqueous veins, is presumably to regulate the blood supply of the anterior segment and to inuence the rate of aqueous outow (Selbach et al., 2000).
5. Composition of the sclera Scleral matrix conforms to a general plan seen in other connective tissues with a scaffold of protein brils, collagen and elastin, and interbrillar proteoglycans and glycoproteins, which surround a diffuse population of cells. Although there has been a recent resurgence of interest in the molecular components present in sclera, particularly in relation to axial development and proper image formation at the retina, far more is known of corneal than scleral composition (Mayne, 2002). 5.1. Collagens Microscopically the sclera is a dense, primarily collagenous tissue. Earlier estimations of human and animal scleral collagen content by weight have varied widely from 50 to 75%, although this may be partly explained by the different techniques employed (Polatnick et al., 1957; Keeley et al., 1984). The collagen family of proteins contains the most abundant proteins in the body. Classically, collagens are dened as molecules contributing to the structure of extracellular tissue matrices (Kielty and Grant, 2002), and are identied as proteins consisting of three polypeptide chains, assembled with triple-helical domains and containing Gly-X-Y amino acid repeat sequences, where X and Y are often proline and hydroxyproline, respectively. Interstitial collagens form the familiar cross-banded brils of tissue matrices by assembly of molecules head to tail with a quarter-stagger overlap of adjacent molecules. Most brils are now recognised to exist as heterotypic interactions of more than one collagen type. Twenty seven different collagens have now been identied from protein and genetic analysis (Pace et al., 2003; for review see Kielty and Grant, 2002), and many more are expected to be discovered from analysis of the human genome sequence. However, many of those found recently have no known structural function. Types I, III, V and VI collagen are present in the sclera, although biochemical analyses have shown that type I predominates, with type III at less than 5% and only trace amounts of other species present (Keeley et al., 1984;
4. Nerve supply of the sclera The nerve supply of the sclera is surprisingly rich for a structure whose main function would appear to be supportive. Consequently, inammation of the sclera is extraordinarily painful, owing both to direct stimulation of the nerve endings by the inammatory process and to distension and stretching of the nerve bundles from tissue swelling and cellular inltration. The primary nerve trunks divide and redivide to emerge in the episclera as single nerve endings. The nerve supply of the posterior sclera is derived from the short ciliary nerves, where they enter the sclera close to the optic nerve. Anteriorly, it is derived from branches of the long ciliary nerves, which accompany the long posterior ciliary nerves. At the equator the long ciliary nerves divide, some return posteriorly in the sclera itself to re-enter the choroid in the region of the lamina fusca. Of those which pass forward, most enter the ciliary body, but some form the nerve loops of Axenfeld (1907). The latter are nerves which, having entered the ciliary body, then pass outward through the full thickness of the sclera and back into the ciliary body through the same canal. These nerves, which are found in 12% of eyes, can form painful tumours when they come to lie in the episclera. The less obvious ones can often be detected on the slit lamp by their squashed mushroom appearance and the faint cuff of pigment which surrounds the nerve. They are usually associated with blood vessels. Their function is unknown, but although they have clinical signicance because they are pigmented and sometimes painful, their removal is not advisable. The rest of the nerves pass distally, penetrating the sclera about 3 mm from the limbus and branching to supply the cornea, trabecular meshwork, Schlemms canal, and episclera. They are very prominent in the tendinous insertion of the muscles. Numerous nerve endings staining for nicotinamide adenine dinucleotide phosphate diaphorate (NADPHd), and thyrotropic hormone (TH), are found on
614
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
Heathcote, 1994). Consistent with these data, types I and III collagen have been identied in human sclera by light microscopy immunolocalisation (Tengroth et al., 1985; Thale and Tillmann, 1993; Thale et al., 1996a,b; White et al., 1997), although some studies have found type III restricted to outermost layers, the lamina cribrosa and an interzone between outer sclera and dura mater (Konomi et al., 1983; Rhenberg et al., 1987). Collagen types I and III were both synthesised by human Tenons capsule broblasts in vitro (Gross, 1999). Localisation of collagens at higher resolution by electron microscopy in macular sclera from aged human eyes showed types I and III collagen to be present in the major D-periodic interstitial brils, with type V at the bril perimeter and type VI in lamentous structures between bre bundles (Marshall et al., 1993). This suggested that copolymerisation of multiple collagen types into complex heterotypic assemblies was present in human sclera, as seen in other tissues, such as cornea and cartilage. The banded brils of scleral stroma, in contrast with those of the cornea, are coarser and exhibit a wider range of diameters, between 25 and 300 nm (Spitznas, 1971; Borcherding et al., 1975; Komai and Ushiki, 1991). Many ultrastructural studies of scleral collagen brils have been carried out using TEM and scanning electron microscopy (SEM) and more recently by atomic force microscopy (AFM, Yamamoto et al., 2000; Meek and Fullwood, 2001; Yamamoto et al., 2002), generally with good agreement on collagen bril dimensions. Different periodicities of corneal and scleral brils of 63 and 67 nm, respectively, have been identied by AFM and attributed to differing inclination angles (15 and 5 8 , respectively), of microbrillar components. Thinner brils are more common in the inner scleral layers and also in two regions of specialised function, namely the lamina cribrosa, where the optic nerve enters the eye, and in the trabecular meshwork in the corneo-scleral angle. In these regions of the sclera, signicant amounts of type III collagen accompany the main type I component, together with types IV, V and VI collagen (Rhenberg et al., 1987; Marshall et al., 1990, 1991; Albon et al., 1995). In the scleral lamina, brils are smaller, more densely packed and uniform in size than elsewhere in the sclera, exhibiting a mean diameter of 47 nm, compared to 146 nm in the equatorial sclera, according to Quigley et al. (1991). SEM showed the circular arrangement of brils around the emerging axons was lost in eyes with glaucoma (Thale et al., 1996a,b). The presence of heterotypic, small-diameter brils, rich in type III collagen, was previously considered to be a specialised adaptation in tissues such as these in the eye, and tendon, where resistance to deformation and elasticity are required (Parry and Craig, 1984). However, our current understanding is that type I:III ratios may show wide diversity in relation to factors such as tissue, location, age and disease, but the association of type I and type III collagens into heterotypic brils is a ubiquitous occurrence in noncartilaginous tissues (Keene et al., 1987).
Many new collagen species have come to light in the last 10 years including several with potential relevance to ocular structure and development (Kielty and Grant, 2002), although in many cases their specic signicance, if any, in relation to the organisation and function of the sclera has yet to be determined. Of these, type XII collagen is thought to be associated with type I brils in human sclera, as well as cornea, but is expressed as different isoforms with only the long form expressed in the sclera (Wessel et al., 1997; Anderson et al., 2000). Types XII and XIV may be important in collagen brillogenesis in development of ocular connective tissues (Young et al., 2002). The type XVIII collagen gene has been implicated in the development of high myopia and is known to be expressed in the human eye (Suzuki et al., 2002). 5.2. Elastin Elastic bres consisting of microbrillar and amorphous components represent an additional brillar system supplementing the collagen framework in the human sclera. At least 19 different proteins can be identied within the elastic bre system (Gimeno et al., 2001). They rst appear as ne microbrils at week 72 in human embryonic development, forming larger composite deposits by week 18 (Sellheyer and Spitznas, 1988). Elastin is composed of nonpolar hydrophobic amino acids such as alanine, valine, isoleucine and leucine and contains little hydroxyproline and no hydroxylysine. It also contains two unique amino acids, desmosine and isodesmosine, which serve to cross-link the polypeptide chains (Postlethwaite and Kang, 1988). Biochemical analysis showed the elastin component of adult human sclera to be around 2%, although this increases to 5% in the scleral spur and trabecular meshwork (Moses et al., 1978). Fibres are most abundant in the lamina fusca and innermost stromal layers and along the tension lines of the extraocular muscles (Marshall, 1995), but also exhibit localised concentrations at the equator, where the sclera is thinnest, at the limbus and optic disc. Morphometric analysis revealed four times as much elastin in the lamina cribrosa as in peripapillary sclera, while in equatorial sclera it was almost absent (Quigley et al., 1991). 5.3. Proteoglycans The interbrillar compartment in the scleral matrix is occupied primarily by proteoglycans (PGs), although they are sparsely represented compared to most other connective tissues with, for example, a four-fold higher concentration in cornea than sclera. Proteoglycans consist of a protein core to which variable numbers of sulphated glycosaminoglycan (GAG) side-chains are covalently attached (for review see rd et al., 2002). Decorin and Biglycan, members of Heinega the small leucine-rich repeat protein (SLRP) family, are the main PGs of human sclera, characterised by the presence of one and two glycosaminoglycan chains, respectively, plus
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
615
