Beruflich Dokumente
Kultur Dokumente
Document Control Procedures
Hochgeladen von
Saya JarotCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Document Control Procedures
Hochgeladen von
Saya JarotCopyright:
Verfügbare Formate
The only official copy of this file is the one on-line in the NSLS Quality Assurance website.
Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Prepared By:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: A. Ackerman 5/15/2008
Approved By:
Page 1 of 11 C. Porretto, S. Hoey, F. Willeke Revision Log
M. Buckley, E. Cheswick Approved By:
*Approval signatures on file with master copy.
Table of Contents 1.0 2.0 3.0 4.0 5.0
Purpose 6.1 Preparing a New Document Forms & Templates
Scope
6.2 Revising an Existing Document
Policy
6.3 Periodic Review of Document List of NSLS Active Controlled Documents and Review Status
References
Appendix A - Document header Instructions Appendix B - EMS/OHSAS Support Documentation Requirements
Definitions
Help
1.0
PURPOSE
To provide instructions for the use and preparation of NSLS or NSLS II controlled documents.
2.0 SCOPE
This department specific procedure is supplemental to the Document Control subject area in SBMS. It applies to NSLS or NSLS II staff who develop new or modify controlled documents, such as policies, manuals, and procedures. In addition, there are special controlled documents such as EMS/OHSAS support documents, operator aids, and forms that need to be controlled. These types of controlled documents have different levels of control requirements and are identified in the steps below as well as in Appendix B. This procedure does not apply to software codes, drawings, plans, specifications, and temporary procedures. Documents of external origin identified as necessary for the planning and operations of the Light Sources Directorate Occupational, Health, Safety, and Environmental management systems are to be controlled in accordance with the SBMS subject for controlled documents. The NSLS and NSLS II QA manuals contain separate procedures that address drawings, specifications, and temporary procedures. The SBMS addresses plans and software code.
3.0 POLICY 3.1
Technical and operating procedures are tools to instruct how to perform a task. They are also used to manage risks and hazards associated with conducting research, operational, and maintenance activities. They are also used to ensure that appropriate quality
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 2 of 11
assurance and other requirements are integrated with work. In addition, some ESH program documents defining requirements or describing program implementation require periodic review and approval and should be treated as controlled documents. NSLS or NSLS II staff should develop formal controlled documents as described in this procedure whenever one of the following criteria applies: Technical, Operating, Occupational, Health, Safety, and Security, or Environmental processes are required to be documented (e.g., by DOE, BNL, EMS, OHSAS, etc.). Policies/program documents are needed to define requirements or to ensure quality, reproducibility, or consistency of a process or design because of safety or programmatic needs.
3.2
When new or modified formalized procedures, policies, or other documents are deemed necessary, these documents must contain the elements stated within this procedure. Unless specified otherwise in this procedure, formalized documents must contain a header that includes a document number, revision letter or number, subject or document title, page numbering (excludes HTML docs), effective date, and name(s) of individual(s) who prepared and authorized the document. The original controlled document must reside with the NSLS Quality Control Coordinator (QCC) or the NSLS II Documents and Records Administrator (DRA) except when specified otherwise within this procedure. The NSLS Quality Manager (QM) or NSLS II Configuration Manager & NSLS QCC or NSLS II DRA will maintain the document control database for tracking and monitoring controlled documents within the system. The NSLS QCC or NSLS II DRA will also generate monthly reports to help notify responsible individuals in advance of upcoming periodic reviews. Controlled Document Use: Obsolete documents shall be removed from circulation and discarded or marked as "reference only" to prevent unintended use. Paper controlled documents should be marked accordingly (e.g. Controlled, Uncontrolled, Reference, Obsolete, etc.). When controlled paper copies are needed in the field, complete a Controlled Document Distribution List (QF-051) form and submit to the NSLS Quality Control Coordinator (QCC) or the NSLS II Documents and Records Administrator (DRA). Web based documents should contain the disclaimer indicated in section 6.1.2, as applicable. Users that print web-based documents must verify that it is the most current version by checking the document effective date on the NSLS website prior to use.
3.3
3.4
4.0
REFERENCES 4.1 4.2 4.3
Guidelines For Developing Procedures, BNL SBMS exhibit Document Control BNL SBMS subject area Forms & Templates:
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 3 of 11
Light Sources Directorate Document Approval Form (DL-QAF-036); Revision Log (QF-037) for paper/PDF documents; Revision Log (QF-038) for html documents; Periodic Document Review Form (QF-055); Light Sources Directorate Document Header template for paper/PDF documents; Light Sources Directorate Document Header template for html documents. Controlled Document Distribution List (QF-051) for requesting paper copies of controlled documents.
