Beruflich Dokumente
Kultur Dokumente
Lecture 12 - Pair Distribution Functions
Hochgeladen von
Robert WexlerCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Lecture 12 - Pair Distribution Functions
Hochgeladen von
Robert WexlerCopyright:
Verfügbare Formate
Lecture 12
Pair distribution functions
Background
Recall that the pair distribution function is ( )
( ( ) )
This quantifies the deviation of (r,r) from the non-interacting/independent result, which is ( ) ( ) ( )
If this system is system, i.e. no external fields, then the joint generic distribution is ( ) ( )
Isotropic, spherically symmetric system
For a general isotropic system, the joint generic distribution is ( ) ( )
If this system is spherically symmetric, then the pair distribution functions can be written as ( where | | ) ( )
g(r) has no center of mass dependence because it does not matter where you
are in the sample, i.e. where the center of mass is. This form of the pair distribution function only depends on the distances between particles.
Examples of g(r) for different pair potentials
Lets choose U as a sum of pair potentials ( ) (| |)
This choice is valid for a spherically symmetric particle/potential and the energy only depends on the distances between particles.
Choices for pair potential u(r)
A. Ideal gas For an ideal gas, u(r)=0 and therefore U(r1,,rN)=0. This means that there are no energy interactions between particles. Lets calculate g(r,r). ( )
This means that the radial distribution function g(r)=1 for r0. A consequence of this finding is the following: ( ) ( ) ( )
The second equality only holds true if the system is isotropic. B. Hard spheres The potential energy of interaction between two hard spheres is defined as follows: ( ) {
where is double the radius of the hard sphere. It turns out that the radial distribution function also depends on the statistical ensemble chosen to describe the system. For the canonical ensemble, ( ) ( | ) ( | ) ( | )
This means that the radial distribution function depends on the distance between particles, density, and temperature. Lets keep this in mind when we are considering g(r).
B-1. Dilute gas, <<3 or 3<<1
The plot of g(r) vs. r looks like a step function.
The distance between the hard spheres is in units of . Configurations where r< get pushed by repulsive forces and bunch up at r= to create the blip in the function.
B-2. Dense liquid, 31
Side note : tells you about the volume per particle : the volume that one particle excludes 31 tells us that the particles are taking up a lot volume, i.e. the allowed volume is almost completely filled by atoms. Its also interesting to note that hard spheres only have two phases, solid and liquid. Here is a schematic plot of g(r) vs. r.
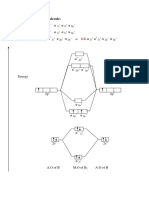
The first bump at r= corresponds to the first solvation shell, which is depicted below for sodium ions being solvated by water.
The second bump corresponds to the second solvation shell, which falls just outside the first solvation shell. The hard sphere model is often used to model colloids and nanoparticles.