oligosaccharides (Coster and Fransson, 1981). The glycan chains in scleral PGs are mostly co-polymers of dermatan sulphate and chondroitin sulphate, although heparan sulphate and the unsulphated GAG hyaluronan have also been reported in small amounts in human sclera (Trier et al., 1990). Dermatan sulphate contains disaccharide repeats of two types: D -glucuronic-N-acetylgalactosamine and L -iduronate-N-acetylgalactosamine. Unlike DSPGs from other tissues, scleral DS is typically O-sulphated at the C-6 position on glucuronate-rich domains (Cheng et al., 1994). Human sclera also contains smaller amounts of the large PG, aggrecan, which is related to the large, aggregating PG of articular cartilage and characterised by the presence of keratan sulphate and chondroitin sulphate GAG chains (Rada et al., 1997). Rotary shadowing electron microscopy showed that the large PG in bovine sclera had similar domain structure to the well-known cartilage aggrecan (Ward et al., 1987). Decorin, biglycan and aggrecan proteoglycans are present throughout the full thickness of the tissue, although aggrecan is most abundant in the posterior sclera (Rada et al., 1997, 2000). SLRP PGs have been implicated in the regulation of collagen brillogenesis and thus may be important in scleral development and repair. Decorin, in particular, has been shown to deccelerate bril growth and increase bril diameter (Neame et al., 2000; Kuc and Scott, 1997). It also binds to TGFb (Takeuchi et al., 1994) and interacts with collagen types I, VI and XIV (Ehnis et al., 1997). A third SLRP PG, lumican, has also been identied in mouse sclera (Austin et al., 2002). This PG is the classical PG of corneal stroma, but its presence in human sclera has not yet been conrmed. Lumican is also able to inuence bril diameter and seems to be involved in determining transparency in the cornea as well (Chakravarti et al., 2000; Quantock et al., 2001). As with collagens, several new SLRPs have been discovered recently, including opticin which was rst
identied in the iris (Friedman et al., 2000). It is a minor component of the trabecular meshwork (Friedman et al., 2002), but has not so far been reported in the scleral stroma. Asporin also is a newly discovered SLRP (Henry et al., 2001), which seems to overlap in its expression with that of the main scleral PGs decorin and biglycan, but which again has not yet been conrmed in the eye. 5.4. Collagen proteoglycan interaction The narrow interbrillar spaces in scleral matrix would seem highly conducive for close apposition and interactions between brillar and nonbrillar components of the matrix. X-ray diffraction techniques applied to analyse collagen organisation and the arrangement of bril-associated structures in human sclera revealed axial density proles very similar to those recorded in rat tail tendon (Quantock and Meek, 1988). Anionic groups on GAG sidechains of matrix PGs have been exploited to visualise scleral PGs and their collagen associations, using cationic dyes, such as cuprolinic and cupromeronic blue (Young, 1985; Van Kuppevelt et al., 1987; Quantock and Meek, 1988). These methods reveal PGs as electron dense laments regularly distributed along the brils and closely-associated with the collagen brils at the d and e bands of the D-periodic cross banded axial pattern (Fig. 2). Application of decorin-specic antibodies and detection by sensitive immunogold particulate markers has since conrmed these structures as decorin PG, periodically associated with the collagen brils in human sclera (Kimura et al., 1995). Decorin binds to a site near the C terminal end of the type I collagen molecule (Keene et al., 2000). In addition, decorin was found to be associated with type VI collagen in the interbrillar space (Kimura et al., 1995). Accumulating evidence supports an important role for small leucine-rich PGs, particularly decorin in regulating
Fig. 2. Normal human sclera showing proteoglycans as ne laments (arrows) associated with collagen brils in longitudinal section. Bar represents 250 nm.
616
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
the topographical organisation of the collagen brillar matrix. Decorin thus appears to have a role in development and wound healing. Signicant reduction in decorin synthesis in the posterior sclera was found to be associated with elongation of the eye and development of myopia in marmosets (Rada et al., 2000). Decorin-null mice exhibit abnormal collagen brils in tendon and skin (Danielson et al., 1997). Surprisingly, lumican deciency in mice also causes scleral anomalies with larger brils in anterior and posterior scleral stroma, in spite of the small amounts of this PG present in the tissue (Austin et al., 2002). In contrast to studies on decorin, there is disagreement in published reports of the binding afnity of Biglycan for collagen type I rd et al., 2002), and no nherr et al., 1995; Heinega (Scho specic studies of biglycan in sclera have been carried out. 5.5. Cellular components All layers of the sclera exhibit low cellularity compared to most vascularised tissues. The indigenous cell is the scleral brocyte. The structural and functional integrity of the scleral connective tissue layers is dependent upon the biosynthetic activity of this cell population. Although few in number, they have extended cytoplasmic extensions which contact adjacent cells, forming a syncytium, similar to that reported in tendon where cells associate by gap junctions (McNeilly et al., 1996). In healthy tissue the cells appear elongate and closely-apposed to the collagen bundles. Only in the lamina fusca there is a noticeable increase in cell numbers, where the sclerocyte population is supplemented by numbers of melanocytes, the importance of which is unclear. Sclerocytes can undergo rapid tranformation into active broblasts following any insult to the sclera. This can be physical trauma, such as surgical incision, or chemotherapeutic as in the topical application of cytotoxic agents to the sclera for the treatment of neoplasia, or in the prevention of post-operative scarring. Scleral broblasts appear as stellate or spindle-shaped cells with a large nucleus and relatively scanty cytoplasm, containing conspicuous mitochondria and rough endoplasmic reticulum with attached ribosomes. The cells vary in size according to function so that, during secretory activity, the cytoplasm becomes lled with a Golgi zone, vacuoles, vesicles and lysosomes. The broblast is able to synthesise all of the component molecules of the matrix. Scleral brocytes, like other connective tissue cells are reactive to a broad range of cytokines including interferon g, growth factors (e.g. Platelet-derived growth factor (PDGF), transforming growth factor (TGFb) and broblast growth factor (FGF)), IL1 and thymocyte derived growth factors. Histiocytes, blast cells, granulocytes, lymphocytes and plasma cells can all occasionally be identied in small numbers in normal scleral stroma. Mast cells and eosinophils, characterised by the structure of their cytoplasmic granules, are also present. Mast cells are present in large quantities at the limbus, around blood vessels traversing
the sclera and in choroid, but are sparse in the iris, ciliary body and retina. Their function within the tissue matrix has not yet been fully dened, but they are active in acute inammatory states, including episcleritis and scleritis and also during the healing of scleral wounds. In response to an inammatory stimulus in the sclera, cells pass rapidly from blood vessels of the choroid and episclera, the rst arriving within minutes at the site of the insult (Watson and Hazleman, 1976). In contrast, choroidal and intraocular tumours rarely seem to penetrate the scleral coat. They may spread out of the globe through the emissary foramen, but usually have to cause a secondary inammation before the cells can penetrate the scleral barrier (Blatt et al., 1958). Inammatory cells readily dissolve intercellular macromolecules, but tumours rarely do so. The tumours do not appear to be conned by an inammatory reaction, but the cells which are able to migrate may well be dealt with elsewhere provided only a few manage to get outside the globe. Intraocular abscesses are conned by the scleral coat in the same way that any abscess will be restricted by a brous envelope. Reactive inammation of the episclera always accompanies intraocular or intrascleral abscesses, so that organisms which pass through the sclera are dealt with in the episclera itself.
6. Scleral hydration and uid transport Hydration of scleral tissue is closely related to the composition of the extracellular matrix. The proteoglycans regulate diffusional transport on account of the hydrophilic nature of their extended glycosaminoglycan side chains, such that the water content of sclera is around 68%. The possibility of drug delivery to the eye through a transcleral route has recently rekindled interest in scleral hydration and permeability (Boubriak et al., 2000). Sclera was found to exhibit a higher permeability to globular proteins than linear dextrans, with diffusion determined by molecular weight and, especially, by molecular radius (Ambati et al., 2000). Tissue hydration, together with brillar organisation is believed to be crucial for corneal transparency. Sclera contains a lower concentration of proteoglycans than cornea, which is reected in the three times greater swelling of cornea over sclera under experimental conditions (Huang and Meek, 1999). If the sclera is dehydrated, as can occur in retinal detachment procedures when the sclera is exposed for a prolonged period, then the sclera becomes more transparent. David Maurice was rst to suggest that this phenomenon might be explained by the increase in concentration of mucoprotein, through dehydration, near to that present in cornea (Maurice, 1969). Intraocular uid transport is vitally important to the health of the eye and involves the sclera via two distinct systems: the rst is represented by the scleral spur and its ciliary muscle and tendon attachments to the trabecular meshwork, in the conventional outow of aqueous to
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
617
Schlemms canal. The second is uveoscleral outow, which accounts for 40% of aqueous outow. This falls with age to between 4 and 14% in those over 60, possibly as a result of the changes which occur within the sclera with age. Uveoscleral outow was demonstrated by Bill (1965) who perfused radio-iodinated albumen into the anterior chamber of cynomolgus monkeys and recovered the tracer from the anterior and posterior sclera within 25 minutes. The uid passes through the spaces between the ciliary muscle bres into the suprachoroidal space and exits around the vortex veins, other emissary channels and through the sclera itself. The driving force for this movement is diffusion. The gradient is jointly determined by the hydrostatic pressure of the aqueous, the permeability of the ciliary muscle and sclera and the osmotic pressure in the suprachoroidal space, which is in turn determined by the concentration of plasma protein in the surrounding vessels. The role of the sclera in uveo-scleral outow is indirectly of considerable importance for the viability of the retinal pigment epithelium and thus the apposition of the retina to this epithelium.
bres crisscross around the axons of the optic nerve to form the lamina cribrosa. The end point of development is determined by the rates of growth, development and function of the adjacent structures, lens, retina and choroid and the production of aqueous by the ciliary body. The postnatal sclera is thin and translucent, allowing the blue colour of the underlying uvea to show through during the rst 3 years of life.