5.0
DEFINITIONS 5.1 5.2 5.3
Active document - A document currently in use and in circulation. HTML - Hypertext Markup Language Periodic Document Review - Active controlled documents are required to be reviewed periodically to verify their accuracy. The frequency of the review depends on occupational, health, safety, environmental, or programmatic risk associated with the document. A successful periodic review will reveal the existing document is current, correct, and does not require any revision/change. Refer to section 6.1.5b for further details. PDF - Adobe portable document files QCC - Quality Control Coordinator DRA Documents and Records Administrator Procedure - A document used to describe a particular series of steps to accomplish a specified manufacturing, inspection, or test operation, process, or activity. SBMS - Standards Based Management System
5.4 5.5 5.6 5.7
5.8
6.0
PROCEDURE
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 4 of 11
6.1
PREPARING A NEW DOCUMENT
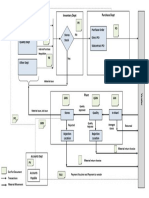
1. Add Elements to Document; 2. Add disclaimer (for web-based documents); 3. Review Document; 4. Approve Document; 5. Complete Revision Log;
6. Submit Document;
6.1.1 Add Elements for the following types of Controlled Documents: 6.1.1.a Policies, manuals, procedures, and requirements: Prepare a controlled document using one of the header formats listed in Appendix A or equivalent and mark the document "DRAFT". Regardless of the style of header used, each controlled document must contain the following Standard Elements:
subject or document title; effective date; page numbers (excludes HTML docs); revision letter or number; document number (obtain from NSLS QCC or NSLS II DRA); names of individual(s) who prepared and authorized (approved) document.
6.1.1.b
Plans, EMS, and OHSAS Support Document: All EMS and OHSAS procedures and manuals as well as JRAs and FRAs must contain the control elements within 6.1.1.a in this procedure. All plans and other applicable EMS and OHSAS support documents must have the following elements:
subject or document title revision date
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 5 of 11
revision letter or number (optional); page numbers (excludes HTML docs) name of individual(s) who authorized document (excludes forms and checklists)
6.1.1.c
Forms:
All forms require at a minimum, a revision date.
6.1.1.d
Operator Aids:
Line supervisors determine if an operator aid is necessary based on the risk, importance, and critical nature of the equipment or operation. The operator aid must contain the name or signature of the person who approved it and the effective date.
Note: Guidance for preparing procedures is available in the "Guidelines for Developing Procedures" SBMS exhibit. 6.1.2 Add document disclaimer (for web-based documents): Include a document control disclaimer at the beginning of each web-based document that addresses the need to verify printed copies are current prior to use. An example of such a disclaimer is "The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website". (See message at the beginning of this document for a disclaimer example). 6.1.3 Review Document: Distribute the draft procedure for review to appropriate individuals. When several individuals will be reviewing the document, some form of tracking mechanism should be used. Determine if a procedure is Safety Significant (A1/A2 ESH&Q risk level) by utilizing the Screening Matrix for ESH&Q Risk Levels. All Safety Significant Documents must be sent to the appropriate NSLS or NSLS II ESH staff member for review. The NSLS Quality Representative or the NSLS II ES&H Coordinator can assist in determining if the document is safety significant. Some examples of safety significant documents but not limited to, are energy control procedures, working with chemicals, interlock testing, and fall protection. Guidance: If the document only affects workers within a group, then the reviews can usually reside with the individuals of that group, the group supervisor, and/or the section head. If the document affects workers within and/or outside of the
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 6 of 11
group developing the document, the review needs to extend to responsible individuals affected and/or higher level management. In either situation, an NSLS or NSLS II ESH staff member needs to review the document if it has been determined Safety Significant. 6.1.4 Approve Document: Upon completion/concurrence of review, obtain approval signatures from appropriate individuals. Remove the "DRAFT" marking from the document. The preparer and authorized individual(s) must provide their approval signature on a Light Sources Directorate Document Approval Form (DL-QAF-036). The graded approach should be used when determining who should authorize documents. At a minimum, the person that prepared the document and responsible manager/supervisor must provide approval signatures. NSLS Section Heads or NSLS II Division Directors can determine if further signatures are required.