Das könnte Ihnen auch gefallen
- Tarasov3 PDFDokument8 SeitenTarasov3 PDFDavid ReyesNoch keine Bewertungen
- Difference Equations in Normed Spaces: Stability and OscillationsVon EverandDifference Equations in Normed Spaces: Stability and OscillationsNoch keine Bewertungen
- Batchelor1982 PDFDokument34 SeitenBatchelor1982 PDFMalik Saqib ShahzadNoch keine Bewertungen
- Vector Field Effective Action in The Open Superstring TheoryDokument38 SeitenVector Field Effective Action in The Open Superstring Theorypepin morenoNoch keine Bewertungen
- Commensurabilities among Lattices in PU (1,n). (AM-132), Volume 132Von EverandCommensurabilities among Lattices in PU (1,n). (AM-132), Volume 132Noch keine Bewertungen
- Mufti 1980Dokument21 SeitenMufti 1980Kousik SasmalNoch keine Bewertungen
- The Penrose Transform: Its Interaction with Representation TheoryVon EverandThe Penrose Transform: Its Interaction with Representation TheoryNoch keine Bewertungen
- Random Close Packing Fractions of Lognormal Distributions of Hard SpheresDokument7 SeitenRandom Close Packing Fractions of Lognormal Distributions of Hard SpheresRodrigo CarvalhoNoch keine Bewertungen
- Lecture MDanalysisDokument42 SeitenLecture MDanalysisCamilo LimaNoch keine Bewertungen
- C*-Algebra Extensions and K-Homology. (AM-95), Volume 95Von EverandC*-Algebra Extensions and K-Homology. (AM-95), Volume 95Noch keine Bewertungen
- Tabata 1972Dokument7 SeitenTabata 1972Zineb SobhyNoch keine Bewertungen
- Pset 10Dokument4 SeitenPset 10jamesNoch keine Bewertungen
- Tomography From Entanglement: PACS Numbers: 11.25.TqDokument6 SeitenTomography From Entanglement: PACS Numbers: 11.25.TqDonneur 1515Noch keine Bewertungen
- Piotr T Chrusciel and Gabriel Nagy - The Hamiltonian Mass of Asymptotically Anti-De Sitter Space-TimesDokument8 SeitenPiotr T Chrusciel and Gabriel Nagy - The Hamiltonian Mass of Asymptotically Anti-De Sitter Space-TimesGijke3Noch keine Bewertungen
- Tmp84e3 TMPDokument4 SeitenTmp84e3 TMPFrontiersNoch keine Bewertungen
- KrigingDokument20 SeitenKrigingDeepblue09Noch keine Bewertungen
- Ward-1983-Completely Solvable Gauge-FieldDokument16 SeitenWard-1983-Completely Solvable Gauge-FieldGuido FranchettiNoch keine Bewertungen
- The Complete Spectrum of The Area From Recoupling Theory in Loop Quantum GravityDokument12 SeitenThe Complete Spectrum of The Area From Recoupling Theory in Loop Quantum GravityPatrick WongNoch keine Bewertungen
- Investigating Complex Networks With Inverse ModelsDokument8 SeitenInvestigating Complex Networks With Inverse ModelsDemian PereiraNoch keine Bewertungen
- P. J. Forrester and B. Jancovici - Generalized Plasmas and The Anomalous Quantum Hall EffectDokument7 SeitenP. J. Forrester and B. Jancovici - Generalized Plasmas and The Anomalous Quantum Hall EffectGreamxxNoch keine Bewertungen
- Statistical Thermodynamics of Rubber ElasticityDokument8 SeitenStatistical Thermodynamics of Rubber Elasticitychiuchan888Noch keine Bewertungen
- Five DimensionsDokument5 SeitenFive DimensionsTBoo Techno-bambooNoch keine Bewertungen
- Resume of Krystynak - 1Dokument6 SeitenResume of Krystynak - 1api-22169845Noch keine Bewertungen
- Chapt 6Dokument15 SeitenChapt 6morphos777Noch keine Bewertungen
- Orthogonal Curvilinear CoordinatesDokument16 SeitenOrthogonal Curvilinear CoordinatesstriaukasNoch keine Bewertungen
- Operador Intercambio Exclusion de PauliDokument4 SeitenOperador Intercambio Exclusion de PauliSoraya BustamanteNoch keine Bewertungen
- 49 Wertheim1984 PDFDokument16 Seiten49 Wertheim1984 PDFNatalia StefaniaNoch keine Bewertungen