8. Age changes in sclera The human sclera reaches its adult size and maximum elasticity at the age of 12 13 years, after which there is a progressive reduction in compliance and an increase in rigidity. Increased scleral rigidity, as in other connective tissues, is the result of a progressive cross-linking of the lysine residues of collagen with age (Keeley et al., 1984), by either enzyme-dependent or independent pathways. As age increases the sclera becomes increasingly yellow as a consequence of the deposition of fat globules between the collagen bres. There seems to be no age-related change in collagen content or type, or between the anterior and posterior segments in the sclera. However, turnover of ocular collagens in general declines with age, although types III and VI may show smaller changes than type I, according to studies of collagen mRNA in ageing mice (Ihanamaki et al., 2001). The collagen bres become thicker and less uniform with increased age, particularly in the region of the muscle insertions. Here the sclera becomes progressively thinned, increasing the colour contrasts between one part of the sclera and the next. If the bres become disrupted then calcium deposition can occur, leading to the production of hyaline plaques. PG components of human sclera increase in concentration until the fourth decade. Thereafter decorin and biglycan undergo a steady decline, with aggrecan concentration maintained until the ninth decade (Rada et al., 2000). The number of elastic bres also falls between the second and seventh decade, particularly in the anterior segment. This is reected in the different behaviour of the surgically incised sclera in the young and the old. Biochemical analysis of the aging lamina cribrosa revealed an increase in total collagen, pentosidine collagen cross-links, and also elastin, and a decrease in type III collagen and sulphated glycosaminoglycans (Albon et al., 1995, 2000a). Assessment of the mechanical properties of aging lamina demonstrated an associated increase in rigidity and loss of compliance of the tissue (Albon et al., 2000b).
7. Scleral development Embryologically, the sclera has dual origins arising from both mesodermal and neural crest primordia. The mesoderm contributes directly to only a small strip of temporal sclera, whilst the extraocular muscles, vascular endothelium and ocular adnexae form entirely from this source. The remaining connective tissues of the eye and the pericytes of the ocular vessels are all derived from the neural crest. As with sclera, many other connective tissues are of neural crest-mesodermal origin, including cartilage, bone, ligament, tendon, dermis and perivascular smooth muscle and their maturation follows a very similar pattern and time scale. This may explain, at least in part, the frequent association of sclera and joints in many systemic diseases. A microscopical study of human embryos and foetuses by Sellheyer and Spitznas (1988) showed that development of the sclera begins anteriorly during the seventh gestational week. It is probable that the differentiating uvea, and in particular the pigment epithelium, is responsible for the induction of the sclera (Gruenwald, 1944). Certainly if the outer layer of the optic vesicle is destroyed, the sclera does not develop (Giroud, 1957). Cellular changes, as indicated by the loss of free ribosomes and polysomes and an increase in the rough endoplasmic reticulum of the developing scleral cells, progress from the presumptive limbus, both posteriorly and from inside outwards. There is a marked increase in glycogen and lipid in the outer (episcleral) sclera from 7 to 10 weeks, but none thereafter. Elastic microbrils are found at week 7, but these do not develop into elastic deposits until week 18 (Sellheyer and Spitznas, 1988), possibly in response to intraocular pressure (Ozanics et al., 1976). By the fourth month the scleral spur appears as circularly oriented bres and by the fth month, scleral
9. Scleral disease Frequently the more that is learnt about any condition the more it is realised that there may be many causes leading to
618
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
a particular clinical state. This is true in scleral disease scleritis is not a single entity. Histologically, it is an inammatory response that can be simulated by a variety of conditions consisting predominantly of lymphocytes with few polymorphs and plasma cells. Importantly, whatever the underlying condition, whether it be Goodpastures syndrome, Wegeners granulomatosis or Herpes zoster, the histological appearance is that of a rheumatoid nodule with a central area of necrosis surrounded by a zone of histiocytes and polymorphs and an outer zone of lymphocytes and plasma cells. Vasculitis is detectable in the majority (Fong et al., 1991). The transformation from normal tissue to necrosis was demonstrated in studies by Young and Watson (1984a,b) in various types of scleritis. The ndings were remarkably similar whatever the underlying disease. Correlative uorescein angiography and electron microscopy were used to study the sclera, at sites from apparently normal tissue to the centre of a necrotic area, in an eye from a 52 year-old man with severe necrotising scleritis (Watson and Young, 1985). Changes were found in both the vasculature and the collagen and proteoglycans. At the periphery of the lesion there was activation of brocytes in the absence of inammatory cells. This broblastic transformation may be one of the earliest events in scleral degradation in necrotising disease. Activation of brocytes is associated with breakdown of proteoglycan linkages between brils and later leads inexorably to complete loss of the proteoglycans from the scleral interbrillar matrix (Young et al., 1988). Removal of proteoglycans allows collagen brils to unwind (Fig. 3), to separate from their fellows, and eventually to be digested. Whether atypical proteoglycans are produced during this process, giving rise to the appearance of brinoid is unclear. The degradation of scleral collagen occurs by both intracellular and
extracellular mechanisms. Cells resembling active broblasts and macrophages can be seen to phagocytose collagen brils into vacuoles associated with dense, intracellular cytoplasmic granules. In the extracellular matrix, collagen brils in large areas of the scleral stroma appear swollen and unravelled or completely solubilised, without close association with stromal cells (Fig. 4). Both activation and degeneration of stromal brocytes are evident in zones of extracellular bril degradation. Closer to the lesion in an area apparently unperfused on the uorescein angiogram, the venules showed high endothelial changes with migration of polymorphs, lymphocytes, plasma cells, macrophages and a large number of mast cells into the surrounding tissue. Adjacent to the ulcerated lesion the vessels appeared to be completely obstructed with the endothelium largely destroyed. These cellular reactions and the nal end point of tissue destruction are strikingly similar to those found in the systemic connective tissue diseases, commonly associated with scleritis (Rao et al., 1985). Many of the aetiological factors that lead to the systemic disorders also apply to the eye. The disorders share common proposed immune aetiologies although, as in the case of scleritis, neither the initiating antigen nor the processes that result in chronicity have been dened. The reason why inammation occurs in sclera is because of its unique anatomical and vascular characteristics, which permit transudation into tissue from which there is sluggish clearance, allowing intense immune reactions to occur and persist. Recent experimental data and clinical observations (alongside comparisons with similar disease processes elsewhere), which have enabled a better understanding of the possible pathogenesis of scleritis have also allowed novel and successful treatment strategies to be formulated.
Fig. 3. Sclera adjacent to a degradative lesion in necrotising scleritis shows loss of proteoglycan laments, separation of collagen brils and appearance of axial striations along brils (arrows), which may be early stages of bril breakdown. Bar represents 300 nm.
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
619
Fig. 4. Advanced degradation of scleral stroma central to a lesion in necrotising scleritis illustrates swelling and unravelling of individual collagen brils. Bar represents 1 mm.