If document is Safety Significant, an NSLS or NSLS II ESH staff member must sign the approval form under the Safety Review section. Guidance: If the controlled document only applies to workers within a group, the group supervisor and/or NSLS Section Heads or NSLS II Division Directors should sign-off on documents. If the controlled document applies to workers in multiple groups, section heads and/or higher level management should sign-off on documents. Note: Signature approvals for support documents, forms, and operator aids, should reside on the copy on file. Approval of forms may be included with the form's master document/procedure.
6.1.5 Complete a "Revision Log" and determine a "Review Frequency". Paper/PDF documents - attach/include a "Revision Log" (QF-037) at the end of each document.
HTML documents - include/link a "Revision Log" (QF-038) file (see the header of this procedure for example). EMS/OHSAS Support Documents:
Specific EMS and OHSAS support documents identified in Appendix B require a
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 7 of 11
revision log. For those documents listed in Appendix B that do not require a revision log, changes to a document should be recorded in the NSLS or NSLS II Assessment Tracking System (ATS) history field.
Forms - Refer to Appendix B for stand-alone forms requiring a revision log. If a form is correlated with a controlled document (e.g. procedure, policy, etc.) utilize that document's revision log to capture any changes to the form. Operator aids: Revision log is not required.
6.1.5.a Include the following information in the Revision Log: 1. Complete the Revision log by including: subject /document title; document number; revision letter or number; description of key changes (Example wording for first revision include "Initial Release", First Release", or "Original Release") describe "Why" a step was added or changes were made if related to Occupational, Health, and Safety or significant environmental aspect. In addition reference source events, when applicable (e.g. Step added or changed due to safety incident or Nonconformance Report # LS-NC-2005-XXXX). 6.1.5.b Determine the required Document Review Frequency1 (time period) and enter this value in the designated location on the form.
1
Controlled documents must be periodically reviewed to verify their accuracy. The cognizant engineer, scientist, or manager shall determine the frequency of review. The review frequency of controlled documents can be set to once every year or up to once every 5 years depending on the Environmental, Safety, Health, or Programmatic Impact. The greater the impact, the smaller the review period and vice versa. Emergency or safety procedures may need to be reviewed more frequently (e.g., 1 year). BNL or external drivers may already require a specific review period for a particular document. For example SBMS requires all energy control procedures to be reviewed at least annually. The NSLS QCC or NSLS II DRA will issue a reminder notice to responsible personnel 2-months prior to the periodic review due date for all documents entered into the NSLS QA database or the NSLS II Document Management System. An automatic reminder will be sent to responsible individuals for controlled documents entered into the NSLS or NSLS II Assessment Tracking System (ATS). 6.1.6 Submit Document to NSLS QCC or NSLS II DRA:
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 8 of 11
Policies, manuals, procedures, and requirements: 1. Completed copy of the Policy, manual, procedure, or requirement; 2. A completed Revision Log; 3. A completed Approval Form (include safety review signature, if applicable); 4. Controlled Document Distribution list (QF-051)- The distribution list is applicable for paper copies only. Paper documents that are distributed through this form will be stamped "Controlled Copy". Note: It is recommended that an ELECTRONIC COPY of the document be sent to the group's secretary/administrative assistant. Use document number and revision letter/number as file name (e.g. DL-QAP-0004_RevA). Plans, EMS, and OHSAS Support Document: 1. EMS/OHSAS Support Documents are submitted as part of the master document or as an EMS/OHSAS record in accordance with QA procedure Light Sources Directorate EMS/OHSAS Records Management (DL-QAP1003). Forms and Operator Aids: 1. Standalone forms are not required to be maintained by the NSLS QCC or NSLS II DRA except where specified in Appendix B.
Notes:
The NSLS QCC or NSLS II DRA will maintain the master paper copy of the controlled document with signature approvals, distribute copies as needed (distribution list must be provided), and enter the document information into the NSLS QA Database, the NSLS II Document Management System or ATS for tracking purposes. Original signed documents must not be placed in circulation.
All controlled documents on file will be maintained by the NSLS QA group or the NSLS II Configuration Manager in accordance with QA procedure Light Sources Directorate EMS/OHSAS Records Management (DL-QAP-1003) and/or the Records Management subject area.