- Modelling of An Homogeneous Equilibrium Mixture Model (HEM) : A. Bernard-Champmartin J.-M. GhidagliaDokument21 SeitenModelling of An Homogeneous Equilibrium Mixture Model (HEM) : A. Bernard-Champmartin J.-M. GhidagliaPedro AlmeidaNoch keine Bewertungen
- A Duality Principle For The Entanglement Entropy of Free Fermion SystemsDokument12 SeitenA Duality Principle For The Entanglement Entropy of Free Fermion SystemsCroco AliNoch keine Bewertungen
- Wolfgang Hasse and Volker Perlick - Gravitational Lensing in Spherically Symmetric Static Spacetimes With Centrifugal Force ReversalDokument18 SeitenWolfgang Hasse and Volker Perlick - Gravitational Lensing in Spherically Symmetric Static Spacetimes With Centrifugal Force ReversalKunma050Noch keine Bewertungen
- Coordination Number in Liquid ArgonDokument10 SeitenCoordination Number in Liquid Argon09187135911Noch keine Bewertungen
- Analytic S-Matrix 5Dokument2 SeitenAnalytic S-Matrix 5FishboneNoch keine Bewertungen
- One Eective Eective NotDokument18 SeitenOne Eective Eective NotspanishramNoch keine Bewertungen
- First Report 1Dokument10 SeitenFirst Report 1Israel Abraham Barragan VidalNoch keine Bewertungen
- Galaxy Fractal DistributionDokument35 SeitenGalaxy Fractal DistributionquatroportexNoch keine Bewertungen
- Discretization To Avoid Singularities in Vibration-Rotation Hamiltonians: Bisector Embedding For AB2 TriatomicsDokument12 SeitenDiscretization To Avoid Singularities in Vibration-Rotation Hamiltonians: Bisector Embedding For AB2 TriatomicsPassammNoch keine Bewertungen
- Presented By: Ercherio Marpaung (14S15056) Dewi Fortuna Simangunsong (14S15007)Dokument30 SeitenPresented By: Ercherio Marpaung (14S15056) Dewi Fortuna Simangunsong (14S15007)Ercherio MarpaungNoch keine Bewertungen
- 8 Non-Abelian Gauge Theory: Perturbative Quantization: YM YM ? A S (R)Dokument31 Seiten8 Non-Abelian Gauge Theory: Perturbative Quantization: YM YM ? A S (R)teo tresNoch keine Bewertungen
- tmp5117 TMPDokument3 Seitentmp5117 TMPFrontiersNoch keine Bewertungen
- Fractal Proptrties of SDSS QuasarsDokument10 SeitenFractal Proptrties of SDSS QuasarscrocoaliNoch keine Bewertungen
- Effective Numerical Treatment of Boundary Integral Equations. J.C Lachat and WatsonDokument15 SeitenEffective Numerical Treatment of Boundary Integral Equations. J.C Lachat and Watsonrahim.sihadjmohandNoch keine Bewertungen
- Kristiansen-Egeland1999 Chapter ObserverDesignForNonlinearOsci PDFDokument16 SeitenKristiansen-Egeland1999 Chapter ObserverDesignForNonlinearOsci PDFShadNoch keine Bewertungen
- Chapter 3. Boundary-Value Problems in Electrostatics: Spherical and Cylindrical GeometriesDokument21 SeitenChapter 3. Boundary-Value Problems in Electrostatics: Spherical and Cylindrical GeometriesapkinobraNoch keine Bewertungen
- Aula 3 - Teoria de Cordas MITDokument7 SeitenAula 3 - Teoria de Cordas MITErick MouraNoch keine Bewertungen
- Five-Dimensional Warped Geometry With A Bulk Scalar Field: DPNU-01-24 Hep-Th/0109040Dokument8 SeitenFive-Dimensional Warped Geometry With A Bulk Scalar Field: DPNU-01-24 Hep-Th/0109040wendel_physicsNoch keine Bewertungen
- Tetsuo Deguchi and Akihisa Yao - Scattering Functions and Correlation Functions of Random KnotsDokument13 SeitenTetsuo Deguchi and Akihisa Yao - Scattering Functions and Correlation Functions of Random KnotsYokdmNoch keine Bewertungen