The immune system operates as an integrated system of protection allowing the effective elimination of microbial pathogens. The innate immune system provides a hardwired defence system that is activated following exposure to characteristic microbial motifs. Cells of the innate immune system, particularly dendritic cells, respond to activation by migrating to local lymph nodes where they can present peptide components from the offending pathogen on surface MHC molecules to cells of the more exible adaptive immune response. The trigger for this response in the sclera can be from without, as with infections or trauma, or within, when it is part of the manifestations of another systemic disease (for reviews, see Foster and Sainz de la Maza, 1993; Watson et al., 2003). The conjunctiva, tears and cornea are in constant contact with bacteria, viruses and other potential antigens, but the defence mechanisms are sophisticated and the episclera and sclera are protected from surface antigens by the conjunctiva. However, Herpes simplex can be cultured from the conjunctival sac in the initial attack in some patients and multiple systemic infections have been reported with scleral disease (Watson and Hayreh, 1976; Sainz de la Maza et al., 1993). Epstein-Barr virus, parvoviruses and mycobacteria have been implicated in the production of rheumatoid arthritis (Alspaugh et al., 1981), but although it is known that the eye can be a portal for entry of viruses into the body, there is no evidence to suggest this is a reason for the onset of scleral disease in patients with rheumatoid arthritis. Equally in Herpes zoster infection, which is frequently associated with scleral disease, it is likely that the scleral inammatory response is induced by virus particles, which have gained retrograde entry into the sclera via the nerves and have induced a localised immune reaction as a response to virally-induced destruction of tissue. Viruses induce a tissue reaction by either changing the host responses once
they become intracellular, or by inducing cellular expression of abnormal proteins, which in turn could render that tissue antigenic (Robb, 1977). Some such mechanism is certainly possible in scleral inammation. Considering the constitutional similarity between the joint and the sclera, it is highly probable that those factors which trigger the onset of joint problems in connective tissue disease, such as rheumatoid arthritis, will also be present in the sclera. Scleral involvement may arise following local trauma, as in surgically-induced necrotising scleritis (SINS). Here, sequestered antigen becomes exposed in an individual already primed to induce a response as a result of systemic disease. Alternatively, a response may be induced because of the unique vascular supply of the anterior segment of the eye. 9.1. Antigen and immune complexes in scleritis No specic antigenic stimulus for the immuno-inammatory cascade in patients with scleritis has yet been detected. In contrast, in Moorens ulcer of the cornea, the defensin protein Calgranulin C is now thought to be the antigenic stimulus (Akpek et al., 2000). A 54 kDa epithelial antigen has been found in association with corneal changes in rheumatoid arthritis (John et al., 1992) and a 70 kDa antigen in Wegeners granulomatosis (John et al., 1992). Further research is required to identify the initial antigenic response in necrotising scleritis. Candidate antigens may reside in matrix proteoglycans and collagens, which as we have shown, are modied early in the disease process (Young et al., 1988). Disruption of proteoglycan collagen interactions is induced by metalloproteinase enzymes released from activated broblasts (Di Girolamo et al., 1995; Riley et al., 1995; Di Girolamo et al., 1997). Once the proteoglycan has been stripped from the bril this collagen
620
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
could become antigenic as it is a sequestered antigen, never having been exposed to the immune systems tolerance mechanisms. These antigens could be presented to the immune system through the antigen presenting cells, such as tissue macrophages. The subsequent T cell response resulting in cytokine release could in turn induce collagenase release from the brocytes. At this stage an acute proinammatory stimulus, such as a virus infection, would perpetuate the response. Such an intense immune response may remain localised, as in Herpes zoster infection or some cases of SINS, or it may become generalised, spreading and progressing to full-blown necrotising disease. The role of immune complexes is uncertain. They were clearly demonstrated in the sclera in an experimental model of scleritis (Hembry et al., 1979). Fibrocytes were activated and the immune complexes were resorbed by the local vessels, inducing a transient but very severe necrotic vasculitic response, which could be detected by angiography and conrmed with histology. As the response did not occur at the site of maximum concentration of antigen, this suggests that the balance of antigen and/or antibody is critical for the vasculitic response to take place. 9.2. Vasculitic response The great majority of patients with any form of scleritis and all those with necrotising disease have a microvascular inammation, the result of either local or systemic disease. The sclera itself does not contain a capillary plexus but derives its nutrition from the episcleral plexuses which overly it and, in the case of the equatorial and posterior sclera, from the choroidal vessels beneath it. The episcleral vessels themselves lose their muscular coat at their origin from the choroidal vessels and consist of simple, walled tubes of endothelial cells surrounded by basement membrane and a discontinuous layer of pericytes. This unusual structure results in the arterial side of the vascular network being thrown into tortuous folds through which the blood ow is turbulent and, because of the artery to artery anastomoses, the circulation is sluggish or oscillatory. As a consequence the normal mechanisms for removing immune complexes and other potentially noxious substances cannot function and this, together with the poor lymphatics, means that inammatory reactions and micro-vascular changes can easily occur and persist at these sites. It is a common clinical observation that scleritis often begins and spreads from the areas between the recti muscles, the area in which the circulation is slowest. Histological examination and angiography of the scleral vessels reveal a variety of changes varying from simple permeability, as in diffuse episcleritis, to complete endothelial destruction and vascular occlusion in severe necrotising scleritis (Nieuwenhuizen et al., 2003). Most specimens show an inammatory microangiopathy with neutrophil inltration in and around the vessel wall in which is deposited IgG. If the inammatory response is severe,
there is evidence of collagen destruction. In addition the endothelial cells rst swell and, in the vaso-occlusive form of the disease, occlude the lumen. This change is usually transient but sometimes a platelet thrombus will form. In the most severe disease the endothelial cells become necrotic, leading to permanent occlusion of the vessels, which are often replaced by new ones, a common feature in patients with a systemic vasculitis. Angiographically, these changes can be detected by the remodelling of the vascular plexus. The swollen endothelium allows almost any cell to move outwards from the vessel into the extravascular space with the consequent release of cytokines and the induction of an inammatory lesion (Michel and Curry, 1999). At the same time, the vascular endothelium upregulates HLA DR Class II and thus the vessel itself becomes a target for attack. If severe this vasculitic process can affect even the largest vessels in the eye. Even though a vessel may appear clinically normal it may still be inamed and incompetent. At present this localised inammation can only be detected by ICG angiography, which may also disclose if the inammation is fully controlled. 9.3. Cellular responses So many reactions can occur within the span of even 1 hr that it is extremely difcult to be certain of the sequence of events when many cell types are present in any one specimen, but the predominant cells found in all those with scleritis are the macrophage and the CD4 T-lymphocyte. (Young and Watson, 1984a; Bernauer et al., 1994; Diaz-Valle et al., 1998). There are few or no macrophages, Langerhans cells, neutrophils or lymphocytes in normal human sclera. After scleral inammation, however, there is a marked increase in T-helper lymphocytes with a high Thelper to T-suppressor ratio (Bernauer et al., 1994). No genetic predisposition to scleritis has been established (Joycey et al., 1997), although there is a possibility that possession of the HLA-DR15(2) phenotype may predispose to corneal ulceration in response to an inammatory stimulus. Immunogenetic susceptibility may however be important in the development of some of the systemic vasculitic disorders associated with scleritis. In rheumatoid arthritis, the connective tissue disorder most frequently associated with scleritis, the class II major histocompatibility complex (MHC) locus is associated with susceptibility to rheumatoid joint disease. A majority of patients with rheumatoid arthritis carry HLA-DR4, HLADR1, or both. HLA-DR4 has ve subtypes, two of which (Dw4 and Dw14) are present in 50 and 35% of patients with rheumatoid arthritis, respectively. Bernauer et al. (1994) analysed the inammatory cellular effector mechanisms in scleritis. The inammatory cells inltrating the scleral tissues were mainly T lymphocytes and macrophages. There was a predominance of CD4 positive cells, but only few lymphocytes were activated (expressed IL-2 receptor). Clusters of B cells were found in
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623
621
perivascular areas. Signs of a granulomatous process with activated macrophages (epithelioid and giant cells) were present in necrotising scleritis. Expression of major histocompatibility, class II molecules (MHC II) was found on lymphocytes and rarely on macrophages. Sainz de la Maza et al. (1994) found similar changes of HLA-DR expression and increased T helper participation in 10 patients, nine of whom had an underlying autoimmune vasculitis systemic disease. The cellular inltrate in scleritis thus shows, at least at certain stages, features compatible with a T cell mediated (autoimmune) disorder.