6.2 REVISING AN EXISTING CONTROLLED DOCUMENT 6.2.1
Upon the need for revising an existing controlled document, the responsible individual or designee prepares a draft procedure as follows:
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 9 of 11
Raise the revision level by a letter/number on the new draft document and review/revise the remaining standard document elements accordingly. circulate the draft document for review as outlined in step 6.1.3; upon concurrence, distribute document for approval as outlined in step 6.1.4; update the revision log as outlined in step 6.1.5. Record key changes in the revision log along with the new revision letter/number and effective date; submit material (signed original documents, revision log, distribution list, electronic copies) to the designated individual as per step 6.1.6.
This process should include the review of requirements/drivers for each document, e.g. SBMS or external, checking hyperlinks, making organizational changes, updating forms, verifying definitions and references. Note: Before removing a step or content from a procedure consider the Occupational, Safety, Health (OSH) and significant environmental aspects. Information should not be removed from these documents without knowing why it was there or changed. Reference the revision log or NSLS or NSLS II ATS for past history to help prevent inadvertent deletion of important text before changing document content.
6.3 PERIODIC REVIEW OF A DOCUMENT 6.3.1
Responsible individuals or designees are responsible for periodically reviewing active controlled documents by the review frequency specified. Upon review of the document the responsible individual or designee does the following: a. If after reviewing the document the result is that the document is accurate as is, up-to-date, reflects current requirements/policy, and changes are not needed, do the following: i. ii. Policies, manuals, procedures, and requirements: Complete a Periodic Document Review Form and submit to the NSLS QCC or NSLS II DRA EMS, and OHSAS Support Document: For EMS/OHSAS Documents identified in Appendix B, submit an ATS closure statement for documents captured in the NSLS or NSLS II Assessment Tracking System (ATS). iii. Forms and Operator Aids: Review should be included with the form's master procedure/document. Standalone Forms and Operator Aids not
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 10 of 11
captured in the NSLS QA Database or NSLS or NSLS II ATS are to be reviewed as the form is used and revised when the changes to the associated process affect the form. Note: Some documents may be reviewed with the master document such as a manual or procedure and some documents will be reviewed as stand alone. Appendix B identifies those EMS/OHSAS documents that will be reviewed with the master document. The Periodic Document Review Form will be maintained in the Central Filing System with the corresponding controlled document and can be signed off multiple times for future reviews. See the NSLS QCC or NSLS II DRA if you wish to follow this method. b. If the result of the periodic review calls for changes, then revise the document as per step 6.2. Note: All review signatures reside on file with the master copy
The only official copy of this file is the one on-line in the NSLS Quality Assurance website. Before using a printed copy, verify that it is the most current version by checking the document effective date on the NSLS QA website. Brookhaven National Laboratory/ Light Sources Directorate
Subject: Number:
Document Preparation and Control
DL-QAP-0414 Revision: A Effective: 5/15/2008 Page 11 of 11
Document Review Frequency 3
Years
Review signatures on file with master copy of controlled document
LIGHT SOURCES DIRECTORATE REVISION LOG Document Number: DL-QAP-0414 Document Preparation and Control Subject Rev