- David Berenstein, Juan Maldacena and Horatiu Nastase - Strings in Flat Space and PP Waves From N 4 Super Yang MillsDokument35 SeitenDavid Berenstein, Juan Maldacena and Horatiu Nastase - Strings in Flat Space and PP Waves From N 4 Super Yang MillsJuazmantNoch keine Bewertungen
- Francisco C. Alcaraz Et Al - Three-Dimensional Spin Systems Without Long-Range OrderDokument26 SeitenFrancisco C. Alcaraz Et Al - Three-Dimensional Spin Systems Without Long-Range OrderImaxSWNoch keine Bewertungen
- Script DynamicsDokument13 SeitenScript DynamicsUMARNoch keine Bewertungen
- A Gauge Field Theory of Chirally Folded Homopolymers With Applications To Folded ProteinsDokument6 SeitenA Gauge Field Theory of Chirally Folded Homopolymers With Applications To Folded ProteinsJoon Suk HuhNoch keine Bewertungen
- Chap8 Stratton-Chu Derivations, Huygen Principle, Far-Field ExpressionsDokument18 SeitenChap8 Stratton-Chu Derivations, Huygen Principle, Far-Field ExpressionsstepannpNoch keine Bewertungen
- Topic 2: Molecular Dynamics of Lennard-Jones System (Continued)Dokument3 SeitenTopic 2: Molecular Dynamics of Lennard-Jones System (Continued)Muklis LatifNoch keine Bewertungen
- Wormholes in Viable F (R) Modified Theories of Gravity and Weak Energy ConditionDokument9 SeitenWormholes in Viable F (R) Modified Theories of Gravity and Weak Energy Conditionrobespjer69Noch keine Bewertungen
- Handout09 A2 UsefulDokument5 SeitenHandout09 A2 UsefulJoyee BasuNoch keine Bewertungen
- Quantum Mechanical Addition of Angular Momenta and SpinDokument42 SeitenQuantum Mechanical Addition of Angular Momenta and Spinمؤيد العليNoch keine Bewertungen
- 2009 Weighted Hardy and Singular Operators in Morrey SpaceDokument17 Seiten2009 Weighted Hardy and Singular Operators in Morrey SpaceUMAIR ASHFAQNoch keine Bewertungen
- Brief Review On Tight-Binding Model: ~ kα ~ k i~ k· (~ R+~ r) α αDokument10 SeitenBrief Review On Tight-Binding Model: ~ kα ~ k i~ k· (~ R+~ r) α αRobert WexlerNoch keine Bewertungen
- Recitation Week 2Dokument2 SeitenRecitation Week 2Robert WexlerNoch keine Bewertungen
- Problem Set 5Dokument2 SeitenProblem Set 5Robert WexlerNoch keine Bewertungen
- 2Dokument18 Seiten2Robert WexlerNoch keine Bewertungen
- D'var Torah - Parsha NassoDokument1 SeiteD'var Torah - Parsha NassoRobert WexlerNoch keine Bewertungen
- Coeficientes Clebsch Gordan PDFDokument1 SeiteCoeficientes Clebsch Gordan PDFHM2P33Noch keine Bewertungen
- Three-Dimensional Hilbert Spaces and An Introduction To Angular MomentumDokument3 SeitenThree-Dimensional Hilbert Spaces and An Introduction To Angular MomentumRobert WexlerNoch keine Bewertungen
- D'var Torah - Parsha NassoDokument1 SeiteD'var Torah - Parsha NassoRobert WexlerNoch keine Bewertungen
- Lecture 5 - Dirac Delta Functions, Characteristic Functions, and The Law of Large NumbersDokument3 SeitenLecture 5 - Dirac Delta Functions, Characteristic Functions, and The Law of Large NumbersRobert WexlerNoch keine Bewertungen
- Uncertainty Principle, Time Evolution, and Quantum Mechanical CurrentDokument4 SeitenUncertainty Principle, Time Evolution, and Quantum Mechanical CurrentRobert WexlerNoch keine Bewertungen
- Young's Double Slit ExperimentDokument1 SeiteYoung's Double Slit ExperimentRobert WexlerNoch keine Bewertungen
- Neutron Diffraction TechniquesDokument30 SeitenNeutron Diffraction TechniquesAgnes maria maniNoch keine Bewertungen
- MSM Photo DetectorDokument16 SeitenMSM Photo DetectorAkash TyagiNoch keine Bewertungen
- 2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VDokument7 Seiten2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VcaptainhassNoch keine Bewertungen