References
Akpek, E.K., Liu, S.H., Gottsch, J.D., 2000. Induction of experimental autoimmume keratitis by adoptive transfer of human corneal antigen specic T cell line. Invest. Ophthalmol. Vis. Sci. 41, 41824188. Albon, J., Karwatowski, W.J., Avery, N., Easty, D.L., Duance, V.C., 1995. Changes in the collagenous matrix of the aging human lamina cribrosa. Br. J. Ophthalmol. 79, 368375. Albon, J., Karwatowski, W.J., Easty, D.L., Sims, T.J., Duance, V.C., 2000a. Age related changes in the non-collagenous components of the extracellular matrix of the human lamina cribrosa. Br. J. Ophthalmol. 84, 311 317. Albon, J., Puslow, P.P., Easty, D.L., Karwatowski, W.S., 2000b. Mechanical compliance of the aging human lamina cribrosa. Br. J. Ophthalmol. 84, 318 323. Alspaugh, M.A., Henle, G., Lennette, E.T., Henle, W., 1981. Elevated levels of antibodies to Epstein-Barr virus antigens in sera and synovial uids of patients with rheumatoid arthritis. J. Clin. Invest. 67, 11341139. Ambati, J., Canakis, C.S., Miller, J.W., Gragoudas, E.S., Edwards, A., Weissgold, D.J., Kim, I., Delori, F.C., Adamis, A.P., 2000. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 41, 11811185. Anderson, D.R., 1969. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch. Ophthalmol. 82, 800 804. Anderson, S., SandarRaj, S., Fite, D., Wessel, H., SundarRaj, N., 2000. Developmentally regulated appearance of spliced variants of type XII collagen in the cornea. Invest. Ophthalmol. Vis. Sci. 41, 55 63. Austin, B.A., Coulon, C., Liu, C.Y., Kao, W.W., Rada, J.A., 2002. Altered collagen bril formation in the sclera of lumican-decient mice. Invest. Ophthalmol. Vis. Sci. 43, 16951701. Axenfeld, K.T., 1907. Accessory episcleral ciliary ganglia. Von Graefe. Arch. Ophthalmol. 34, 300. Bernauer, W., Watson, P.G., Daiker, B., Lightman, S., 1994. Cells perpetuating the inammatory response in scleritis. Br. J. Ophthalmol. 78, 381 385. Bill, A., 1965. Movement of albumin and dextran through the sclera. Arch. Ophthalmol. 74, 248 252. Blatt, N., Ursu, A., Popovici, V., 1958. Invasion potential of malignant t. Augenheil. 132, 808 828. intraocular tumours. Klin. Monatsbla Borcherding, M.S., Blacik, L.J., Sittig, R.A., Bizzel, J.W., Breen, M., Weinstein, H.G., 1975. Proteoglycans and collagen bre organization in human corneoscleral tissue. Exp. Eye Res. 21, 5970. Boubriak, O.A., Urban, J.P., Akhtar, S., Meek, K.M., Bron, A.J., 2000. The effect of hydration and matrix composition on solute diffusion in rabbit sclera. Exp. Eye Res. 71, 503 514. Bussacca, A., 1947. Les vaisseaux lymphatiques de la conjonctivite bulbaire etudies par la methode des injections vitals be Bleu Tripan. Bull. Soc. Fr. Ophthalmol. 60, 8487. Chakravarti, S., Petroll, W.M., Hassell, J.R., Jester, J.V., Lass, J.H., Paul, J., Birk, D.E., 2000. Corneal opacity in lumican-null mice: defects in
collagen bril structure and packing in the posterior stroma. Invest. Ophthalmol. Vis. Sci. 41, 33633373. rd, D., Malmstrom, A., Schmidtchen, A., Yoshida, K., Cheng, F., Heinega Fransson, L.-A., 1994. Patterns of uronosyl epimerization and 4-/6-Osulphation in chondroitin/dermatan sulphate from decorin and biglycan of various bovine tissues. Glycobiology 4, 685 696. Coster, L., Fransson, L.-A., 1981. Isolation and characterization of dermatan sulphate proteoglycans from bovine sclera. Biochem. J. 193, 143153. Danielson, K.G., Baribault, H., Holmes, D.F., Graham, H., Kadler, K.E., Iozzo, R.V., 1997. Targetted disruption of decorin leads to abnormal collagen bril morphology and skin fragility. J. Biol. Chem. 136, 729 743. Di Girolamo, N., Lloyd, A., McCluskey, P., Filipic, M., Wakeeld, D., 1997. Increased expression of matrix metalloproteinases in vivo in scleritis tissue and in vitro in cultured human scleral broblasts. Am. J. Pathol. 150, 653 658. Di Girolamo, N., McCluskey, P.J., Lloyd, A., et al., 1995. Localisation of stromelysin matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase TIMP-1 mRNA in scleritis. Ocul. Immunol. Inamm. 3, 181 184. Diaz-Valle, D., Benitez de Castillo, J.M., Castillo, A., 1998. Immunologic and clinical evaluation of post surgical necrotic scleral ulceration. Cornea 17, 371375. Ehnis, T., Dieterich, W., Bauer, M., Kresse, H., Schuppan, D., 1997. Localization of a binding site for the proteoglycan decorin on collagen XIV (undulin). J. Biol. Chem. 272, 20414 20419. Fong, L.P., Sainz de la Maza, M., Rice, B.A., Kupferman, A.E., Foster, C.S., 1991. Immunopathology of scleritis. Ophthalmology 98, 472 476. Foster, C.S., Sainz de la Maza, M., 1993. The Sclera. Chapters 6 and 7, Springer, Berlin. Friberg, T.R., Lace, J.W., 1988. A comparison of the elastic properties of human choroid and sclera. Exp. Eye Res. 47, 429436. Friedman, J.S., Ducharme, R., Raymond, V., Walter, M.A., 2000. Isolation of a novel iris-specic and leucine-repeat protein (oculoglycan) using differential selection. Invest. Opthalmol. Vis. Sci. 41, 20592066. Friedman, J.S., Faucher, M., Hiscott, P., Biron, V.L., Malenfant, M., Turcotte, P., Raymond, V., Walter, M.A., 2002. Protein localization in the human eye and genetic screen of opticin. Hum. Mol. Genet. 15, 13331342. Gimeno, M.J., Bellon, J.M., Bujan, J., 2001. Ocular changes associated with connective tissue disorders: role of the elastic and collagen components. Arch. Soc. Esp. Oftalmol. 76, 459469. nome ` nes dinduction et leurs perturbations chez Giroud, M., 1957. Phe ` res. Acta Anat. 30, 297306. mammife Gross, R.L., 1999. Collagen type I and III synthesis by Tenons capsule broblasts in culture: individual patient characteristics and response to mitomycin C, 5-uorouracil and ascorbic acid. Trans. Am. Ophthalmol. Soc. 97, 513 543. Gruenwald, P., 1944. Studies on developmental pathology II. Sporadic unilateral microphthalmia and associated malformations in chick embryos. Am. J. Anat. 74, 217 257. Hamanaka, T., 1989. Scleral spur and ciliary muscle in man and monkey. Jpn. J. Ophthalmol. 33, 221 236. Heathcote, J.G., 1994. Collagen and its disorders. In: Garner, A., Klintworth, G.K. (Eds.), Pathobiology of Ocular Disease. A Dynamic Approach, 2nd Ed., Marcel Dekker, New York, pp. 10331084. n, A., Lorenzo, P., 2002. Glycosylated rd, D., Aspberg, A., Franze Heinega matrix proteins. In: Royce, R.M., Steinmann, B. (Eds.), Connective Tissue and its Heritable Disorders, 2nd Ed., Wiley-Liss, pp. 271291, Chapter 4. Hembry, R.M., Playfair, J., Watson, P.G., Dingle, J.T., 1979. Experimental model for scleritis. Arch. Ophthalmol. 97, 13371340. Henry, S.P., Takanosu, M., Boyd, T.C., Mayne, P.M., Eberspaecher, H., Zhou, W., de Crombrugghe, B., Hook, M., Mayne, R., 2001. Expression pattern and gene characterization of asporin, a newly discovered
622
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623 Michel, C.C., Curry, F.E., 1999. Microvascular permeability. Physiol. Rev. 79, 703706. Morrison, J.C., Van Birskirk, M., 1983. Anterior collateral circulation in the primate eye. Ophthalmology 90, 707715. Moses, R.A., Grodzki Jr., W.J., Starcher, B.C., Galione, M.J., 1978. Elastin content of the scleral spur, trabecular mesh, and sclera. Invest. Ophthalmol. Vis. Sci. 17, 817818. Neame, P.J., Kay, C.J., McQuillan, D.J., Beales, M.P., Hassell, J.R., 2000. Independent modulation of collagen brillogenesis by decorin and lumican. Cell Mol. Life Sci. 57, 859 863. Newton, R.H., Meek, K.M., 1998. Circumcorneal annulus of collagen brils in the human limbus. Invest. Ophthalmol. Vis. Sci. 39, 11251134. Nieuwenhuizen, J., Watson, P.G., Jager, M., Emmanouilidis-van der Spek, K., Keunen, J., 2003. The value of combining anterior segment uorescein angiography with indocyanine green angiography in scleral inammation. Ophthalmology 110:1653 1666. Ozanics, V., Rayborn, M., Sagun, D., 1976. Some aspects of corneal and scleral differentiation in the primate. Exp. Eye Res. 22, 305 327. Pace, J.M., Corrado, M., Missero, C., Byers, P.H., 2003. Identication, characterization and expression of a new brillar collagen gene, COL27A1. Matrix Biol. 22, 314. Parry, D.A.D., Craig, A.S., 1984. Growth and development of collagen brils in connective tissue. In: Ruggeri, A., Motta, P. (Eds.), Ultrastructure of the Connective Tissue Matrix, Martinus Nijhoff, Boston, MA, pp. 34 64. Polatnick, J., Tessa, A.J., Katzin, H.M., 1957. Comparison of collagen preparations from beef cornea and sclera. Biochim. Biophys. Acta 26, 365 369. Postlethwaite, E.A., Kang, A.H., 1988. Fibroblasts. In: Gallin, J.I., Goldstein, I.M., Snyderman, R. (Eds.), Inammation: Basic Principles and Clinical Correlates, Raven, New York, pp. 747 774. Quantock, A.J., Meek, K.M., 1988. Axial electron density of human scleral collagen. Location of proteoglycans by X-ray diffraction. Biophys. J. 54, 159164. Quantock, A.J., Meek, K.M., Chakravarti, S., 2001. An X-ray diffraction investigation of corneal structure in lumican-decient mice. Invest. Ophthalmol. Vis. Sci. 42, 17501756. Quigley, H.A., Dorman-Pease, M.E., Brown, A.E., 1991. Quantitative study of collagen and elastin of the optic nerve head and sclera in human and experimental monkey glaucoma. Curr. Eye Res. 10, 877888. Rada, J.A., Achen, V.R., Perry, C.A., Fox, P.W., 1997. Proteoglycans in the human sclera. Evidence for the presence of aggrecan. Invest. Ophthalmol. Vis. Sci. 38, 17401751. Rada, J.A., Achen, V.R., Penugonda, S., Schmidt, R.W., Mount, B.A., 2000. Proteoglycan composition in the human sclera during growth and aging. Invest. Ophthalmol. Vis. Sci. 41, 1639 1648. Rao, N., Marak, G., Hidayat, A., 1985. Necrotising scleritis: a clinicopathological study of 41 cases. Ophthalmoogy 92, 15421549. Riley, G.P., Harrall, R.L., Hazleman, B.L., Watson, P.G., 1995. Collagenase (MMP1) and (TIMP) in destructive corneal disease associated with rheumatoid arthritis. Eye 9, 703718. Rhenberg, M., Ammitzboll, T., Tengroth, B., 1987. Collagen distribution in the lamina cribrosa and the trabecular meshwork of the human eye. Br. J. Ophthalmol. 71, 886892. Robb, J.A., 1977. Virus-cell interactions: a classication of virus causing human disease. Prog. Med. Virol. 23, 5160. Roth, A., Muhlendyck, H., de Gottrau, P., 2002. The function of Tenons capsule revisited. J. Fr. Ophthalmol. 25, 968 976. Sainz de la Maza, M., Foster, C.S., Jabbur, N.S., 1994. Scleritis associated with rheumatoid arthritis and with other systemic autoimmune diseases. Ophthalmology 101, 1281 1286. Sainz de la Maza, M., Hemady, R.K., Foster, C.S., 1993. Infectious scleritis: report of four cases. Doc. Ophthalmol. 83, 33 41. nherr, E., Witsch-Prehm, P., Harrach, B., Robenek, H., Rauterberg, J., Scho Kresse, H., 1995. Interaction of biglycan with type I collagen. J. Biol. Chem. 270, 27762783.