A
Description
Initial Document for Light Source Directorate see LS-QAP-0414
Date
5/15/2008
Das könnte Ihnen auch gefallen
- Quality Assurance FrameworkDokument9 SeitenQuality Assurance FrameworkAinil MardiahNoch keine Bewertungen
- Product Qualification Requirements 2.0Dokument3 SeitenProduct Qualification Requirements 2.0Amit SinghNoch keine Bewertungen
- PassThru API 1Dokument83 SeitenPassThru API 1andaposa9Noch keine Bewertungen
- ISO 9001:2008 Standard Operating Procedures Manual: A P & C, IDokument88 SeitenISO 9001:2008 Standard Operating Procedures Manual: A P & C, IBuenoflor GrandeaNoch keine Bewertungen
- Developing E-Business Systems and ArchitecturesDokument147 SeitenDeveloping E-Business Systems and ArchitecturesNikola LanjakNoch keine Bewertungen
- SopDokument87 SeitenSopskynyrd75100% (3)
- P Deviation ProcedureDokument4 SeitenP Deviation Procedureshakti sindhu100% (1)
- Standard Operating Procedures (SOP) - Back Bone of Pharmaceutical IndustriesDokument37 SeitenStandard Operating Procedures (SOP) - Back Bone of Pharmaceutical Industriessaininavdeep077Noch keine Bewertungen
- Good Agriculture Practice (An Approach)Dokument28 SeitenGood Agriculture Practice (An Approach)Robinson GultomNoch keine Bewertungen
- Software Quality AssuranceDokument23 SeitenSoftware Quality AssuranceAbhishek sonuneNoch keine Bewertungen
- Measuring Safety Performance SlidesDokument66 SeitenMeasuring Safety Performance SlidesRobinson Gultom100% (1)
- The Cost of Production - Chapter 7Dokument18 SeitenThe Cost of Production - Chapter 7rajesh shekarNoch keine Bewertungen
- Asq What Is A Quality PlanDokument3 SeitenAsq What Is A Quality PlanCloud RedfieldNoch keine Bewertungen
- Purpose / Scope:: Information Technology Management Approval: Managing DirectorDokument17 SeitenPurpose / Scope:: Information Technology Management Approval: Managing DirectorMuhammad AsimNoch keine Bewertungen
- 2018423-ISO 9001 OLD Vs NEWDokument6 Seiten2018423-ISO 9001 OLD Vs NEWAnand K. MouryaNoch keine Bewertungen
- 6ChangeRequestTemplate EPMO 03162009Dokument3 Seiten6ChangeRequestTemplate EPMO 03162009Phạm TuyếtNoch keine Bewertungen
- Analysis Report TemplateDokument2 SeitenAnalysis Report Templatemounit121Noch keine Bewertungen
- Columbus Europe's Laboratory On The International Space StationDokument39 SeitenColumbus Europe's Laboratory On The International Space StationBob AndrepontNoch keine Bewertungen
- WIPO DL-401 Managing Intellectual Property in The Book Publishing Industry Final ExamDokument3 SeitenWIPO DL-401 Managing Intellectual Property in The Book Publishing Industry Final ExamSingh SudipNoch keine Bewertungen
- Design Mee2008 VusDokument42 SeitenDesign Mee2008 VusRajesh RJNoch keine Bewertungen
- Change Control Request Form Change Request Number:: General InformationDokument2 SeitenChange Control Request Form Change Request Number:: General Informationarunrpatil0465450% (1)
- Juniper RMA Return Procedure 9060008-EnDokument2 SeitenJuniper RMA Return Procedure 9060008-EnAdnan MohammadNoch keine Bewertungen
- The Complete Guide To Iso 13485 For Medical DevicesDokument22 SeitenThe Complete Guide To Iso 13485 For Medical DevicesCendur AbithanNoch keine Bewertungen
- Business Requirements Document TemplateDokument9 SeitenBusiness Requirements Document TemplateMeghan ChandlerNoch keine Bewertungen
- Revision Record Sheet: TitleDokument4 SeitenRevision Record Sheet: TitleSanjay MalhotraNoch keine Bewertungen
- Developing Operational Review Programmes For Managerial and Audit UseDokument38 SeitenDeveloping Operational Review Programmes For Managerial and Audit UseRoxanne CerioNoch keine Bewertungen
- GMP Quality Documentation Control Tracking and Distribution QMS-025 SampleDokument13 SeitenGMP Quality Documentation Control Tracking and Distribution QMS-025 SampleMostafa FawzyNoch keine Bewertungen