- 2008 Physics NotesDokument12 Seiten2008 Physics NotesAbdullionNoch keine Bewertungen
- Semiconductors: DIODES, SMD, Transient Voltage SuppressionDokument5 SeitenSemiconductors: DIODES, SMD, Transient Voltage SuppressionMike BravoNoch keine Bewertungen
- Fundamentals of Modern Manufacturing 6th Edition Groover Solutions ManualDokument3 SeitenFundamentals of Modern Manufacturing 6th Edition Groover Solutions ManualJacobTorresbipkz100% (14)
- Seminar Report 2Dokument23 SeitenSeminar Report 2Mayank S.H88% (8)
- Synchrotron X Ray Absorption Spectroscopy and Electrochemical Study of Bi2O2Se Electrode For Lithium - Potassium Ion StorageDokument11 SeitenSynchrotron X Ray Absorption Spectroscopy and Electrochemical Study of Bi2O2Se Electrode For Lithium - Potassium Ion Storage沙漠Noch keine Bewertungen
- Fine StructureDokument17 SeitenFine StructureVivek BelaNoch keine Bewertungen
- Graphene Tight-Binding Model-1ny95f1Dokument8 SeitenGraphene Tight-Binding Model-1ny95f1Nitish KumarNoch keine Bewertungen
- VL2022230501086 DaDokument2 SeitenVL2022230501086 DabihbugvNoch keine Bewertungen
- Properties of Matter and Its Various FormsDokument29 SeitenProperties of Matter and Its Various FormsKim InumerablesNoch keine Bewertungen
- Li3BaSrLn3 (MoO4) 8 SM V1Dokument18 SeitenLi3BaSrLn3 (MoO4) 8 SM V1sivaji naikNoch keine Bewertungen
- Gschneidner K. A., Eyring L. - Handbook On The Physics and Chemistry of Rare Earths Vol. 20 (1995) (470s) PDFDokument472 SeitenGschneidner K. A., Eyring L. - Handbook On The Physics and Chemistry of Rare Earths Vol. 20 (1995) (470s) PDFxxalikayaxxNoch keine Bewertungen
- Re CrystallizationDokument476 SeitenRe CrystallizationJosé RamírezNoch keine Bewertungen
- 14.1 Matter & Thermal Energy (Press Read Only To Open)Dokument104 Seiten14.1 Matter & Thermal Energy (Press Read Only To Open)Abdullah Alqahtani100% (1)
- BSES26Dokument7 SeitenBSES26jerico.lapurgaNoch keine Bewertungen
- Powder X-Ray Diffraction: Assist - Prof.Drnawal Al-KarwiDokument21 SeitenPowder X-Ray Diffraction: Assist - Prof.Drnawal Al-Karwiفرح محمدNoch keine Bewertungen
- Ceramics International: Mahamoud S. Alkathy, Rahul Gayam, K.C. James RajuDokument10 SeitenCeramics International: Mahamoud S. Alkathy, Rahul Gayam, K.C. James RajuRachna SelvamaniNoch keine Bewertungen
- ET4117 Electrical Machines and Drives Lecture5Dokument31 SeitenET4117 Electrical Machines and Drives Lecture5farhan beighNoch keine Bewertungen
- CHEM F111 (Handout Revised)Dokument3 SeitenCHEM F111 (Handout Revised)shrey shahNoch keine Bewertungen
- 1 MP Crystal Range (720P) : Price ListDokument4 Seiten1 MP Crystal Range (720P) : Price ListFuture InnovationsNoch keine Bewertungen
- Chapter 6 Solar Cell Part 1Dokument83 SeitenChapter 6 Solar Cell Part 1Puvanesvararam Puvanes PuvaNoch keine Bewertungen
- P N Junction DiodeDokument3 SeitenP N Junction DiodeAsogwa ChinazaNoch keine Bewertungen
- Factors Affecting The Growth ProcessDokument23 SeitenFactors Affecting The Growth ProcessBhaagi Sird100% (1)
- Effect of Substrate-Induced Strain in The Transport Properties of Algan/Gan HeterostructuresDokument5 SeitenEffect of Substrate-Induced Strain in The Transport Properties of Algan/Gan HeterostructuresA Mohan BabuNoch keine Bewertungen
- Molecular Orbital Diagram For B, C, N, O, F and NeDokument12 SeitenMolecular Orbital Diagram For B, C, N, O, F and NeGods ArmyNoch keine Bewertungen
- EE2203 Electronic Devices and Circuits Lecture Notes PDFDokument377 SeitenEE2203 Electronic Devices and Circuits Lecture Notes PDFRabin Vaidhya100% (1)