member of the leucine-rich repeat protein family. J. Biol. Chem. 276, 1221212221. Hogan, M.J., Alvarado, J.A., Weddell, J.E., 1971. Histology of the Human Eye, The Sclera, Saunders, Philadelphia, PA, Chapter 5, p. 194. Huang, Y., Meek, K.M., 1999. Swelling studies on the cornea and sclera: the effects of pH and ionic strength. Biophys. J. 77, 16551665. Ihanamaki, T., Salminen, H., Saamanen, A.M., Pelliniemi, L.J., Hartmann, D.J., Sandberg-Lall, M., Vuorio, M.E., 2001. Age-dependent changes in the expression of matrix components in the mouse eye. Exp. Eye Res. 72, 423 431. John, S.L., Morgan, K., Tullo, A.B., Holt, P.J., 1992. Corneal autoimmunity in patients with peripheral ulcerative keratitis in association with rheumatoid arthritis and Wegeners Granulomatosis. Eye 6, 630636. Joycey, V.C., Roger, J.H., Ashwoth, F., Watson, P.G., 1997. Parrallel studies of HLA antigens in patients with rheumatic disease and scleritis: comparison with three control populations. J. Rheumatol. Suppl. 3, 8488. Keeley, F.W., Morin, J.D., Vesely, S., 1984. Characterisation of collagen from normal human sclera. Exp. Eye Res. 39, 533 542. chinger, H.P., Burgeson, R.E., 1987. Type III Keene, D.R., Sakai, L.Y., Ba collagen can be present on banded collagen brils regardless of bril diameter. J. Cell. Biol. 105, 23932402. Keene, D.R., San Antonio, J.D., Mayne, R., McQuillan, D.J., Sarris, G., Santoro, S.A., Iozzo, R.V., 2000. Decorin binds near the C-terminus of type I collagen. J. Biol. Chem. 275, 2180121804. Kielty, C.M., Grant, M.E., 2002. The collagen family: structure, assembly and organization in the extracellular matrix. In: Royce, R.M., Steinmann, B. (Eds.), Connective Tissue and its Heritable Disorders, Wiley-Liss, pp. 159221, Chapter 2, Part 1. Kimura, S., Kobayashi, M., Nakamura, M., Hirano, K., Awaya, S., Hoshino, T., 1995. Immunoelectron microscopic localization of decorin in aged human corneal and scleral stroma. J. Electron Microsc. 44, 445 449. Komai, Y., Ushiki, T., 1991. The three-dimensional organisation of collagen brils in the human cornea and sclera. Invest. Ophthalmol. Vis. Sci. 32, 22442258. Konomi, H., Hayashi, T., Sano, J., Terato, K., Nagai, Y., Arima, M., Nakayasu, K., Tanaka, M., Nakajima, A., 1983. Immunohistochemical localization of type I, III and IV collagens in the sclera and choroids of bovine, rat, and normal and pathological human eyes. Biomed. Res. 4, 451 458. Kuc, I.M., Scott, P.G., 1997. Increased diameters of collagen brils precipitated in vitro in the presence of decorin from various connective tissues. Connect. Tissue Res. 36, 287296. Marshall, G.E., 1995. Human scleral elastic system: an immunoelectron microscopic study. Br. J. Ophthalmol. 79, 5764. Marshall, G.E., Konstas, A.G., Lee, W.R., 1990. Immunogold localisation of type IV collagen and laminin in the aged human outow system. Exp. Eye Res. 51, 691 699. Marshall, G.E., Konstas, A.G., Lee, W.R., 1991. Immunogold ultrastructural localisation of collagens in the aged human outow system. Ophthalmology 98, 692700. Marshall, G.E., Konstas, A.G., Lee, W.R., 1993. Collagens in the aged human macular sclera. Br. J. Ophthalmol. 12, 143153. Maurice, D.M., 1969. The cornea and sclera. In: Davson, H., (Ed.), 2nd Ed., The Eye, vol. 1. Little Brown, Boston, MA, pp. 489600. Mayne, R., 2002. Morphological and chemical composition of connective tissue: the eye. In: Royce, R.M., Steinmann, B. (Eds.), Connective Tissue and its Heritable Disorders, 2nd Ed., Wiley-Liss, pp. 145157, Chapter 1. McNeilly, C.M., Banes, A.J., Benjamin, M., Ralphs, J.R., 1996. Tendon cells in vivo form a three-dimensional network of cell processes linked by gap junctions. J. Anat. 189, 593 600. Meek, K.M., Fullwood, N.J., 2001. Corneal and scleral collagensa microscopists perspective. Micron 32, 261272. Meyer, P.A.R., 1988. Patterns of blood ow in episcleral vessels studied by low dose uorescein videoangiography. Eye 2, 533536.