- Voltage Stress in Power SystemsDokument29 SeitenVoltage Stress in Power Systemssorry2qazNoch keine Bewertungen
- Understanding Document Control by Adanma Kadiri.Dokument12 SeitenUnderstanding Document Control by Adanma Kadiri.Kelechi OchuloNoch keine Bewertungen
- Boiler MaintenanceDokument6 SeitenBoiler MaintenanceRamalingam PrabhakaranNoch keine Bewertungen
- How To Write SOPDokument4 SeitenHow To Write SOPYousifNoch keine Bewertungen
- ERP Life CycleDokument9 SeitenERP Life CycleChintu Sisodia100% (1)
- Quality Register: Project Name: Date: Release: Author: Owner: ClientDokument3 SeitenQuality Register: Project Name: Date: Release: Author: Owner: ClientfarazNoch keine Bewertungen
- Change Request FormDokument2 SeitenChange Request FormMadjid MansouriNoch keine Bewertungen
- ISO 13485 PurchasingDokument5 SeitenISO 13485 PurchasingSubhashNoch keine Bewertungen
- Change Request FlowDokument1 SeiteChange Request Flowyash shahNoch keine Bewertungen
- Change Request: Project Title: Date PreparedDokument2 SeitenChange Request: Project Title: Date PreparedDiggerNoch keine Bewertungen
- COP5 - F06 Level 3 Audit ChecklistDokument9 SeitenCOP5 - F06 Level 3 Audit ChecklistEdgar G. Sánchez A.Noch keine Bewertungen
- Vendor CertificationDokument5 SeitenVendor CertificationAliqahwashNoch keine Bewertungen
- V&V Plan TemplateDokument12 SeitenV&V Plan TemplateYaw Choon KitNoch keine Bewertungen
- Vendor Assessment Questionnaire: Pt. Bakrie Pipe IndustriesDokument7 SeitenVendor Assessment Questionnaire: Pt. Bakrie Pipe IndustriesBang UcuppNoch keine Bewertungen
- An Overview of IEEE Software Engineering Standards and Knowledge ProductsDokument46 SeitenAn Overview of IEEE Software Engineering Standards and Knowledge ProductsNava GrahazNoch keine Bewertungen
- QMS 010 Classification Definition and Approval Matrix of GMP Documents SampleDokument5 SeitenQMS 010 Classification Definition and Approval Matrix of GMP Documents SampleRosella Planta100% (1)
- 53 Arc 0000Dokument27 Seiten53 Arc 0000Mustafa AlHalfawiNoch keine Bewertungen
- Customer Experience MakeoverDokument11 SeitenCustomer Experience MakeoverWaqas AzizNoch keine Bewertungen
- IEEE STD 12207 PDFDokument12 SeitenIEEE STD 12207 PDFMilorad PavlovicNoch keine Bewertungen
- QT9 QMS Proposal 11 Apr 17 - PT Timas Suplindo - IlhamDokument15 SeitenQT9 QMS Proposal 11 Apr 17 - PT Timas Suplindo - IlhamIlham YahyaNoch keine Bewertungen
- QMS ProposalDokument22 SeitenQMS Proposalflawlessy2kNoch keine Bewertungen
- ISO 14641 1 A Complete Guide - 2019 EditionVon EverandISO 14641 1 A Complete Guide - 2019 EditionBewertung: 1 von 5 Sternen1/5 (1)
- Control of documents-QMP-SYS-01Dokument5 SeitenControl of documents-QMP-SYS-01Rohit VishwakarmaNoch keine Bewertungen
- IDCT01 Infrastructure Project DevelopmentDokument16 SeitenIDCT01 Infrastructure Project DevelopmentPauline Caceres AbayaNoch keine Bewertungen
- Abnormal Audit Fee and Audit QualityDokument23 SeitenAbnormal Audit Fee and Audit QualityYohaNnesDeSetiyanToNoch keine Bewertungen
- Clinical Research Organisation CultureDokument3 SeitenClinical Research Organisation CultureZain MalikNoch keine Bewertungen
- Electronic Document Management in ConstructionDokument13 SeitenElectronic Document Management in Constructionxai_hafeezNoch keine Bewertungen
- RFC TemplateDokument2 SeitenRFC TemplateGustavo Lama ValdiviaNoch keine Bewertungen
- Change Management Plan TemplateDokument7 SeitenChange Management Plan TemplateAndrés PacompíaNoch keine Bewertungen
- ERP WorkFlowDokument1 SeiteERP WorkFlownagarajs50Noch keine Bewertungen
- Documentation and RecordsDokument9 SeitenDocumentation and RecordsWambi DanielcollinsNoch keine Bewertungen
- Template Nonconformity FormDokument3 SeitenTemplate Nonconformity Formavinash_k007Noch keine Bewertungen
- Internal Audit ProcDokument7 SeitenInternal Audit ProcVera BoshovaNoch keine Bewertungen