- Grade 6 Answer KeyDokument8 SeitenGrade 6 Answer KeyKaren AnneNoch keine Bewertungen
- A Brief History of Time: From the Big Bang to Black HolesVon EverandA Brief History of Time: From the Big Bang to Black HolesBewertung: 4 von 5 Sternen4/5 (2193)
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceVon EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceBewertung: 4 von 5 Sternen4/5 (51)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessVon EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessBewertung: 4 von 5 Sternen4/5 (6)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseVon EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseBewertung: 3.5 von 5 Sternen3.5/5 (69)
- The Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectVon EverandThe Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectBewertung: 4.5 von 5 Sternen4.5/5 (20)
- Summary and Interpretation of Reality TransurfingVon EverandSummary and Interpretation of Reality TransurfingBewertung: 5 von 5 Sternen5/5 (5)
- Giza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyVon EverandGiza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyNoch keine Bewertungen
- The Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsVon EverandThe Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsBewertung: 5 von 5 Sternen5/5 (5)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldVon EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldBewertung: 3.5 von 5 Sternen3.5/5 (64)
- Black Holes: The Key to Understanding the UniverseVon EverandBlack Holes: The Key to Understanding the UniverseBewertung: 4.5 von 5 Sternen4.5/5 (13)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterVon EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterBewertung: 4.5 von 5 Sternen4.5/5 (410)
- Packing for Mars: The Curious Science of Life in the VoidVon EverandPacking for Mars: The Curious Science of Life in the VoidBewertung: 4 von 5 Sternen4/5 (1395)
- Too Big for a Single Mind: How the Greatest Generation of Physicists Uncovered the Quantum WorldVon EverandToo Big for a Single Mind: How the Greatest Generation of Physicists Uncovered the Quantum WorldBewertung: 4.5 von 5 Sternen4.5/5 (8)
- The Beginning of Infinity: Explanations That Transform the WorldVon EverandThe Beginning of Infinity: Explanations That Transform the WorldBewertung: 5 von 5 Sternen5/5 (60)
- The Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismVon EverandThe Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismBewertung: 4 von 5 Sternen4/5 (500)
- Lost in Math: How Beauty Leads Physics AstrayVon EverandLost in Math: How Beauty Leads Physics AstrayBewertung: 4.5 von 5 Sternen4.5/5 (125)
- Chernobyl 01:23:40: The Incredible True Story of the World's Worst Nuclear DisasterVon EverandChernobyl 01:23:40: The Incredible True Story of the World's Worst Nuclear DisasterBewertung: 4 von 5 Sternen4/5 (264)
- Strange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsVon EverandStrange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsBewertung: 4 von 5 Sternen4/5 (94)
- Quantum Physics: What Everyone Needs to KnowVon EverandQuantum Physics: What Everyone Needs to KnowBewertung: 4.5 von 5 Sternen4.5/5 (49)
- The Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceVon EverandThe Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceBewertung: 4.5 von 5 Sternen4.5/5 (23)
- Bedeviled: A Shadow History of Demons in ScienceVon EverandBedeviled: A Shadow History of Demons in ScienceBewertung: 5 von 5 Sternen5/5 (5)