P.G. Watson, R.D. Young / Experimental Eye Research 78 (2004) 609623 Selbach, J.M., Gottanka, J., Wittman, M., Lutjen-Drecoll, E., 2000. Efferent and afferent innervation of primate trabecular meshwork and scleral spur. Invest. Ophthalmol. Vis. Sci. 41, 2184 2191. Selbach, J.M., Schonfelder, U., Funk, R.H., 1998. Arteriovenous anastomoses of the episcleral vasculature in the rabbit and rat eye. J. Glaucoma 7, 5070. Sellheyer, K., Spitznas, M., 1988. Development of the human sclera. A morphological study. Graefes. Arch. Clin. Exp. Ophthalmol. 226, 89100. Shauly, Y., Miller, B., Lichtig, C., Modan, M., Meyer, E., 1992. Tenons capsule: ultrastructure of collagen brils in normals and infantile esotropia. Invest. Ophthalmol. Vis. Sci. 33, 651 656. Spitznas, M., 1971. The ne structure of human scleral collagen. Am. J. Ophthalmol. 71, 68. Stone, R.A., Kuwayama, Y., Laties, A.M., 1987. Regulatory peptides in the Eye. Experientia 43, 791 800. Suzuki, O.T., Sertie, A.L., der Kaloustian, V.M., Kok, F., Carpenter, M., Murray, J., Czeizel, A.E., Klieman, S.E., Rosemberg, S., Monteiro, M., Olsen, B.R., Passos-Bueno, M.R., 2002. Molecular analysis of collagen XVIII reveals novel mutations, presence of a third isoform, and possible genetic heterogeneity in Knobloch syndrome. Am. J. Hum. Genet. 71, 13201329. Takeuchi, Y., Kodama, Y., Matsumoto, T., 1994. Bone matrix decorin binds transforming growth factor-beta and enhances its bioactivity. J. Biol. Chem. 269, 3263432638. Tengroth, B., Rehnberg, M., Amitzboll, T., 1985. A comparative analysis of the collagen type and distribution in the trabecular meshwork sclera lamina cribrosa and the optic nerve in the human eye. Acta Ophthalmol. Suppl. 173, 9193. Thale, A., Tillmann, B., 1993. The collagen architecture of the sclera SEM and immunohistochemical studies. Anat. Anz. 175, 215 220. Thale, A., Tillmann, B., Rochels, R., 1996a. Scanning electron microscopic studies of the collagen architecture of the human scleranormal and pathological ndings. Ophthalmologica 2103, 137141. Thale, A., Tillmann, B., Rochels, R., 1996b. SEM studies of the collagen architecture of the human lamina cribrosa: normal and pathological ndings. Ophthalmologica 210, 142147. Trier, K., Olsen, E.B., Ammitzboll, T., 1990. Regional glycosaminoglycan composition of the human sclera. Acta Ophthalmol. 68, 304 306. Van Kuppevelt, T.H., Rutten, T.L., Kuyper, C.M., 1987. Ultrastructural localization of proteoglycans in tissue using cuprolinic blue according
623
to the critical electrolyte concentration method: comparison with biochemical data from the literature. Histochem. J. 19, 520 526. ster, L., 1987. Dermatan sulphate proteoglycans Ward, N.P., Scott, J.E., Co from sclera examined by rotary shadowing and electron microscopy. Biochem. J. 242, 761 766. Watson, P.G., Hazleman, B.L., 1976. The Sclera and Systemic Disorders, 1st Ed., Saunders, London, pp. 198199. Watson, P.G., Hazleman, B.L., Pavasio, C., Green, R., 2003, Second Ed, The Sclera and Systemic Disorders, 9. Butterworth-Heinemann, London, Chapters 8 and 9. Watson, P.G., Hayreh, S.S., 1976. Scleritis and episcleritis. Br. J. Ophthalmol. 60, 163191. Watson, P.G., Young, R.D., 1985. Changes at the periphery of a lesion in necrotising scleritis: anterior segment uorescein angiography correlated with electron microscopy. Br. J. Ophthalmol. 69, 656663. Wessel, H., Anderson, S., Fite, D., Halvas, E., Hempel, J., SundarRaj, N., 1997. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest. Ophthalmol. Vis. Sci. 38, 24082422. White, J., Werkmeister, J.A., Ramshaw, J.A., Birk, D.E., 1997. Organization of brillar collagen in the human and bovine cornea: collagen types V and III. Connect. Tissue Res. 36, 165174. Yamamoto, S., Hashizume, H., Hitomi, J., Shigeno, M., Sawaguchi, S., Abe, H., Ushiki, T., 2000. The subbrillar arrangement of corneal and scleral collagen brils as revealed by scanning electron and atomic force microscopy. Arch. Histol. Cytol. 63, 127 135. Yamamoto, S., Hitomi, J., Sawaguchi, S., Abe, H., Shigeno, M., Ushiki, T., 2002. Observation of human corneal and scleral collagen brils by atomic force microscopy. Jpn. J. Ophthalmol. 46, 496 501. Young, B.B., Zhang, G., Koch, M., Birk, D.E., 2002. The roles of type XII and XIV collagen in brillogenesis and matrix assembly in the developing cornea. J. Cell Biochem. 87, 208 220. Young, R.D., 1985. The ultrastructural organisation of proteoglycans and collagen in human and rabbit scleral matrix. J. Cell Sci. 74, 95 104. Young, R.D., Powell, J., Watson, P.G., 1988. Ultrastructural changes in scleral proteoglycans precede destruction of the collagen bril matrix in necrotizing scleritis. Histopathology 12, 7584. Young, R.D., Watson, P.G., 1984a. Microscopical studies of necrotising scleritis. I. Cellular aspects. Br. J. Ophthalmol. 68, 770780. Young, R.D., Watson, P.G., 1984b. Microscopical studies of necrotising scleritis. II. Collagen degradation in the scleral stroma. Br. J. Ophthalmol. 68, 781789.
Das könnte Ihnen auch gefallen
- Mens Health - Fat Burn WorkoutDokument1 SeiteMens Health - Fat Burn WorkoutonebzaNoch keine Bewertungen
- INTRODUCTION NeewDokument33 SeitenINTRODUCTION NeewMuhammad MustafaNoch keine Bewertungen
- Glaucoma EssayDokument5 SeitenGlaucoma Essaysujataravi6Noch keine Bewertungen
- Major Review: Ocular ColobomataDokument20 SeitenMajor Review: Ocular ColobomataPriyankaNoch keine Bewertungen
- Vitreous FloatersDokument35 SeitenVitreous FloatersnadiasalimaNoch keine Bewertungen
- Review: Pathogenesis of Rhegmatogenous Retinal Detachment Predisposing Anatomy and Cell BiologyDokument12 SeitenReview: Pathogenesis of Rhegmatogenous Retinal Detachment Predisposing Anatomy and Cell BiologyFathirNoch keine Bewertungen
- Anatomy VitreousDokument6 SeitenAnatomy VitreousPa Mei Lai100% (1)
- Corneal Vascularization: David G. CoganDokument9 SeitenCorneal Vascularization: David G. CoganPraful ChaudharyNoch keine Bewertungen
- Pemicu 1 PENGINDRAAN C2Dokument113 SeitenPemicu 1 PENGINDRAAN C2CcNoch keine Bewertungen
- Michael G. Glasspool FRCS, DO (Auth.) - Atlas of Ophthalmology-Springer Netherlands (1982) PDFDokument117 SeitenMichael G. Glasspool FRCS, DO (Auth.) - Atlas of Ophthalmology-Springer Netherlands (1982) PDFInna BujorNoch keine Bewertungen
- Resume Ulkus CorneaDokument10 SeitenResume Ulkus CorneaNita RosianiNoch keine Bewertungen
- Senile Cataract (Age-Related Cataract) - Practice Essentials, Background, PathophysiologyDokument5 SeitenSenile Cataract (Age-Related Cataract) - Practice Essentials, Background, PathophysiologyAhmad FahroziNoch keine Bewertungen
- Anatomy & Physiology of The Vitreous & The Vitreoretinal InterfaceDokument11 SeitenAnatomy & Physiology of The Vitreous & The Vitreoretinal InterfaceEva Marini SimbolonNoch keine Bewertungen
- The Multifunctional ChoroidDokument38 SeitenThe Multifunctional ChoroidyinvilllNoch keine Bewertungen
- Cet 5Dokument4 SeitenCet 5jumi26Noch keine Bewertungen
- The Histology and Biology of The LensDokument8 SeitenThe Histology and Biology of The LensIdham BaharudinNoch keine Bewertungen
- GlaucomaDokument18 SeitenGlaucomaOncología CdsNoch keine Bewertungen
- Ophthalmology: Anatomy of The LensDokument35 SeitenOphthalmology: Anatomy of The Lensمحمد عبدالوهاب ابراهيم الطباطبائيNoch keine Bewertungen
- Extracapsular Cataract Extraction (ECCE) Is A Category of Eye Surgery in WhichDokument13 SeitenExtracapsular Cataract Extraction (ECCE) Is A Category of Eye Surgery in WhichMa Christina RabayaNoch keine Bewertungen
- CataractDokument5 SeitenCataractcarls burg a. resurreccionNoch keine Bewertungen
- Primary and Secondary Lacrimal Canaliculitis A Review of LiteratureDokument6 SeitenPrimary and Secondary Lacrimal Canaliculitis A Review of LiteratureaflsnoxorNoch keine Bewertungen
- Mature CataractDokument24 SeitenMature CataractmethadamayNoch keine Bewertungen
- Treatment Options For Lower Eyelid FestoonsDokument9 SeitenTreatment Options For Lower Eyelid FestoonsBFF BotoxNoch keine Bewertungen
- Age Related or Senile Cataract Pathology, Mechanism and ManagementDokument8 SeitenAge Related or Senile Cataract Pathology, Mechanism and ManagementEricNoch keine Bewertungen
- AVICENNIA LBM 4 Mata SGD 20Dokument28 SeitenAVICENNIA LBM 4 Mata SGD 20Maylinda PutriNoch keine Bewertungen