- Stakeholder Engagement A ToolkitDokument38 SeitenStakeholder Engagement A ToolkitNelsonNúñezVidalNoch keine Bewertungen
- Legal Department Request FormDokument1 SeiteLegal Department Request FormCloudette BaguiwenNoch keine Bewertungen
- What Is Iso CertificationDokument4 SeitenWhat Is Iso CertificationShailesh GuptaNoch keine Bewertungen
- Conformity Assessment (Management System Certification)Dokument5 SeitenConformity Assessment (Management System Certification)Talal AhmedNoch keine Bewertungen
- BA Handover ChecklistDokument1 SeiteBA Handover ChecklistSumber UnduhNoch keine Bewertungen
- Permentan No 11 Tahun 2015 Tentang ISPODokument9 SeitenPermentan No 11 Tahun 2015 Tentang ISPORobinson Gultom0% (1)
- CITES Appendices I, II, III Valid From Apr-3-2012Dokument46 SeitenCITES Appendices I, II, III Valid From Apr-3-2012Robinson GultomNoch keine Bewertungen
- I Have A Website Called GravitywriteDokument6 SeitenI Have A Website Called GravitywriteNirmal KumarNoch keine Bewertungen
- Energy Management and Heat Storage For Solar Adsorption CoolingDokument11 SeitenEnergy Management and Heat Storage For Solar Adsorption CoolingRendy Adhi RachmantoNoch keine Bewertungen
- Cosivis eDokument208 SeitenCosivis eZadiel MirelesNoch keine Bewertungen
- Project Report (IoT)Dokument20 SeitenProject Report (IoT)gayatri kamatNoch keine Bewertungen
- List Lay Cable For ControlDokument5 SeitenList Lay Cable For ControlBayu SeptiadiNoch keine Bewertungen
- Unit I - Basics of C ProgrammingDokument44 SeitenUnit I - Basics of C ProgrammingHarsh DeshwalNoch keine Bewertungen
- UKOOA Telecoms GuidelinesDokument35 SeitenUKOOA Telecoms GuidelinesmaarutzNoch keine Bewertungen
- Release 6.0: Technical Solution ManualDokument14 SeitenRelease 6.0: Technical Solution ManualcosconorNoch keine Bewertungen
- RTN 300 TrainingDokument21 SeitenRTN 300 TrainingJim Jr . OyolaNoch keine Bewertungen
- It 14Dokument3 SeitenIt 14Izza WrapNoch keine Bewertungen
- Technical Specification: ConfigurationDokument7 SeitenTechnical Specification: ConfigurationAhmedRamadanNoch keine Bewertungen
- Hospital Equipment and Its Management System A MinDokument4 SeitenHospital Equipment and Its Management System A MinPaola LorenzattoNoch keine Bewertungen
- Baggage X RayDokument4 SeitenBaggage X RayShaaban HassanNoch keine Bewertungen
- PDG 40S QeDokument2 SeitenPDG 40S QeSamuel S. RoxasNoch keine Bewertungen
- SRM University Smart Grids: Nihal Farhan Arnab Dash A.Vinod Kumar P.Upendra 1041110454 ECE-H (3 YR)Dokument7 SeitenSRM University Smart Grids: Nihal Farhan Arnab Dash A.Vinod Kumar P.Upendra 1041110454 ECE-H (3 YR)Primearnab DashNoch keine Bewertungen
- Blue Eyes Technology ReportDokument31 SeitenBlue Eyes Technology ReportShubham Chauhan S.Noch keine Bewertungen
- 30 Rat BCU TechsheetDokument2 Seiten30 Rat BCU TechsheetBryan OngNoch keine Bewertungen
- MCQ OsDokument5 SeitenMCQ Oskushagra sharmaNoch keine Bewertungen
- Riso Page CalculatorDokument19 SeitenRiso Page CalculatorNana Kwabena Ampem DarkoNoch keine Bewertungen
- Future MuseumDokument12 SeitenFuture MuseumPatriciaNoch keine Bewertungen
- 01 OnePlus Mobiles by United Mobile Flyer 05821Dokument2 Seiten01 OnePlus Mobiles by United Mobile Flyer 05821Malik Amir AbbasNoch keine Bewertungen
- 14 MibsDokument38 Seiten14 MibsSherif_SalamaNoch keine Bewertungen
- Product Data Sheet: Gypframe® MF5 Ceiling SectionDokument2 SeitenProduct Data Sheet: Gypframe® MF5 Ceiling SectionJanna BakeerNoch keine Bewertungen
- 10ECL47 MICROCONTROLLER Lab ManualDokument142 Seiten10ECL47 MICROCONTROLLER Lab ManualVishalakshi B HiremaniNoch keine Bewertungen
- CV Iqbal 2016Dokument3 SeitenCV Iqbal 2016Muhammad Iqbal ImaduddinNoch keine Bewertungen
- Samco CarryallDokument4 SeitenSamco CarryallbillNoch keine Bewertungen
- CS-C12BKPG Cu-2c24bkp5gDokument70 SeitenCS-C12BKPG Cu-2c24bkp5gDũng LêNoch keine Bewertungen