- AVICENNIA LBM 4 Mata SGD 20Dokument28 SeitenAVICENNIA LBM 4 Mata SGD 20Maylinda Putri100% (1)
- The Physical Examination of The EyeDokument16 SeitenThe Physical Examination of The EyeriveliNoch keine Bewertungen
- AnatomyDokument9 SeitenAnatomyannora daffaNoch keine Bewertungen
- University of Baghdad Alkindy College of Medicine Fifth Stage / GroupDokument14 SeitenUniversity of Baghdad Alkindy College of Medicine Fifth Stage / GroupNoor Al Zahraa AliNoch keine Bewertungen
- Li LBM 4 Mata 2Dokument30 SeitenLi LBM 4 Mata 2Salma SavitaNoch keine Bewertungen
- Diseases of LensDokument42 SeitenDiseases of LensAmmad ShahidNoch keine Bewertungen
- Vol 11 R2 EyelidDokument56 SeitenVol 11 R2 Eyelidsalibagustavo100% (1)
- Referat Cystoid Macular Edema Periode 17 Juni - 20 Juli 2019Dokument25 SeitenReferat Cystoid Macular Edema Periode 17 Juni - 20 Juli 2019CharlotteGraceNusiferaNoch keine Bewertungen
- 02 The Cornea and ScleraDokument6 Seiten02 The Cornea and ScleramoraadoreneNoch keine Bewertungen
- The Anatomy of The LimbusDokument8 SeitenThe Anatomy of The LimbusBukaNoch keine Bewertungen
- Cataract Morphology: Dip HE Rehabilitation Work (Visual Impairment)Dokument9 SeitenCataract Morphology: Dip HE Rehabilitation Work (Visual Impairment)blinkbumbumNoch keine Bewertungen
- Mac HoleDokument21 SeitenMac HoleSwati RamtekeNoch keine Bewertungen
- Discuss Physiology of Aqueous Formation and Its Drainage and Relate It To How Intraocular Pressure Is ControlledDokument8 SeitenDiscuss Physiology of Aqueous Formation and Its Drainage and Relate It To How Intraocular Pressure Is ControlledPrince ChipunguNoch keine Bewertungen
- The Eye 1Dokument42 SeitenThe Eye 1Aunties Hyd Kka DeewanaNoch keine Bewertungen
- Pemicu 1 Pengindraan c2Dokument113 SeitenPemicu 1 Pengindraan c2CcNoch keine Bewertungen
- Lens by DR - Haider AswadDokument7 SeitenLens by DR - Haider AswadcwkmrqkhjjNoch keine Bewertungen
- Cornea Duanes Ophthalmology 2337 2337Dokument75 SeitenCornea Duanes Ophthalmology 2337 2337Endre Shitaye KulkiNoch keine Bewertungen
- Aging Vit Struct 1987Dokument5 SeitenAging Vit Struct 1987JLoNoch keine Bewertungen
- Primary Open-Angle Glaucoma: SeminarDokument10 SeitenPrimary Open-Angle Glaucoma: SeminarFebry LuthunananaNoch keine Bewertungen
- Senile Cataract (Age-Related Cataract) : Practice Essentials, Background, PathophysiologyDokument6 SeitenSenile Cataract (Age-Related Cataract) : Practice Essentials, Background, PathophysiologyadliahghaisaniNoch keine Bewertungen
- Hapter 73 Eyelid Abnormalities: Ectropion, Entropion, TrichiasisDokument21 SeitenHapter 73 Eyelid Abnormalities: Ectropion, Entropion, TrichiasisEllysabet DianNoch keine Bewertungen
- Cataracts: Jay Thompson,, Naheed LakhaniDokument15 SeitenCataracts: Jay Thompson,, Naheed LakhaniwhiezardNoch keine Bewertungen
- Retinal Detachment: PathogenesisDokument8 SeitenRetinal Detachment: PathogenesisRashellya RasyidaNoch keine Bewertungen
- Cataract For Medical StudentsDokument7 SeitenCataract For Medical StudentsJustin Michal DassNoch keine Bewertungen
- Corneal Transplantation: The Forgotten Graft: MinireviewDokument8 SeitenCorneal Transplantation: The Forgotten Graft: MinireviewadityaprakosoNoch keine Bewertungen
- Ocular Biochemistry UpdatedDokument42 SeitenOcular Biochemistry UpdatedDivine OkolieNoch keine Bewertungen
- Squisis MacularDokument8 SeitenSquisis MacularremotmNoch keine Bewertungen
- Ocular Biochemistry LECTURE NOTE-1Dokument44 SeitenOcular Biochemistry LECTURE NOTE-1Divine OkolieNoch keine Bewertungen
- Oxidative Damage and The Prevention ofDokument11 SeitenOxidative Damage and The Prevention ofFernando NugrohoNoch keine Bewertungen
- Odontogenic Keratocyst: Submitted By: Digcha Shree Rai Roll No: 22Dokument31 SeitenOdontogenic Keratocyst: Submitted By: Digcha Shree Rai Roll No: 22Rajat NangiaNoch keine Bewertungen
- Retinal Detachment: Case Report Operating RoomDokument30 SeitenRetinal Detachment: Case Report Operating RoomCarlo Joseph Intal LlacerNoch keine Bewertungen
- Aging-Related Cataracts: General FeaturesDokument19 SeitenAging-Related Cataracts: General FeaturespatriciamaisNoch keine Bewertungen
- Collagen Cross Linking For Keratoconus ArticleDokument5 SeitenCollagen Cross Linking For Keratoconus ArticleHirendra VaishnavNoch keine Bewertungen
- Stmcls 28 3 597Dokument15 SeitenStmcls 28 3 597Prematura Sri AnasaryNoch keine Bewertungen
- Suprachoroidal Space InterventionsVon EverandSuprachoroidal Space InterventionsShohista SaidkasimovaNoch keine Bewertungen
- Effect of Mcconnell Taping On Pain, Rom & Grip Strength in Patients With Triangular Fibrocartilage Complex InjuryDokument9 SeitenEffect of Mcconnell Taping On Pain, Rom & Grip Strength in Patients With Triangular Fibrocartilage Complex InjuryDr. Krishna N. SharmaNoch keine Bewertungen
- M2X M5X PDFDokument19 SeitenM2X M5X PDFEdgar Rojas EspejoNoch keine Bewertungen
- Anatomy of TMJ and Its Role in ProsthodonticsDokument72 SeitenAnatomy of TMJ and Its Role in ProsthodonticsBaishali GhoshNoch keine Bewertungen
- Rehabilitation of Muscle Dysfunction in HemophiliaDokument14 SeitenRehabilitation of Muscle Dysfunction in HemophiliadavidcorzoNoch keine Bewertungen
- Upper LimbDokument20 SeitenUpper Limbhafsah sidi abdullahiNoch keine Bewertungen
- RA For Tiling WorkDokument12 SeitenRA For Tiling WorkSaeed AhmadNoch keine Bewertungen
- IPC Cases For Discussion PDFDokument393 SeitenIPC Cases For Discussion PDFDolly Singh OberoiNoch keine Bewertungen
- Right Inguinal Hernia RepairDokument1 SeiteRight Inguinal Hernia Repairsgod34Noch keine Bewertungen
- Autumn Off Duty SafetyDokument20 SeitenAutumn Off Duty SafetyDeepu RavikumarNoch keine Bewertungen
- Pipkin Article - Femoral Head FractureDokument17 SeitenPipkin Article - Femoral Head Fractureakb601Noch keine Bewertungen
- Nursing Interview Guide To Collect Subjective Data From The Client Questions RationaleDokument19 SeitenNursing Interview Guide To Collect Subjective Data From The Client Questions RationaleKent Rebong100% (1)
- Barbarian Prince Compiled Fan VariantsDokument10 SeitenBarbarian Prince Compiled Fan VariantsSlev00Noch keine Bewertungen
- Mechanical Asphyxia 3 - Strangulation, ThrottlingDokument19 SeitenMechanical Asphyxia 3 - Strangulation, Throttlingvarshu ramaniNoch keine Bewertungen
- Damage Done: By: Ty Setty English 1000 Mrs. MansfieldDokument5 SeitenDamage Done: By: Ty Setty English 1000 Mrs. Mansfieldapi-302127192Noch keine Bewertungen
- Complications of Total Hip ReplacementDokument22 SeitenComplications of Total Hip ReplacementNitya KrishnaNoch keine Bewertungen
- Mourngul: Description AbilitiesDokument1 SeiteMourngul: Description AbilitiesFininNoch keine Bewertungen
- Daniel Medina BarselonaDokument24 SeitenDaniel Medina BarselonaDusan OrescaninNoch keine Bewertungen
- Abdominal Aortic Aneurysm Vascular SurgeryDokument2 SeitenAbdominal Aortic Aneurysm Vascular SurgeryzipzipzipNoch keine Bewertungen
- Physical Rehabilitation 2.2 Rehabilitation of Lower Limb Musculoskeletal DisordersDokument6 SeitenPhysical Rehabilitation 2.2 Rehabilitation of Lower Limb Musculoskeletal DisordersJAIRISH YZABELLE SALVADORNoch keine Bewertungen
- CTR Master Class CourseDokument15 SeitenCTR Master Class CourseCool N CoolNoch keine Bewertungen
- Adventure Deck Savage Worlds PDFDokument8 SeitenAdventure Deck Savage Worlds PDFPierre-Eric Raby100% (3)
- MySims - Manual - WIIDokument6 SeitenMySims - Manual - WIIasdasdNoch keine Bewertungen
- Mines and Geosciences Bureau: Regional Office No. XIIDokument3 SeitenMines and Geosciences Bureau: Regional Office No. XIIErmelyn Jane CelindroNoch keine Bewertungen
- Sectional Anatomy by MRI and CT - NodrmDokument627 SeitenSectional Anatomy by MRI and CT - NodrmEsteban Balcero Zuluaga100% (2)
- Types of JointsDokument2 SeitenTypes of JointsJayhan TanNoch keine Bewertungen
- Pe ReviewerDokument10 SeitenPe ReviewerLean Amara VillarNoch keine Bewertungen
- Adaptation of Locomotor and Non-Locomotor SkillsDokument8 SeitenAdaptation of Locomotor and Non-Locomotor SkillsLyneth Ann RuleNoch keine Bewertungen
- Ladder and Agility Drills Week 5Dokument9 SeitenLadder and Agility Drills Week 5munichrangersNoch keine Bewertungen
- Santo Lin I 2020Dokument13 SeitenSanto Lin I 2020Jose Tellez HuertaNoch keine Bewertungen